Abstract
The aim of this study was to investigate the extent of cortical and subcortical lesions in amyotrophic lateral sclerosis (ALS) using, in combination, voxel based diffusion tensor imaging (DTI) and voxel based morphometry (VBM). We included 15 patients with definite or probable ALS and 25 healthy volunteers. Patients were assessed using the revised ALS Functional Rating Scale (ALSFRS‐R). In patients, reduced fractional anisotropy was found in bilateral corticospinal tracts, the left insula/ventrolateral premotor cortex, the right parietal cortex and the thalamus, which correlated with the ALSFRS‐R. Increased mean diffusivity (MD) was found bilaterally in the motor cortex, the ventrolateral premotor cortex/insula, the hippocampal formations and the right superior temporal gyrus, which did not correlate with the ALSFRS‐R. VBM analysis showed no changes in white matter but widespread volume decreases in grey matter in several regions exhibiting MD abnormalities. In ALS patients, our results show that subcortical lesions extend beyond the corticospinal tract and are clinically relevant.
In amyotrophic lateral sclerosis (ALS), extension of the cortical lesions and their correlations with motor dysfunction remain unclear.
Diffusion tensor imaging (DTI) studies using a regions of interest method showed a bilateral reduction in anisotropy along the corticospinal tracts (CST)1,2,3,4,5,6,7,8; however, its correlation with motor disability is still debated.1,3,4,8 Studies using voxel based methods reported essentially a reduced anisotropy in the CST.9,10
Voxel based morphometry (VBM) studies have provided conflicting results. Reduction in grey matter (GM), found in several regions of the frontal lobe,11,12,13,14 was not confirmed by others.15 Volume reduction15 or increased density12 of white matter (WM) was found in motor and non‐motor regions, but was not detected in other studies.9,11,13,14
In this study, we used whole brain voxel based DTI to study mean diffusivity (MD) and fractional anisotropy (FA) in combination with VBM to assess morphological changes in both GM and WM. The extent of the lesion was correlated with the degree of motor disability, assessed by a validated functional scale, the revised ALS Functional Rating Scale (ALSFRS‐R).16
Methods
Patients and controls
Fifteen ALS patients (nine men and six women; mean age 51.8 (8.7) years (range 37–69); mean disease duration 30.9 (15.9) months (range 11–66)) with definite (n = 9) or probable (n = 6) (http://wfnals.org) sporadic ALS were included. No patient fulfilled the clinical criteria of frontotemporal lobe dementia. The site of onset was bulbar in four patients, the upper limbs in five patients and the lower limbs in six patients. The mean ALSFRS‐R score was 30 (6) (range 16–36). The control group included 25 healthy volunteers with no history of neurological disorders (11 women and 14 men; mean age 44.9 (12.4) years (range 31–68); no significant difference in age compared with the patients). The study was approved by the local ethics committee and in accordance with the Declaration of Helsinki. Informed written consent to participation in the study was obtained from all patients and healthy volunteers.
Imaging protocol
Conventional MRI and DTI data were acquired on a 1.5 T scanner (GE, Milwaukee, Wisconsin, USA). Patients underwent structural T1 weighted (Inversion Recovery‐Fast SPGR) and T2 FLAIR images that were reviewed to exclude potential abnormalities in control subjects. For T1 weighted images, 124 axial slices were obtained using the following parameters: TR 10.3 ms, TE 2.1 ms, inversion time 400 ms, flip angle 10°, acquisition matrix 256×192, reconstruction matrix 256×256, FOV 24×18 cm, in plane resolution 0.937×0.937 mm2, slice thickness 1.5 mm, no gap.
Diffusion weighted spin echo, echo planar images (EPI) were acquired with a standard head coil for signal reception. Twenty axial slices were obtained using the following parameters: TR 6500 ms, TE 85 ms, flip angle 90°, acquisition matrix 128×128, reconstruction matrix 256×256, FOV 32×32 cm, in plane resolution 1.25×1.25 mm2, slice thickness 5 mm, no gap. Diffusion weighting was performed along 23 optimised non‐colinear directions. A single b value of 700 s/mm2 was applied. A reference image with no diffusion weighting was also obtained (b0 image). Raw diffusion weighted data were corrected for geometric distortions secondary to eddy currents using a registration technique based on the geometric model of distortions.17
Voxel based diffusion data analysis
To allow voxel based statistical comparisons, the EPI images (T2 weighted images obtained for b = 0) of all subjects were spatially normalised to a customised template. This template was created by normalising EPI images of patients and control subjects to the standard EPI template provided in SPM2 using an affine transformation with 12 degrees of freedom. The 40 EPI images were then averaged and smoothed with an 8 mm Gaussian kernel to create a study specific template. Diffusion maps were then normalised using non‐linear warp with a 25 mm cut‐off and 16 iterations. The FA and MD maps were then normalised using the parameters determined from normalisation of the b0 image and smoothed with a 10 mm isotropic Gaussian kernel.
Age and sex were used as confounding variables in all statistical analysis. Group comparisons were performed in SPM2 using ANCOVA. Multiple regressions were performed in patients for correlation of diffusion changes with the ALSFRS‐R score.
For group analysis, a threshold of p<0.05, corrected for multiple comparisons at the voxel level, was applied using the False Discovery Rate. Correlations between diffusion abnormalities and the ALSFRS‐R score were examined in the regions previously found to be abnormal in the group comparison. For this purpose, an inclusive mask was built using the control versus patient statistical map with a statistical threshold of p<0.05 uncorrected. The mask was smoothed using a 5 mm Gaussian kernel. Within this mask, the clusters were considered significant at p<0.05 (height threshold) and corrected for multiple comparisons at the cluster level at p<0.05.
Voxel based morphometry
We determined the GM and WM volume using the modulation step described by Good and colleagues,18 with some modification for SPM2 (see http://dbm.neuro.uni‐jena.de/vbm). The same parameters and thresholds as for the DTI analysis were used for image normalisation, smoothing and statistical analysis.
Results
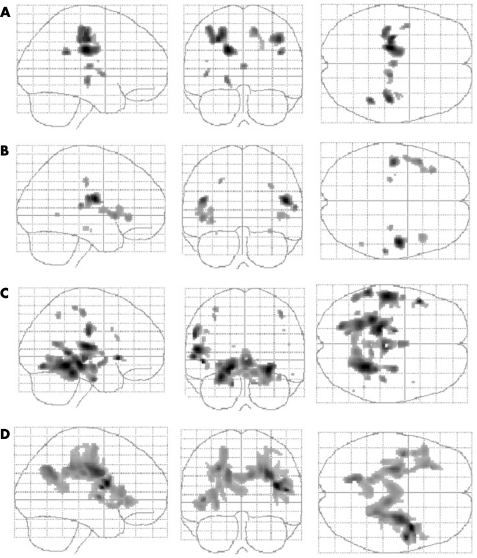
In patients compared with controls, anisotropy was decreased bilaterally along the CST (WM underneath the precentral gyrus, the centrum semiovale and the internal capsule), in the thalamus and in the WM underneath the left insula/ventrolateral premotor cortex and the right parietal cortex (table 1, fig 1). A trend was found for the right ventrolateral premotor cortex and the left parietal cortex. No increased FA was found in patients.
Table 1 Brain regions exhibiting diffusion and voxel based morphometry abnormalities.
| Cortical area | Side | MNI coordinates x, y, z | T score | p Value |
|---|---|---|---|---|
| Group comparison: decreased FA | ||||
| Corticospinal tract underneath motor cortex | R | 40, −24, 44 | 5.35 | 0.012 |
| R | 36, −14, 44 | 4.11 | 0.029 | |
| R | 38, −8, 36 | 3.87 | 0.041 | |
| L | −30, −20, 42 | 5.55 | 0.012 | |
| Corticospinal tract in centrum semiovale | L | −18, −22, 30 | 5.98 | 0.012 |
| L | −24, −22, 48 | 5.41 | 0.012 | |
| R | 20, −22, 42 | 3.94 | 0.037 | |
| Corticospinal tract in the internal capsule | L | −18, −20, −6 | 4.75 | 0.015 |
| WM underneath motor cortex (medial part) | R | 14, −22, 52 | 4.69 | 0.016 |
| Thalamus | 0, −18, 12 | 4.54 | 0.018 | |
| WM underneath parietal cortex | R | 42, −44, 28 | 5.01 | 0.013 |
| L | −44, −50, 28 | 3.70 | 0.051 | |
| Insula | L | −42, −2, 2 | 4.40 | 0.021 |
| WM underneath ventrolateral premotor cortex | L | −42, −8, 18 | 3.85 | 0.042 |
| R | 40, 10, −2 | 3.68 | 0.052 | |
| Group comparison: increased MD | ||||
| Precentral gyrus (primary motor cortex) | R | 48, −10, 20 | 6 | 0.023 |
| L | −44, −16, 20 | 5.21 | 0.029 | |
| Ventrolateral premotor cortex/insula (post part) | R | 42, 18, 2 | 4.28 | 0.048 |
| L | −46, 14, 0 | 4.64 | 0.042 | |
| Ventrolateral premotor cortex/insula (ant part) | L | −34, 28, −2 | 4.41 | 0.047 |
| Superior temporal gyrus | R | 60, −26, 12 | 4.77 | 0.036 |
| R | 50, −54, 2 | 4.17 | 0.048 | |
| Corticospinal tract in centrum semiovale | L | −24, −20, 42 | 4.29 | 0.048 |
| R | 30, −18, 38 | 4.28 | 0.048 | |
| Hippocampal formation | R | 32, −20, −14 | 4.01 | 0.048 |
| L | −30, −14, −18 | 3.92 | 0.049 | |
| Positive correlation between FA and ALSFRS | ||||
| WM underneath the lateral part of the precentral gyrus | R | 40, 4, 18 | 7.66 | 4568 |
| Corticospinal tract in centrum semiovale | R | 30, −8, 32 | 6.15 | |
| L | −26, −16, 38 | 4.87 | ||
| Corticospinal tract underneath motor cortex | L | −18, −10, 58 | 3.04 | |
| R | 28, −32, 56 | 2.19 | ||
| Insula | L | −42, −6, 4 | 4.45 | |
| L | −30, 20, 6 | 3.48 | ||
| WM underneath ventrolateral premotor cortex | R | 50, 16, 4 | 2.29 | |
| L | −46, 18, −4 | 3.20 | ||
| Precuneus | L | −14, −60, 32 | 4.58 | |
| Cingulum (posterior part) | L | −14, −42, 30 | 3.18 | |
| Corpus callosum | R | 10, −22, 30 | 4.05 | |
| L | −4, −16, 28 | 3.07 | ||
| Group comparison: decreased GM volume | ||||
| Hippocampal formation | L | −18, −28, −10 | 5.04 | 0.034 |
| R | 30, −34, −6 | 4.34 | 0.034 | |
| Temporal isthmus | L | −22, −52, −10 | 4.50 | 0.034 |
| R | 28, −44, −10 | 4.96 | 0.034 | |
| Thalamus | R | 6, −22, −2 | 4.34 | 0.034 |
| L | −12, −14, 16 | 3.33 | 0.046 | |
| Inferior frontal gyrus | L | −48, 16, 22 | 3.46 | 0.043 |
| R | 44, 10, 34 | 3.31 | 0.046 | |
| Precentral gyrus (primary motor cortex) | L | −56, −18, 28 | 4.52 | 0.034 |
| L | −34, −34, 58 | 3.71 | 0.037 | |
| R | 42, −24, 52 | 3.80 | 0.036 | |
| Ventrolateral premotor cortex/insula (post part) | L | −54, −2, 4 | 3.71 | 0.037 |
| Ventrolateral premotor cortex/insula (ant part) | L | −48, 18, −2 | 4.77 | 0.034 |
| R | 50, 20, 2 | 3.18 | 0.052 | |
| Parietal cortex | L | −34, −54, 48 | 3.58 | 0.040 |
| Occipital lobe | L | −20, −72, −12 | 4.36 | 0.034 |
| Cerebellum | R | 10, −56, −14 | 3.82 | 0.036 |
| Superior temporal gyrus | L | −54, −20, 12 | 4.92 | 0.034 |
| Superior temporal sulcus | L | −52, −56, 16 | 3.90 | 0.036 |
ALSFRS, Amyotrophic Lateral Sclerosis Functional Rating Scale; Ant, anterior; FA, fractional anisotropy; GM, grey matter; L, left; MD, mean diffusivity; Post, posterior; R, right; WM, white matter.
Coordinates are in MNI space. Only the higher peaks per region are given. Uncorrected clusters are shown in italics.
Figure 1 Glass brain representation of the group comparison between patients with amyotrophic lateral sclerosis and controls (clusters significant at p<0.05, FDR correction at the voxel level) for (A) fractional anisotropy maps showing areas of decreased anisotropy in patients, (B) mean diffusivity maps showing areas of increased diffusivity in patients and (C) grey matter volume maps showing areas of decreased volume in patients. Statistical parametric maps (D) for the positive correlation between fractional anisotropy and the revised Amyotrophic Lateral Sclerosis Functional Rating Scale score (p<0.05, corrected for cluster extent). The left of the images corresponds to the patient's left.
A bilateral and symmetric increased MD was found along the opercular regions of the frontal lobe, including the ventrolateral premotor cortex, the insula and the precentral gyrus. The other regions involved were the centrum semiovale, the hippocampal formation bilaterally and the right superior temporal gyrus. No decreased MD was found in patients.
A positive correlation between FA and the ALSFRS‐R score was found bilaterally in the upper part of the CST (underneath the motor cortices and centrum semiovale), in the WM underneath the insula/ventrolateral premotor cortex, in the lateral part of the right precentral gyrus, in the cingulum, in the precuneus and in the splenium of the corpus callosum. No negative correlation was found.
MD abnormalities did not correlate with ALSFRS‐R score.
FA correlated only negatively with disease duration in the corpus callosum and centrum semiovale bilaterally. MD did not correlate with disease duration.
Decreased GM volume was found bilaterally in the hippocampal formations, temporal isthmus, thalamus, inferior frontal gyrus and precentral gyrus. Other regions exhibiting a decreased GM volume were the left ventrolateral premotor cortex/insula with a trend on the right side, the left superior temporal gyrus, the left parietal and occipital cortex and the right cerebellum. No increase in GM was found in patients.
No abnormalities were found for maps of WM volume.
Discussion
This study showed a reduced anisotropy along the CST correlating with the ALSFRS‐R in patients with ALS. FA was decreased in subcortical regions beyond the limits of the primary motor areas and correlated with the degree of motor disability. MD was increased in the opercular motor and premotor areas bilaterally, and did not correlate with motor disability. Reduced GM volume in regions exhibiting MD abnormalities suggests that MD abnormalities reflected an atrophic process.
The reduced FA along the CST in ALS patients confirms previous DTI studies.1,2,3,4,5,6,7,8,9,10 Our observation of the lack of WM volume loss confirms that anisotropy changes resulted from a loss of fibre integrity caused by axonal degeneration.9 In previous studies, the magnitude of diffusion was reported to be unchanged4,7 or increased.1,3 These results suggest that FA is a more reliable marker of axonal degeneration than the magnitude of diffusion in ALS.
Our study confirms the correlation between the reduction in FA in the CST and the functional severity of the disease, as assessed using the ALSFRS‐R score, in agreement with previous studies.1,4,8 However, correlation analysis in SPM should be interpreted cautiously and our results using the ALSFRS‐R could also reflect the global progression of the disease. However, the lack of correlation with disease duration does not support this hypothesis.
Reduced FA extended largely into the subcortical WM, far beyond the primary motor areas. Such a large extension could not be demonstrated in studies using region of interest analysis which did not look for these regions.1,2,3,4,5,6,7,8 Using whole brain methods, no10 or limited extramotor abnormalities were found9 but the first study included only seven patients10 and the other analysed only a subvolume of the brain.9
We observed a bilateral increase in MD in the frontal opercular regions which corresponds to regions where cell loss was reported in vivo19,20 and in neuropathological studies.21
Using VBM, we found a reduction in GM volume in several of the regions exhibiting MD abnormalities. In common with others,9,11,13,14 we found no hemispheric WM abnormalities along the CST. We observed a decrease in WM volume in the brainstem but an increased volume in the optic radiations and the medial prefrontal cortex, possibly related to structural modifications of the frontal lobe inducing a remodelling of the subcortical WM.
Abnormalities outside the primary motor system are in agreement with the view that ALS is a multisystem motor degeneration disease.19,20,21 Their correlation with motor dysfunction suggests that these regions may also be involved in motor function.
Our VBM results suggest that MD but not FA abnormalities may be related to brain atrophy. Longitudinal studies of brain lesions using DTI would help in the understanding of the pathogenesis of ALS and determine the potential of DTI as a marker of disease extent and severity.
Acknowledgements
We wish to thank Dr Larbi Lala and the technical staff of the department of neuroradiology for their collaboration and assistance. This work was supported by the IFR 49 and by a grant from the Collège des Enseignants de Neurologie (LT).
Abbreviations
ALS - amyotrophic lateral sclerosis
ALSFRS‐R - revised Amyotrophic Lateral Sclerosis Functional Rating Scale
CST - corticospinal tracts
DTI - diffusion tensor imaging
EPI - echo planar images
FA - fractional anisotropy
GM - grey matter
MD - mean diffusivity
VBM - voxel based morphometry
WM - white matter
Footnotes
Competing interests: None.
References
- 1.Ellis C M, Simmons A, Jones D K.et al Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999531051–1058. [DOI] [PubMed] [Google Scholar]
- 2.Jacob S, Finsterbusch J, Weishaupt J H.et al Diffusion tensor imaging for long‐term follow‐up of corticospinal tract degeneration in amyotrophic lateral sclerosis. Neuroradiology 200345598–600. [DOI] [PubMed] [Google Scholar]
- 3.Toosy A T, Werring D J, Orrell R W.et al Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003741250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham J M, Papadakis N, Evans J.et al Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004632111–2119. [DOI] [PubMed] [Google Scholar]
- 5.Karlsborg M, Rosenbaum S, Wiegell M.et al Corticospinal tract degeneration and possible pathogenesis in ALS evaluated by MR diffusion tensor imaging. Amyotroph Lateral Scler Other Motor Neuron Disord 20045136–140. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y H, Lee K W, Sung J J.et al Diffusion tensor MRI as a diagnostic tool of upper motor neuron involvement in amyotrophic lateral sclerosis. J Neurol Sci 200422773–78. [DOI] [PubMed] [Google Scholar]
- 7.Yin H, Lim C C, Ma L.et al Combined MR spectroscopic imaging and diffusion tensor MRI visualizes corticospinal tract degeneration in amyotrophic lateral sclerosis. J Neurol 20042511249–1254. [DOI] [PubMed] [Google Scholar]
- 8.Cosottini M, Giannelli M, Siciliano G.et al Diffusion‐tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology 2005237258–264. [DOI] [PubMed] [Google Scholar]
- 9.Sach M, Winkler G, Glauche V.et al Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004127340–350. [DOI] [PubMed] [Google Scholar]
- 10.Abe O, Yamada H, Masutani Y.et al Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel‐based analysis. NMR Biomed 200417411–416. [DOI] [PubMed] [Google Scholar]
- 11.Ellis C M, Suckling J, Amaro E., Jret al Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 2001571571–1578. [DOI] [PubMed] [Google Scholar]
- 12.Kassubek J, Unrath A, Huppertz H J.et al Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel‐based morphometry of 3‐D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord 20056213–220. [DOI] [PubMed] [Google Scholar]
- 13.Chang J L, Lomen‐Hoerth C, Murphy J.et al A voxel‐based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 20056575–80. [DOI] [PubMed] [Google Scholar]
- 14.Grosskreutz J, Kaufmann J, Fradrich J.et al Widespread sensorimotor and frontal cortical atrophy in amyotrophic lateral sclerosis. BMC Neurol 2006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrahams S, Goldstein L H, Suckling J.et al Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005252321–331. [DOI] [PubMed] [Google Scholar]
- 16.Cedarbaum J M, Stambler N, Malta E.et al The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 199916913–21. [DOI] [PubMed] [Google Scholar]
- 17.Haselgrove J C, Moore J R. Correction for distortion of echo‐planar images used to calculate the apparent diffusion coefficient. Magn Reson Med 199636960–964. [DOI] [PubMed] [Google Scholar]
- 18.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd C M, Richardson M P, Brooks D J.et al Extramotor involvement in ALS: PET studies with the GABA(A) ligand [(11)C]flumazenil. Brain 20001232289–2296. [DOI] [PubMed] [Google Scholar]
- 20.Turner M R, Rabiner E A, Hammers A.et al [11C]‐WAY100635 PET demonstrates marked 5‐HT1A receptor changes in sporadic ALS. Brain 2005128896–905. [DOI] [PubMed] [Google Scholar]
- 21.Maekawa S, Al‐Sarraj S, Kibble M.et al Cortical selective vulnerability in motor neuron disease: a morphometric study. Brain 20041271237–1251. [DOI] [PubMed] [Google Scholar]