Abstract
Whipple disease is a granulomatous infectious disease caused by Tropheryma whipplei. The bacteria accumulate within macrophages, preferentially in the intestinal mucosa. Disease manifestation seems to be linked to immunological abnormalities of macrophages. We describe a patient with cerebral Whipple disease who presented with changes in mental status, confusion, inverse sleep–wake cycle, bilateral ptosis and vertical gaze palsy. Endoscopic biopsy sampling revealed Whipple disease in the gastric antrum but not in the duodenum. Whole blood stimulation displayed reactivity to T whipplei that was at the lower end of healthy controls while reactivity of duodenal lymphocytes was not diminished. We propose that in cases of neurological symptoms suspicious of Whipple disease with normal duodenal and jenunal findings, biopsy sampling should be extended to the gastric mucosa. The robust reactivity of duodenal lymphocytes may have prevented our patient from developing small bowel disease, whereas the impaired reactivity in peripheral blood lymphocytes might yet explain the bacterial spreading to the central nervous system leading to the rare case of predominant neurological symptoms without relevant systemic involvement.
Whipple disease (WD) is a multisystemic granulomatous infectious disease caused by Tropheryma whipplei, a rod shaped, gram positive actinomycete. It preferentially involves joints and the gastrointestinal tract, but may spread to other organs, including the heart, lung and brain. The bacteria accumulate within macrophages in the intestinal mucosa causing diarrhoea, malabsorption and abdominal pain.1T whipplei infection of the central nervous system (CNS) occurs in up to 40% of patients2 but clinical presentation of isolated neurological symptoms is rare.3 As T whipplei is ubiquitously present in the environment, a predisposition for the disease manifestation seems to be linked to immunological abnormalities, in particular deficits of macrophages.1,4
Case report
A 53‐year‐old man presented with a 4 year history of low grade fever. Over several months before admission, progressive changes in mental status were noted which included apathy, fluctuating level of consciousness, inappropriate social behaviour and transient confusion. He showed marked nocturnal insomnia of only 3 h sleep and an inverse sleep–wake cycle. No gastrointestinal symptoms were elicited.
The neurological examination revealed bilateral ptosis and vertical gaze palsy involving saccadic and smooth pursuit movements with preserved vestibulo‐ocular reflexes. Horizontal saccades were slow with a reduced range of movement while horizontal vestibulo‐ocular reflexes were intact. Cerebral MRI and routine laboratory examinations showed no pathological findings. CSF analysis showed slight pleocytosis and no synthesis of intrathecal oligoclonal immunoglobulin. PCR examinations of CSF were positive for T whipplei.
Upper gastrointestinal endoscopy with antral, corpus, duodenal and jejunal biopsies was performed. Histological examination of duodenal and jejunal specimens showed widely normal tissue according to Marsh‐0 grading,5 and PCR for T whipplei was negative. To minimise sampling error, as WD involvement can be patchy, endoscopy with extensive duodenal and upper jejunal biopsies (n = 12) was repeated and confirmed negative PCR and periodic acid–Schiff (PAS) staining. However, antral biopsies from both endoscopies revealed marginal intestinal metaplasia and foamy macrophages that were stuffed with intracellular PAS positive granules (fig 1C). PCR assay for T whipplei performed on these biopsies was positive. Corpus biopsies showed no signs of WD, and gastric Helicobacter pylori and mycobacterial infection were excluded.
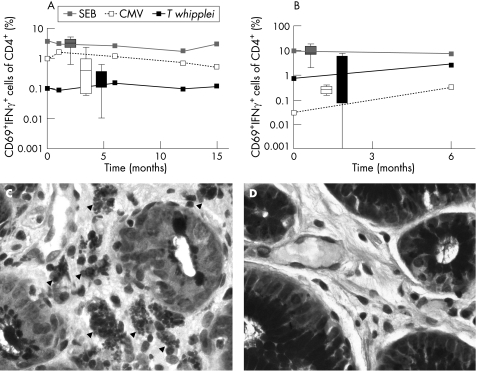
Figure 1 Stimulation of whole blood (A) and duodenal lymphocytes (B) with staphylococcus enterotoxin B (SEB 2 μg/ml), cytomegalovirus (CMV 1.2 μg/ml) and Tropheryma whipplei lysate (T whipplei 107 bacteria/ml) (for methods see Working Group on Coeliac Disease of the United European Gastroenterology Week in Amsterdam5). The curves represent the time course of per cent CD69+ interferon γ+ (IFNγ) cells of CD4+ lymphocytes of the patient, and box and whisker values of unaffected controls (10 subjects during follow‐up of ulcer disease without Whipple disease served as controls). (C, D) Periodic acid–Schiff (PAS) staining of biopsies from the antrum. (C) T whipplei infected type I macrophages (arrowheads, see also von Herbay and colleagues9) in the antrum before the onset of therapy. (D) PAS negative antrum 18 months after the onset of therapy.
Antibiotic therapy with ceftriaxone (2 g/day intravenously) was given for 14 days, followed by oral trimethoprim–sulfamethoxazole (160/800 mg twice a day) for 6 months. Three months after initiation of therapy (3 month follow‐up), the patient was fully orientated with markedly improved drive and restored sleep–wake cycle, with total sleep duration increased to 6 h. The neuropsychological examination revealed deficits in sustained attention and anterograde memory. However, the ophthalmoparesis deteriorated further, resulting in almost complete loss of voluntary vertical and horizontal eye movements.
At the 6 month follow‐up, examination revealed complete supranuclear gaze palsy, slight pleocytosis of the CSF with identical oligoclonal bands in CSF and serum, negative T whipplei PCR of CSF and a decreasing number of PAS positive macrophages in the lamina propria of the gastric antrum. Because of deterioration of the oculomotor symptoms, antibiotic treatment was extended, and meropenem (1000 mg three times a day) was given for 14 days. Between the 9 and 15 month follow‐up, only minimal improvement in ophthalmoparesis was seen. PCR of CSF remained negative for T whipplei. On follow‐up endoscopy after 18 months, gastric biopsies revealed no PAS positive material (fig 1D).
Lymphocyte stimulation
T cell reactivity was determined by four colour flow cytometry, as described previously.6 The short period (6 h) of whole blood stimulation with staphylococcus enterotoxin B and cytomegalovirus revealed normal reactivity of CD4+ T cells (%CD69+ interferon γ+ of CD4+ cells) compared with healthy subjects, whereas reactivity to T whipplei was at the low end of normal compared with healthy subjects (fig 1A). The antigen specific reactivity did not change in blood cells during 18 months of follow‐up, with an impaired T whipplei specific reaction despite treatment and clinical improvement. The absolute number of T cells, their subtypes (49.19% CD4+, 42.03% CD8+ of CD3+) and the percentage of CD4+CD25high regulatory T cells (2.51% of CD4+ cells) were equivalent to normal controls. Duodenal lymphocytes (intraepithelial and lamina propria lymphocytes) revealed a stable reactivity to staphylococcus enterotoxin B during the 6 months of follow‐up that was similar to healthy controls. The cytomegalovirus specific reactivity was below the reactivity of healthy controls before antibiotic therapy but increased to normal after 6 months of follow‐up. In contrast with other WD patients, duodenal lymphocytes did not show a diminished T whipplei specific reactivity compared with healthy subjects before treatment. In addition, the T whipplei specific reactivity increased after 6 months (fig 1B).
Discussion
The predominant neurological symptoms of our patient with WD in the absence of gastrointestinal problems represented a clinical challenge, even more so as routine endoscopy with biopsy sampling from both the proximal and distal duodenum and the upper part of the jejunum revealed no PAS positive material and normal PCR results. However, antral biopsies showed foamy macrophages with intracellular PAS positive granules. Only a few patients with cerebral WD have been described as lacking pathognomonic PAS positive material in small bowel macrophages,7 and only single reports have described WD with prominent involvement of the oesophagus, terminal ileum or colon.8 To the best of our knowledge, there has been no previous report of positive gastric biopsies in patients with WD. Thus the present findings suggest a very unusual material distribution in the digestive tract. We therefore propose that in cases of neurological symptoms suspicious of WD with normal duodenal and jejunal findings, biopsy sampling should be extended to the gastric mucosa.
The clinical manifestations in WD patients seem to result from immunological abnormalities with defective T‐helper cell immunity of type 1 that may be caused by disturbed macrophage functions.1,4 We recently established T whipplei specific stimulation of CD4+ T cells that was shown to be induced by proteins of T whipplei and did not reflect cross reactivity to phylogenetically related actinomycetes.6 In the present case, whole blood stimulation displayed reactivity to T whipplei that was at the lower end of healthy controls, underscoring the known antigen specific impaired immune response (fig 1A). However, reactivity of duodenal lymphocytes was not diminished compared with healthy subjects (fig 1B). These immunological results contrast with data from other patients as our patient revealed the highest T whipplei specific reactivity in both peripheral blood and duodenal lymphocytes among all patients tested to date. Usually both whole blood and duodenal lymphocytes show markedly impaired reactivity in WD patients.6 The robust reactivity of duodenal lymphocytes might have prevented our patient from developing small bowel disease and consequently gastrointestinal symptoms such as malabsorption. Moreover, the impaired reactivity in peripheral blood lymphocytes might yet explain bacterial spreading to the CNS leading to the rare case of predominant neurological symptoms without relevant systemic involvement. However, as the reactivity of cerebral lymphocytes could not be determined because of the low cell numbers in the CSF and gastric reactivity was not investigated, we cannot comment on the immunological features of further organs and tissues in this patient which might participate in the unusual clinical WD manifestation. Thus further examination of patients with isolated CNS WD is needed to confirm this hypothesis of local immune protection.
Neurological symptoms in WD patients are frequently interpreted as relapse after insufficient systemic antibiotic treatment. In this case, however, the patient had not received prior antibiotic treatments. Moreover, the presence of type I macrophages9 within the antral mucosa supports the assumption of untreated WD. Taken together, the present case suggests that tissues might be affected atypically in cerebral WD. Thus gastrointestinal samples (both antrum and corpus) not routinely used for diagnosis of WD should be considered for histological examination in patients with negative duodenal biopsies.
Abbreviations
CNS - central nervous system
PAS - periodic acid–Schiff
WD - Whipple disease
Footnotes
Competing interests: None.
References
- 1.Fenollar F, Puechal X, Raoult D. Whipple's disease. N Engl J Med 200735655–66. [DOI] [PubMed] [Google Scholar]
- 2.Louis E D, Lynch T, Kaufmann P.et al Diagnostic guidelines in central nervous system Whipple's disease. Ann Neurol 199640561–568. [DOI] [PubMed] [Google Scholar]
- 3.Suzer T, Demirkan N, Tahta K.et al Whipple's disease confined to the central nervous system: case report and review of the literature. Scand J Infect Dis 199931411–414. [DOI] [PubMed] [Google Scholar]
- 4.Desnues B, Ihrig M, Raoult D.et al Whipple's disease: a macrophage disease. Clin Vaccine Immunol 200613170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Working Group on Coeliac Disease of the United European Gastroenterology Week in Amsterdam When is a coeliac a coeliac? Eur J Gastroenterol Hepatol 2001131123–1128. [DOI] [PubMed] [Google Scholar]
- 6.Moos V, Kunkel D, Marth T.et al Reduced peripheral and mucosal Tropheryma whipplei‐specific Th1 response in patients with Whipple's disease. J Immunol 20061772015–2022. [DOI] [PubMed] [Google Scholar]
- 7.Galldiks N, Burghaus L, Vollmar S.et al Novel neuroimaging findings in a patient with cerebral Whipple's disease: a magnetic resonance imaging and positron emission tomography study. J Neuroimaging 200414372–376. [DOI] [PubMed] [Google Scholar]
- 8.Marcial M A, Villafana M. Whipple's disease with esophageal and colonic involvement: endoscopic and histopathologic findings. Gastrointest Endosc 199746263–266. [DOI] [PubMed] [Google Scholar]
- 9.von Herbay A, Maiwald M, Ditton H J.et al Histology of intestinal Whipple's disease revisited. A study of 48 patients. Virchows Arch 1996429335–343. [DOI] [PubMed] [Google Scholar]