Short abstract
Myotonic dystrophy type 1 (DM1) is the most common type of muscular dystrophy in adults. Approximately 60% of individuals report either having difficulty performing or being unable to carry out some activities related to home management, mobility and transportation, work and leisure. Employment, educational level and income are, on average, lower than in the general population. The complexity and variability of disease manifestations in DM1 undoubtedly pose a challenge as regards anticipating all potential problems and developing a plan for health and community management. This article presents a conceptual model for DM1 management as well as a brief discussion of an approach for developing interdisciplinary health and community services.
Myotonic dystrophy type 1 (DM1, OMIM 160900) is the most common type of muscular dystrophy in adults. Its estimated prevalence ranges between 2.1 and 14.3 per 100 000 worldwide, but reaches 189 per 100 000 in the Saguenay–Lac‐St‐Jean region of the province of Quebec, Canada.1,2 DM1 is an autosomal dominant disease caused by an unstable trinucleotide repeat expansion of the cytosine–thymine–guanine [CTG]n located in the 3′ untranslated region of chromosome 19q13.3.3 DM1 was first described as a muscle disease with gonadal involvement in 1909. Since then, it has been recognised as a multisystemic disorder with various impairments, especially in the muscular, respiratory, cardiac, central nervous, endocrine and ocular systems.4 Typical symptoms of the disease include progressive loss of muscle strength, usually distal to proximal, ptosis, weakness of facial and anterior neck muscles, myotonia, daytime somnolence and cataracts.4 DM1 may also affect the ability of patients to carry out certain daily activities and social roles. Approximately 60% of individuals report either having difficulty performing or being unable to carry out some activities related to home management, mobility and transportation, work and leisure.5 Employment, educational level and income are, on average, lower than in the general population.6
The [CTG]n expansion responsible for DM1 can vary from 50 to over 1000 repetitions, and contributes to a broad range of different phenotypes. Four different clinical phenotypes are recognised in DM1 according to age of onset in conjunction with [CTG]n repeats: congenital, childhood, classic (adult) and late onset forms.7
The diagnosis of DM1 must be considered across the entire age range of a person's lifespan, and the age at diagnosis will bear management implications according to the severity of the manifestations of the disease. Onset clinical manifestations display great variation between and within each phenotype. While some individuals may consult a neurologist for typical symptoms of severe muscle weakness and myotonia, others may only see an ophthalmologist for cataracts. Anticipation, defined as the earlier onset of symptoms of increasing severity in successive generations within a family, also contributes to the complexity of the clinical manifestations of DM1.2 This complexity not only poses a challenge in establishing a diagnosis of DM1, but also in managing the wide variety of disease manifestations.
Over the past 20 years, recommendations regarding diagnosis, management and care delivery for individuals with genetic disorders have stemmed from the disciplines of medical genetics, paediatrics and neurology.8 Under optimal circumstances, fundamental components in the clinical care of patients with genetic diseases, such as DM1, need to include: (a) appropriate clinical–genetic screening; (b) specific preventive treatments; (c) guidance and anticipation of future care needs; (d) social evaluations to monitor patients for complications; and (e) effective communication and trust with patients and their families. The complexity and variability of disease manifestations in DM1 undoubtedly pose a challenge as regards anticipating all potential problems and developing a plan for health and community management. DM1 patient follow‐ups have been described as fragmented, inadequate or even deficient for many patients.4,9 Numerous factors, namely health (multisystemic disease with several disabilities), economic (low income), social (poor social support network) and low educational level, may contribute to this situation. These different factors emphasise the need for a comprehensive management approach in DM1 patient care. This article presents a conceptual model for DM1 management as well as a brief discussion of an approach for developing interdisciplinary health and community services.
Development of a conceptual framework for management of myotonic dystrophy
Developing integrated approach plans for managing various aspects of DM1 disease manifestations is important to ensure optimal care. Such an integrated plan can be enhanced through the use of a conceptual framework illustrating the essential items in overall management and, more importantly, the relationship between these different aspects of care. A conceptual approach of this type has been used in diseases such as spina bifida to evaluate its usefulness in integrating management of impairment, disability and restrictions in participation. Such an approach has helped develop standards of care, integrated services and policies.10 In DM1, this conceptual framework may help clinicians: (1) assess all aspects of management at a glance; (2) establish links between several factors influencing social participation; and (3) improve anticipatory guidance through an adequate interdisciplinary health and community management plan.
The traditional medical care model focuses on treatment of impairments. However, more recent models, including the International Classification of Functioning, Disability and Health (ICF), have explained the process of disablement not only in terms of the presence of impairments and disabilities per se, but also in terms of changes in the physical and social environments of affected individuals.11,12 We will discuss briefly two disability models—the ICF and the application of the Disability Creation Process (DCP) model to the management of DM1.
International Classification of Functioning, Disability and Health
The World Health Organisation (WHO) recently issued the final version of the ICIDH‐2, now called the ICF.11 Many studies have used the ICF model since its adoption13,17 but there have been some criticisms.14 Firstly, the model kept parts of its linearity, which may lead the disablement process to be viewed as static, sequential and unidirectional. Secondly, environment is not seen as a definite dimension of the model but as a contextual factor, which does not sufficiently emphasise the enormous role of environmental factors.15 As DM1 is overrepresented in the poorer strata of society,6 the influence of environmental factors (community management), such as allocation of healthcare resources or family and social support, must be integrated in the global management plan, and the conceptual framework must recognise the impact of environmental factors on the life of patients with DM1. Thirdly, the possible confusion between the terms activity and participation has been pointed out.14,16 The ICF model has the same list of items for activity and participation domains, and the distinction has to be made by the user. However, Jette et al demonstrated that activity and participation are distinct dimensions and should be treated as such in the WHO model if it is to be used as a scientific model.14 To our knowledge, in neuromuscular disorders, the ICF role has been limited to classifying the impairments and disabilities and has not been used as a model explaining the various consequences (biological, functional and social) of the disease.9
Application of the Disability Creation Process model to DM1
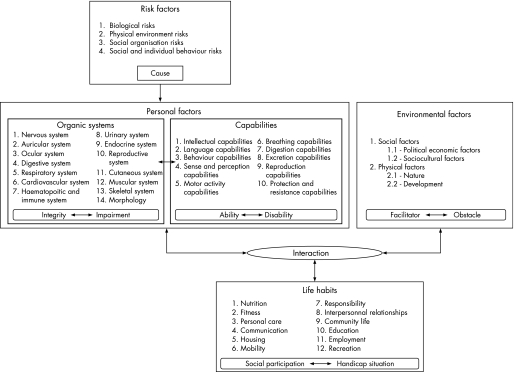
The DCP model (fig 1) resulted from the work conducted by the Quebec Committee on the revision of the WHO International Classification of Impairments, Disabilities and Handicap model.12 The DCP model emerged from a human development model assuming that individuals will experience some type of handicap situation throughout their lives.12 The model has proven useful in populations of patients with spinal cord injury, cerebral palsy as well as in older adults, with and without functional limitations, to document the occurrence of handicap situations and the association with personal characteristics.18,19,20 The DCP model also serves as a platform for the design and implementation of policies and services for various populations.
Figure 1 Disability Creation Process model (conceptual scheme).
This article presents the specific health and community aspects of DM1 management according to the DCP model. Our model has sought to use an evidence based approach. Our tables illustrate many internationally accepted clinical characteristics related to this disorder as well as more recent findings. However, the literature on DM1 has been categorised as poor, and available information on the subject is limited. Hence adaptation of the DCP model has solely incorporated factors with a presumed impact on management. Although quality of life is a crucial dimension of rehabilitation, it is not part of the DCP model and will not be discussed, as it will require a paper on its own.
The model includes four domains, which are briefly outlined with clinical examples relevant to DM1.
Risk factors
A risk factor (table 1) is an element related to the individual or the environment that is likely to give rise to a disease or an injury.12 In DM1, the mutation responsible for the disease (number of CTG repeats on chromosome 19q13.3) is a risk factor that partly determines the severity of DM1 symptoms, including muscular impairment (r = 0.46 to 0.51; p<0.05)81 and age of onset (r = −0.82 to 0.57; p<0.01).7,81 Anticipation and the congenital phenotype primarily transmitted by affected women are examples of how the gene defect modulates health outcomes in this disease. In addition, life expectancy is greatly reduced in DM1 patients, particularly in those with early age of onset of disease and the concomitant involvement of proximal muscular weakness.45
Table 1 Risk factors for myotonic dystrophy type 1 and organic system involvement.
| Risk factors |
| Dominant autosomal disease |
| CTG expansion at 19q13.33 |
| Anticipation2 |
| Organic systems |
| Muscular |
| Variations in fibre size, atrophy of type 1 fibres |
| Ringed fibres, increased central nuclei, nuclear chains21 |
| Nervous |
| General and focal cerebral atrophy, progressive ventricular dilatation, white matter lesions22,23,24,25 |
| Reduced cortical glucose utilisation26 |
| Reduced blood flow in fronto‐temporal regions bilaterally25,26,27 |
| Central motor control involved28 |
| Auricular |
| Bilateral high tone hearing loss29 |
| Ocular |
| Early cataracts (78–97%), frequent symmetrical ptosis2,30 |
| Retinopathy, blepharitis, corneal lesions2 |
| Digestive |
| Locking of the jaw, weakness of the palate and decreased chewing ability2,31 |
| Smooth muscle dysfunction affecting all parts of the GI tract32 |
| Slow oesophageal transit and gastric emptying33 |
| Small intestine and colonic dysmotility,33 colonic pseudo‐obstruction |
| Bile acid malabsorption, high incidence of gall bladder stones33 |
| Anal sphincter weakness and myotonia34 |
| Respiratory |
| Alveolar hypoventilation, marked hypercapnia35 |
| Respiratory muscle weakness35 and myotonia36 |
| Restrictive respiratory disease (58%)35 |
| Central37 and obstructive sleep apnoea38 |
| Cardiovascular |
| Extensive involvement of cardiac conducting tissue with fatty infiltration, fibrosis and degenerative change39 |
| Abnormal ECG (65%)40,41,42 |
| AV conduction disturbances, heart block, atrial flutter and fibrillation39,43 |
| Ventricular tachy/brady‐arrhythmias42 |
| Hypotension44 |
| Sudden death (8–30%)39,43,45 (2nd cause of death)45 |
| Endocrine |
| Hypogonadism, testicular atrophy (60–90%)2 |
| Hyperinsulinaemia, diabetes2 |
| HPA axis disturbance, abnormal diurnal rhythm of cortisol46 |
| Growth hormone secretion disturbance,47 hyperleptinaemia48 |
| Increased interleukin 6 and tumour necrosis factor α49 |
| Decreased DHEA and DHEA sulphate50 |
| Urinary |
| Urgency, frequency and stress incontinence51 |
| Reproductive |
| Uterus; incoordinate contraction in labour and in vivo2 |
| Skeletal |
| Talipes (CF)2 |
| Cutaneous |
| Premature balding2 |
CF, congenital form only; CTG, cytosine–thymine–guanine; DHEA, dehydroepiandrosterone; HPA, hypothalamic–pituitary–adrenal
% = reported frequency in the literature.
Personal factors
A personal factor (tables 1, 2) refers to a person's intrinsic characteristics, such as, age, sex, sociocultural characteristics, organic systems and capabilities.12 An organic system is defined as a group of biological components sharing a common function, such as the muscular system, with a measurement scale ranging from integrity to impairment. A capability is defined as a person's potential to accomplish a mental or physical activity. A capability can be measured on a scale ranging from optimal ability to total disability.12 Typically, symptoms become evident during mid‐life, but signs of DM1 can be detectable in the first or second decades of life.
Table 2 Disabilities in myotonic dystrophy type 1.
| Disabilities |
|---|
| Motor activity |
| 1.2% loss per year of muscle strength with a distal to proximal pattern52 |
| Oro‐facio‐pharyngeal and anterior neck muscle weakness53,54 |
| Myotonia, frequent presenting symptom (36–75.9%)2,55 |
| Decreased mobility and locomotion, frequent fall,56 abnormal hip motion57 |
| Difficulty handling and releasing objects properly58 |
| Intellectual |
| Excessive daytime sleepiness (33–39%)59 |
| Mental retardation (congenital form and those with severe symptoms)44 |
| Learning difficulties (infantile and adult form)60 |
| Executive and frontal lobe function impairment27 |
| Behaviour |
| Apathy,61,62 poor motivation62 |
| Rigidity, impulsivity, avoidance27 |
| Passive–aggressive traits63 |
| Sense and perception |
| Diminution of visual acuity2 |
| Frequent cataract surgery |
| Hearing difficulty64 |
| Digestion |
| Oropharyngeal dysphagia (33–57%) |
| Nausea, vomiting (35%)33 |
| Abdominal pain (55%), constipation33 |
| Breathing |
| Bronchial aspiration and pneumonia (1st cause of death)45 |
| Post‐anaesthesia respiratory complications65 |
| Reproduction |
| Preterm birth (30–35%), uterine atonia, caesarean section (31–36%)66,67 |
| Erectile dysfunction, male infertility2 |
| Language |
| Flaccid dysarthria and decreased intelligibility with nasal speech2,68 |
| Excretion |
| Diarrhoea (30%), anal incontinence (30%), faecal impaction33 |
| Protection and resistance |
| Excessive fatigue (50.5%)62,69,70 |
| Decreased physical and mental endurance |
| Cold sensitivity2 |
Muscular system and motor activity
Clinical myotonia, facial weakness, atrophy, ptosis, nasal speech and weakness of the sternomastoid and neck flexor muscles, long finger flexors and foot dorsiflexor muscles are the earlier muscular features of DM1. As the disease progresses, other distal muscles of the upper and lower limbs become involved, and later proximal weakness and more severe distal weakness occur.2 The majority of patients with DM1 are able to carry out most basic motor activities but they will perform these tasks more slowly than control subjects, and their performance is likely to deteriorate over time.68,82
Nervous system and intellectual and behavioural capabilities
Intellectual functioning in adult DM1 patients falls within the range of the normal population.83 In many cases, DM1 patients have great difficulties with abstraction and new concept formation, resulting in a tendency to rigidity and perseveration. The incidence of anxiety disorders and depression is also higher in this population.84 Although the physiopathology of hypersomnia remains unclear, excessive daytime sleepiness is a prominent feature of DM1,30 sometimes preceding muscular involvement by many years.85 Symptoms of severe sleepiness may markedly impair social or occupational functions.86 Fatigue is frequently reported on clinical assessments in DM187; its diagnosis and evaluation are rarely if ever specifically addressed.88 Emotional factors and personality as well as education, facial appearance, nasal voice, introversion, apathy and excessive daytime sleepiness create the popular label of a lower intelligence. This label in turn tends to influence DM1 patients in their learning or social interactions.89
Ocular system
Cataracts may be the only presenting symptom in the late onset form of DM1. The presence of cataracts is almost always present in adults but rare under the age of 10 years.2
Digestive system
Digestive symptoms, including abdominal pain and diarrhoea, may disrupt daily life and prevent participation in many social activities. Gastrointestinal disturbances are the most disabling impairment in 25% of patients.33
Respiratory system
Involvement of the upper airway and expiratory muscles may occur early in the course of DM1.35 Later in the evolution of the disease, the weakness of respiratory muscles decreases the vital capacity and increases the risk of alveolar hypoventilation.35 There is an increased risk of perioperative pulmonary complications.65
Cardiovascular system
Cardiac arrhythmias and conduction defects are well known and frequent complications of DM1, and they can lead to sudden death. Pacemaker implantation is necessary in approximately 5% of patients. The risk of conduction disturbances and sudden death increases with duration of the disease and age, but cases of exercise induced tachycardias have been reported in childhood.90
Endocrine system
Hypogonadism is the most frequent endocrine abnormality in DM1 patients.84 In adult males, markedly elevated follicle stimulating hormone levels and moderately low testosterone levels are frequently observed. Significant reductions in serum adrenal androgen levels are often observed in affected patients, and these deficiencies may contribute to cognitive impairment.46,50
Reproductive system
Pregnancy in women with the adult form of DM1 is often accompanied by obstetric complications. The risk of perinatal loss is approximately 15% compared with 1.9% in the reference population.67
Environmental factors
An environmental factor (table 3) is defined as a physical or social dimension that determines a society's organisation and context. Environmental social factors include political/economic factors and sociocultural factors. Physical environmental factors are divided into the nature group (weather, time, etc.) and the development group (architecture, vehicles). Environmental factors are assessed on a qualitative scale ranging from being an optimal facilitator to a total obstacle. A facilitator helps while an obstacle hinders the performance of life habits, in interaction with personal factors.12
Table 3 Environmental factors in myotonic dystrophy type 1.
| Environmental factors |
|---|
| Social factors |
| Economic system: social welfare (43.6%)6 |
| Residence: underprivileged area (61.8%)6 |
| Genetic counselling: large variation where between 30% and 90% receive services71 although most reported being important and will decide to be tested over again72 |
| Medical care and rehabilitation: poor follow‐up in general30,71 |
| Community social services: poor provision of social and home based services for daily living |
| Family support: progressive social deterioration of the family73 |
| Association and support: paucity of specific support groups for information,74 support and financial help |
| Low community awareness of the disease72 |
| Social attitude: negative impact of physical appearance74 |
| Physical factors |
| Residential and public building adaptation: restricted access to residential adaptation financial help |
| Adapted equipment: restricted access to orthosis, cane and transfer equipment |
Poverty and social exclusion are frequently observed social environmental factors in DM1.6 A much larger proportion of affected individuals (43.6%) than in the general population (12.2%) depend on social welfare, which places them below the poverty line.6 Employment opportunities are also limited by their educational level. More than half of the DM1 population has no high school diploma.80 It has been suggested that the progressive social deterioration of DMI families occurs over several generations as more severe forms of the disease appear.
Because DM1 is still relatively unknown among medical and paramedical resources, the provision of healthcare and community services is underdeveloped. Care is inconsistent, problematic and in many areas only given yearly, at best.4,30 A multidisciplinary neuromuscular clinic is rarely the main care provider for the day‐to‐day management of this patient population.
Social participation
Social participation (table 4) is described and assessed using the concept of life habits, which are defined as daily activities or social roles valued by the person according to his/her sociocultural context and characteristics (age, sex, sociocultural identity, etc). The DCP model assumes that social participation is the product of the interaction between personal and environmental factors. The accomplishment of life habits ranges from full social participation to a total handicap situation.12
Table 4 Life Habits in myotonic dystrophy type 1.
| Life habits |
|---|
| Nutrition: dysphagia related difficulties, malnutrition |
| Fitness: irregular sleep–wake schedule75 |
| Personal care: difficulties in many areas of personal care (17%)76 |
| Communication: speech difficulty related to dysarthria68 and difficulty being understood by others |
| Housing: difficulty with housework (58%)76 |
| Mobility |
| Restricted walking over time requiring mobility aids (most)76 |
| Requiring a wheelchair (⩽50%)77 |
| Driving security issues56 |
| Responsibility |
| Difficulty with budget management and taking charge of one's life |
| Difficulty with family's demands (adult form)60 |
| Difficulty with motherhood and child education for DM1 mother67 |
| Interpersonal relationships: decreased marriage eligibility for men78 |
| Community life: decreased social interaction, social isolation79 |
| Education: low level of education (63%)6,73 |
| Employment: frequent unemployment (77–88%)73,80 |
| Recreation: restricted participation in leisure activities (63%)76 |
DM1, myotonic dystrophy type 1.
In DM1, various life habits are known to be disrupted in one way or another in the congenital, childhood and adult forms of the disease. Only a small percentage of this population is able to maintain an active and fulfilling life.5 In the adult form, daily activities related to mobility and nutrition are often the first to be disrupted. With the progression of muscular involvement, patients usually need a wheelchair for short or long distances and have considerable difficulty in carrying out their daily living activities.
With respect to the accomplishment of social roles, the clinical picture often seen in the adult form is that of a sedentary person with few relationships except for family members. Participation in the community is severely restricted. These individuals typically have left school early, and have rarely held a steady job or taken part in community activities. Fulfilment of their daily responsibilities is often difficult, even more so when several family members are affected by DM1. A common situation is that of an affected mother with a severely affected child. She is not able to cope with the daily care of her family and is confronted with the progressive deterioration of their social participation.
The present conceptual framework illustrated the management elements needed to improve care of this underserved population. Potential solutions for optimal organisation and planning of services are discussed below.
Development of a comprehensive health and community management framework for DM1
From physician oriented outcomes to patient oriented outcomes
Health supervision has now been established as part of the clinical practice's foundation and is important in terms of performing appropriate screenings, applying specific preventive measures and developing a relationship with families.8 The mainstay of care is anticipatory guidance and monitoring for treatable complications. Literature and clinical experiences worldwide clearly demonstrate that DM1 patients and their families are provided with suboptimal health management, including insufficient anticipatory guidance. The multiplicity and variability of the disease manifestations, the health professionals' limited knowledge of DM1, time constraints and difficulty in targeting guidance on the topics of greatest concern to patients are some of the factors explaining this observation.
Integrating all of the clinical manifestations into a conceptual framework is not sufficient, as each aspect clearly does not deserve the same attention in DM1 health management. Some assessment protocols focusing on impairments have already been developed.91,92 Clinical experience suggests that myotonia and muscle strength, although important to assess, are not the main concern of DM1 patients.4 The resulting difficulties in daily activities and access to services that help overcome them, which are often not properly addressed, are far more important to patients and their families. When properly identified and included in a continuum of care, improvement in dealing with health, social and family environmental factors can partly counterbalance existing impairments and disabilities, and prevent the development of handicap situations in life habits. Development and implementation of environmental facilitators, such as access to high quality healthcare and community services, can be a key concept in support provided to people with DM1. When assessment moves from a focus on impairment to a focus on social participation, the integration of an interdisciplinary team into the health management programme becomes mandatory.
Community based case management: a model to be developed in DM1
In order to implement a health management programme, service development needs to be carefully reviewed. A health management programme must provide support to both the patient and family through an interdisciplinary team, which includes genetic and medical resources as well as community resources.91 Such programmes needs to be concerned not only with treating patients during discrete care episodes but also with providing high quality care across the continuum. It is common knowledge that currently available healthcare resources in many countries do not permit appropriate evaluation and follow‐up by an interdisciplinary team. In Canada, and probably in many other countries, the solution may lie in an organisation of care more centred on the nursing staff, with correspondingly increased responsibility in the evaluation and referral procedures related to healthcare and community resources.92 A community based nursing case management programme, as part of a health management programme, has been developed as a method of care delivery for several chronic diseases.93 The nurse is able to act as a case manager to help achieve goals set by the interdisciplinary team, namely by planning follow‐up individual assessments for service referrals, needed services and resource identification, and strengthening the links between resources and patients, thus ensuring a continuum of care.94 Services needed by DM1 patients include genetic counselling, anticipatory guidance, referrals to medical and healthcare professionals, patient and family support within the healthcare system and referral to community services. Practically, it means moving from a single physician management approach to interdisciplinary management coordinated by a nurse. Such measures may also enable the release of medical staff to concentrate on more specialised areas of clinical genetic services, such as diagnosis.92 Before such an approach can be implemented, research is needed to develop appropriate health management protocols and an intervention decision tree to support the nurse's evaluation and assess the validity and efficacy of this community based nursing case management programme.
Conclusion
Myotonic dystrophy is a complex disease that needs to be addressed with a comprehensive conceptual framework, which simultaneously considers several aspects of the individual's life in order to help the person achieve optimal social participation according to his/her expectations.
The DCP model offers a unique perspective to help understand the reasons and processes explaining why social participation is disrupted among many DM1 patients. The close relationships between some personal factors, such as fatigue, motivation and decreased strength, must be seen in relation to their interaction with environmental factors and impact on different aspects of social participation. The model not only points out impairments and disabilities, but also the role of environmental factors, such as access to healthcare, family support and income, as possible explanations for their low social participation. A health management programme based on this conceptual framework needs to be developed in order to improve services provided to this underserved population. Within such a health management programme, a community based nursing case management programme may be an interesting model to develop. The most important message conveyed by the DCP model is to shift DM1 management from traditional physician oriented outcomes at the medical clinic to patient oriented outcomes in the community, where there are tremendous opportunities to improve individuals' social participation.
Acknowledgements
This research was supported by the Neuromuscular Partnership Program of Muscular Dystrophy Canada, the Canadian Institutes of Health Research (CIHR) (#MOP49556) and ECOGENE‐21, a research programme in community genetics and genomics supported by the Canada Research Chairs Program and the CIHR (#CAR43283). CG holds a PhD Scholarship from the Canadian Institutes of Health Research.
Footnotes
Competing interests: None.
References
- 1.Mathieu J, De Braekeleer M, Prevost C. Genealogical reconstruction of myotonic dystrophy in the Saguenay‐Lac‐Saint‐Jean area (Quebec, Canada). Neurology 199040839–842. [DOI] [PubMed] [Google Scholar]
- 2.Harper P.Myotonic dystrophy, 3rd edn. London: WB Saunders, 2001
- 3.Fu Y H, Pizzuti A, Fenwick R G., Jret al An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 19922551256–1258. [DOI] [PubMed] [Google Scholar]
- 4.Harper P. Myotonic dystrophy: a multisystemic disorder. In: Harper P, Van Engelen B, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 20043–13.
- 5.Natterlund B, Ahlstrom G. Activities of daily living and quality of life in persons with muscular dystrophy. J Rehabil Med 200133206–211. [DOI] [PubMed] [Google Scholar]
- 6.Perron M, Veillette S, Mathieu J. Myotonic dystrophy: I. Socioeconomic and residential characteristics of the patients. Can J Neurol Sci 198916109–113. [PubMed] [Google Scholar]
- 7.Harley H G, Rundle S A, MacMillan J C.et al Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet 1993521164–1174. [PMC free article] [PubMed] [Google Scholar]
- 8.Carey J C. Health supervision and anticipatory guidance for children with genetic disorders (including specific recommendations for trisomy 21, trisomy 18, and neurofibromatosis I). Pediatr Clin North Am 19923925–53. [DOI] [PubMed] [Google Scholar]
- 9.Fowler W M, Jr, Carter G T, Kraft G H. The role of physiatry in the management of neuromuscular disease. Phys Med Rehabil Clin N Am 199891–8, v. [PubMed] [Google Scholar]
- 10.Kinsman S L, Levey E, Ruffing V.et al Beyond multidisciplinary care: a new conceptual model for spina bifida services. Eur J Pediatr Surg 200010(Suppl 1)35–38. [DOI] [PubMed] [Google Scholar]
- 11.WHO International Classification of Functionning, Disability and Health: ICF Geneva: WHO, 2001
- 12.Fougeyrollas P, Cloutier R, Bergeron H.et alThe Quebec classification: Disability Creation Process. Lac‐St‐Charles, Quebec: International Network on the Disability Creation Process, 1999
- 13.Steiner W A, Ryser L, Huber E.et al Use of the ICF model as a clinical problem‐solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002821098–1107. [PubMed] [Google Scholar]
- 14.Jette A M, Haley S M, Kooyoomjian J T. Are the ICF activity and participation dimensions distinct? J Rehabil Med 200335145–149. [DOI] [PubMed] [Google Scholar]
- 15.Fougeyrollas P, Beauregard L. Disability: An interactive person‐environment social creation. In: Albrecht GL, Seelman KD, Burry M, eds. Handbook of disability studies. London: Sage, 2001171–194.
- 16.Dahl T H. International classification of functioning, disability and health: an introduction and discussion of its potential impact on rehabilitation services and research. J Rehabil Med 200234201–204. [DOI] [PubMed] [Google Scholar]
- 17.Fransen J, Uebelhart D, Stucki G.et al The ICIDH‐2 as a framework for the assessment of functioning and disability in rheumatoid arthritis. Ann Rheum Dis 200261225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noreau L, Fougeyrollas P. Long‐term consequences of spinal cord injury on social participation: the occurrence of handicap situations. Disabil Rehabil 200022170–180. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers J, Noreau L, Rochette A. Social participation of older adults in Québec. Aging Clin Exp Res 200416406–412. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers J, Noreau L, Rochette A.et al Predictors of handicap situations following post‐stroke rehabilitation. Disabil Rehabil 200224774–785. [DOI] [PubMed] [Google Scholar]
- 21.Dubowitz V.Muscle biopsy, 2nd edn. Philadelphia: WB Saunders, 1985
- 22.Di Costanzo A, Di Salle F, Santoro L.et al Brain MRI features of congenital‐ and adult‐form myotonic dystrophy type 1: case‐control study. Neuromuscul Disord 200212476–483. [DOI] [PubMed] [Google Scholar]
- 23.Kornblum C, Reul J, Kress W.et al Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol 2004251710–714. [DOI] [PubMed] [Google Scholar]
- 24.Kassubek J, Juengling F D, Hoffmann S.et al Quantification of brain atrophy in patients with myotonic dystrophy and proximal myotonic myopathy: a controlled 3‐dimensional magnetic resonance imaging study. Neurosci Lett 200334873–76. [DOI] [PubMed] [Google Scholar]
- 25.Antonini G, Mainero C, Romano A.et al Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J Neurol Neurosurg Psychiatry 2004751611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meola G, Sansone V, Perani D.et al Reduced cerebral blood flow and impaired visual–spatial function in proximal myotonic myopathy. Neurology 1999531042–1050. [DOI] [PubMed] [Google Scholar]
- 27.Meola G, Sansone V, Perani D.et al Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM‐1) and in proximal myotonic myopathy (PROMM/DM‐2). Neuromuscul Disord 200313813–821. [DOI] [PubMed] [Google Scholar]
- 28.Ono S, Kanda F, Takahashi K.et al Neuronal loss in the medullary reticular formation in myotonic dystrophy: a clinicopathological study. Neurology 199646228–231. [DOI] [PubMed] [Google Scholar]
- 29.Lassaletta L, Fernandez‐Zubilaga A, Gonzalez T.et al Internal auditory canal hyperostosis in myotonic dystrophy. Otol Neurotol 200526310–311. [DOI] [PubMed] [Google Scholar]
- 30.Hilton‐Jones D. Myotonic dystrophy—forgotten aspects of an often neglected condition. Curr Opin Neurol 199710399–401. [DOI] [PubMed] [Google Scholar]
- 31.Kiliaridis S, Katsaros C. The effects of myotonic dystrophy and Duchenne muscular dystrophy on the orofacial muscles and dentofacial morphology. Acta Odontol Scand 199856369–374. [DOI] [PubMed] [Google Scholar]
- 32.van Engelen B, Brunner H G. Gastrointestinal dysfunction in myotonic dystrophy. In: Harper P, van Engelen B, Eymard B, eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 2004113–125.
- 33.Ronnblom A, Forsberg H, Danielsson A. Gastrointestinal symptoms in myotonic dystrophy. Scand J Gastroenterol 199631654–657. [DOI] [PubMed] [Google Scholar]
- 34.Eckardt V F, Nix W. The anal sphincter in patients with myotonic muscular dystrophy. Gastroenterology 1991100424–430. [DOI] [PubMed] [Google Scholar]
- 35.Begin P, Mathieu J, Almirall J.et al Relationship between chronic hypercapnia and inspiratory‐muscle weakness in myotonic dystrophy. Am J Respir Crit Care Med 1997156133–139. [DOI] [PubMed] [Google Scholar]
- 36.Zifko U A, Hahn A F, Remtulla H.et al Central and peripheral respiratory electrophysiological studies in myotonic dystrophy. Brain 1996119(Pt 6)1911–1922. [DOI] [PubMed] [Google Scholar]
- 37.Finnimore A J, Jackson R V, Morton A.et al Sleep hypoxia in myotonic dystrophy and its correlation with awake respiratory function. Thorax 19944966–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bégin P, Laforte M, Beaudry M.et al Sleep apnea and daytime respiratory function are associated with CTG repeat in myotonic dystrophy. Proccedings of the American Thoracic Association 2005A674
- 39.Nguyen H H, Wolfe J T, III, Holmes D R., Jret al Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J Am Coll Cardiol 198811662–671. [DOI] [PubMed] [Google Scholar]
- 40.Bushby K, Muntoni F, Bourke J P. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th‐9th June 2002, Naarden, the Netherlands. Neuromuscul Disord 200313166–172. [DOI] [PubMed] [Google Scholar]
- 41.Johnson E R, Abresch R T, Carter G T.et al Profiles of neuromuscular diseases. Myotonic dystrophy. Am J Phys Med Rehabil 199574(5 Suppl)S104–S116. [PubMed] [Google Scholar]
- 42.Groh W J, Lowe M R, Zipes D P. Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 correlates with age and CTG repeat length. J Cardiovasc Electrophysiol 200213444–448. [DOI] [PubMed] [Google Scholar]
- 43.Sabovic M, Medica I, Logar N.et al Relation of CTG expansion and clinical variables to electrocardiogram conduction abnormalities and sudden death in patients with myotonic dystrophy. Neuromuscul Disord 200313822–826. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien T A, Harper P S. Course, prognosis and complications of childhood‐onset myotonic dystrophy. Dev Med Child Neurol 19842662–67. [DOI] [PubMed] [Google Scholar]
- 45.Mathieu J, Allard P, Potvin L.et al A 10‐year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999521658–1662. [DOI] [PubMed] [Google Scholar]
- 46.Johansson A, Henriksson A, Olofsson B O.et al Adrenal steroid dysregulation in dystrophia myotonica. J Intern Med 1999245345–351. [DOI] [PubMed] [Google Scholar]
- 47.Gomez‐Saez J M, Fernandez‐Real J M, Navarro M A.et al GH secretion status in myotonic dystrophy. Psychoneuroendocrinology 199318183–190. [DOI] [PubMed] [Google Scholar]
- 48.Johansson A, Ahren B, Forsberg H.et al Testosterone and diurnal rhythmicity of leptin, TNF‐alpha and TNF‐II receptor in insulin‐resistant myotonic dystrophy patients. Int J Obes Relat Metab Disord 2002261386–1392. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez‐Real J M, Molina A, Broch M.et al Tumor necrosis factor system activity is associated with insulin resistance and dyslipidemia in myotonic dystrophy. Diabetes 1999481108–1112. [DOI] [PubMed] [Google Scholar]
- 50.Carter J N, Steinbeck K S. Reduced adrenal androgens in patients with myotonic dystrophy. J Clin Endocrinol Metab 198560611–614. [DOI] [PubMed] [Google Scholar]
- 51.Sakakibara R, Hattori T, Tojo M.et al Micturitional disturbance in myotonic dystrophy. J Auton Nerv Syst 19955217–21. [DOI] [PubMed] [Google Scholar]
- 52.Mathieu J, Boivin H, Richards C L. Quantitative motor assessment in myotonic dystrophy. Can J Neurol Sci 200330129–136. [DOI] [PubMed] [Google Scholar]
- 53.Nitz J, Burns Y, Jackson R V. Development of a reliable test of neck muscle strength and range in myotonic dystrophy subjects. Physiother Theory Pract 199511239–244. [Google Scholar]
- 54.Ertekin C, Yuceyar N, Aydogdu I.et al Electrophysiological evaluation of oropharyngeal swallowing in myotonic dystrophy. J Neurol Neurosurg Psychiatry 200170363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathieu J, De Braekeleer M, Prevost C.et al Myotonic dystrophy: clinical assessment of muscular disability in an isolated population with presumed homogeneous mutation. Neurology 199242203–208. [DOI] [PubMed] [Google Scholar]
- 56.Phillips M F, Mathieu J. Physical disability in myotonic dystrophy. In: Harper P, Van Engelen B, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford Press University, 200468–82.
- 57.Wright R B, Yoder D M, Costa J L.et al Characterization of gait parameters in adult‐onset myotonic dystrophy: abnormal hip motion. Arch Phys Med Rehabil 19957633–38. [DOI] [PubMed] [Google Scholar]
- 58.Nitz J, Burns Y, Jackson R. The validity of button fastening as a test of hand disability in myotonic dystrophy. Aust J Physiother 199844117–121. [DOI] [PubMed] [Google Scholar]
- 59.Laberge L, Begin P, Montplaisir J.et al Sleep complaints in patients with myotonic dystrophy. J Sleep Res 20041395–100. [DOI] [PubMed] [Google Scholar]
- 60.de Die‐Smulders C E. Congenital and childhood‐onset myotonic dystrophy. In: Harper P, van Engelen B, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 2004162–175.
- 61.Rubinsztein J S, Rubinsztein D C, Goodburn S.et al Apathy and hypersomnia are common features of myotonic dystrophy. J Neurol Neurosurg Psychiatry 199864510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Werf S, Kalkman J, Bleijenberg G.et al The relation between daytime sleepiness, fatigue, and reduced motivation in patients with adult onset myotonic dystrophy. J Neurol Neurosurg Psychiatry 200374138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delaporte C. Personality patterns in patients with myotonic dystrophy. Arch Neurol 199855635–640. [DOI] [PubMed] [Google Scholar]
- 64.Verhagen W I, ter Bruggen J P, Huygen P L. Oculomotor, auditory, and vestibular responses in myotonic dystrophy. Arch Neurol 199249954–960. [DOI] [PubMed] [Google Scholar]
- 65.Mathieu J, Allard P, Gobeil G.et al Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology 1997491646–1650. [DOI] [PubMed] [Google Scholar]
- 66.Rudnik‐Schöneborn S, de Die‐Smulders C E. Pregnancy and perinatal problems in myotonic dystrophy. In: Harper P, Van Engelen BGM, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 2004153–161.
- 67.Rudnik‐Schoneborn S, Zerres K. Outcome in pregnancies complicated by myotonic dystrophy: a study of 31 patients and review of the literature. Eur J Obstet Gynecol Reprod Biol 200411444–53. [DOI] [PubMed] [Google Scholar]
- 68.Dahlbom K, Ahlstrom G, Barany M.et al Muscular dystrophy in adults: a five‐year follow‐up. Scand J Rehabil Med 199931178–184. [DOI] [PubMed] [Google Scholar]
- 69.Mathieu J, Jean S, Gagnon C.et al Fatigue is linked to disease severity in myotonic dystrophy. J Sleep Res 200413(Suppl 1)1 [Google Scholar]
- 70.Laberge L, Bégin P, Richer L.et al Fatigue and daytime sleepiness in patients with myotonic dystrophy type 1: to lump or split? Arch Neurol. In press [DOI] [PubMed]
- 71.Donze C, Delattre S, Viet G.et al Neuromuscular disease: health care accessibility in the Nord‐Pas‐de‐ Calais region. Rev Neurol (Paris) 19991551063–1070. [PubMed] [Google Scholar]
- 72.Prevost C, Veillette S, Perron M.et al Psychosocial impact of predictive testing for myotonic dystrophy type 1. Am J Med Genet A 200412668–77. [DOI] [PubMed] [Google Scholar]
- 73.Veillette S, Perron M, Desbiens F.La dystrophie myotonique: Étude épidémiologique et socio‐démographique au Saguenay‐Lac‐St‐Jean. Jonquière: Cégep de Jonquière, 1986
- 74.Lord S M. Support groups for myotonic dystrophy and their role: an American family perspective. In: Harper P, van Engelen B, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 2004
- 75.van Hilten J J, Kerkhof G A, van Dijk J G.et al Disruption of sleep–wake rhythmicity and daytime sleepiness in myotonic dystrophy. J Neurol Sci 199311468–75. [DOI] [PubMed] [Google Scholar]
- 76.Nätterlund B, Ahlström G. Problem‐focused coping and satisfaction with activities of daily living in individuals with muscular dystrophy and postpolio syndrome. Scand J Caring Sci 19991326–32. [PubMed] [Google Scholar]
- 77.de Die‐Smulders C E, Howeler C J, Thijs C.et al Age and causes of death in adult‐onset myotonic dystrophy. Brain 1998121(Pt 8)1557–1563. [DOI] [PubMed] [Google Scholar]
- 78.Veillette S, Perron M, Mathieu J. Myotonic dystrophy: II. Marital status, fertility and gene transmission. Can J Neurol Sci 198916114–118. [PubMed] [Google Scholar]
- 79.Veillette S, Perron M, Mathieu J.et al Socio‐cultural factors influencing the spread of myotonic dystrophy in the Saguenay‐Lac‐St‐Jean region of the province of Quebec (Canada). In: Roberts DF, Bittles AH, eds. Genetics, demography and health in minority populations. London: MacMillan, 199183–100.
- 80.Fowler W M, Jr, Abresch R T, Koch T R.et al Employment profiles in neuromuscular diseases. Am J Phys Med Rehabil 19977626–37. [DOI] [PubMed] [Google Scholar]
- 81.Marchini C, Lonigro R, Verriello L.et al Correlations between individual clinical manifestations and CTG repeat amplification in myotonic dystrophy. Clin Genet 20005774–82. [DOI] [PubMed] [Google Scholar]
- 82.Lindeman E, Leffers P, Reulen J.et al Quadriceps strength and timed motor performances in myotonic dystrophy, Charcot‐Marie‐Tooth disease, and healthy subjects. Clin Rehabil 199812127–135. [DOI] [PubMed] [Google Scholar]
- 83.Turnpenny P, Clark C, Kelly K. Intelligence quotient profile in myotonic dystrophy, intergenerational deficit, and correlation with CTG amplification. J Med Genet 199431300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harper P.Myotonic dystrophy, 2nd edn. London: WB Saunders Co, 1989
- 85.Phillips M F, Steer H M, Soldan J R.et al Daytime somnolence in myotonic dystrophy. J Neurol 1999246275–282. [DOI] [PubMed] [Google Scholar]
- 86.Hilton‐Jones D, Damian M, Meola G. Somnolence and its management. In: Harper PS, Van Engelen BGM, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy. Oxford: Oxford University Press, 2004135–149.
- 87.Chaudhuri A, Behan P O. Fatigue in neurological disorders. Lancet 2004363978–988. [DOI] [PubMed] [Google Scholar]
- 88.Machuca‐Tzili L, Brook D, Hilton‐Jones D. Clinical and molecular aspects of the myotonic dystrophies: A review. Muscle Nerve 2005321–18. [DOI] [PubMed] [Google Scholar]
- 89.Portwood M M, Wicks J J, Lieberman J S.et al Psychometric evaluation in myotonic muscular dystrophy. Arch Phys Med Rehabil 198465533–536. [PubMed] [Google Scholar]
- 90.Bassez G, Lazarus A, Desguerre I.et al Severe cardiac arrhythmias in young patients with myotonic dystrophy type 1. Neurology 2004631939–1941. [DOI] [PubMed] [Google Scholar]
- 91.Rogers M T, Mathieu J. Follow‐up and assessment protocols for myotonic dystrophy. In: Harper P, van Engelen B, Eymard B, et al eds. Myotonic dystrophy: present management, future therapy Oxford: Oxford University Press, 200458–67.
- 92.Campbell H, Bradshaw N, Davidson R.et al Evidence based medicine in practice: lessons from a Scottish clinical genetics project. J Med Genet 200037684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rohrbach J I. Critical pathways as an essential part of a disease management program. J Nurs Care Qual 19991411–15. [DOI] [PubMed] [Google Scholar]
- 94.Taylor P. Comprehensive nursing case management. An advanced practice model. Nurs Case Manag 199942–10 quiz 113. [PubMed] [Google Scholar]