Abstract
Objectives
To review the angiographic and clinical outcome of patients with unruptured intracranial aneurysm(s) (UIA) with regard to complications and successful obliteration by surgical clipping or endovascular coiling.
Methods
Data were derived from a prospective database of intracranial aneurysms from June 1999 to May 2005. All patients were followed‐up for 6 months using the modified Rankin Scale (mRS). Favourable outcome was classified as mRS 0–2. From a total of 691 patients included in the database, 173 harboured 206 UIA of whom 118 patients (133 UIA) were treated.
Results
Primary treatment assignment was surgical repair in 91 UIA and endovascular treatment in 42. In 3 UIA (7.1%), endovascular treatment was not feasible and had to be abandoned. Definite treatment was surgery in 94 UIA (81 patients) and endovascular obliteration in 39 UIA (37 patients). There were no deaths related to any treatment. Immediately after treatment, 6.4% of the surgical and 7.7% of the endovascular patients showed new neurological deficits, mainly related to cerebral ischaemia. After 6 months, 3 (2.3%) patients had a treatment related unfavourable outcome, defined as mRS >2, 2 patients after surgical and 1 patient after endovascular aneurysm repair (not statistically different, p = 0.3; Fisher's exact test). This led to an overall satisfactory outcome in 97.9% of surgically and 97.4% of endovasculary treated UIA. After surgical clipping, complete occlusion of the aneurysm was achieved in 88 (93.6%) and near complete (small residual neck) in 4 (4.3%) of 94 UIA. Two small posterior communicating artery aneurysms with a fetal type posterior communicating artery were wrapped. After endovascular treatment, obliteration was complete in 26 (66.7%). Small residual neck was seen in 13 (33.3%), but none of the UIA showed residual aneurysm filling. Five patients in the endovascular group (13.9%) underwent repeated endovascular treatment after aneurysm recanalisation.
Conclusions
If patients are carefully selected and individually assigned to their optimum treatment modality, UIA can be obliterated by surgery or endovascular treatment in the majority of patients, with a low percentage of unfavourable outcomes. In this series, the outcome was not dependent on treatment. However, the rate of recanalisation of UIA is higher after endovascular obliteration. After diagnosis of an UIA, an individual interdisciplinary decision is essential for each patient to provide the optimum management.
An increasing number of patients with unruptured intracranial aneurysm (UIA) are diagnosed by modern non‐invasive imaging procedures, mostly performed because of unspecific symptoms not related to the aneurysm. Thus the majority of patients with UIA present without neurological deficits and therefore prophylactic treatment of UIA remains a challenge for neurosurgeons and endovascular neuroradiologists. The results of the International Study of Unruptured Intracranial Aneurysm (ISUIA),1,2 analysing the natural history of UIA and treatment related morbidity and mortality, were inconclusive, causing a dilemma for both treating physicians and patients about whether treatment of UIA can be recommended and, if it is, which method of aneurysm obliteration (clipping or coiling) should be performed. Reviewing the literature in smaller series, the results of obliteration and outcome of patients seems to be biased by the treating physician, which means that surgery is promoted by neurosurgeons and endovascular treatment by endovascular neuroradiologists.3,4,5,6,7,8,9 Thus the optimal treatment modality remains controversial.
However, data derived from ISUIA2 indicate that in patients less than 50 years old with small and medium sized aneurysms, similar proportions of adverse outcomes after clipping and coiling are observed, while clipping gives a higher percentage of definite and complete aneurysm obliteration. To recommend a certain treatment for a given patient, it is a prerequisite to know the individual data from a cerebrovascular centre, not only with regard to the number of complete obliterations but also in terms of the number and severity of periprocedural complications.
The aim of this study was to analyse the results of UIA treatment of patients in a single cerebrovascular centre in a given period where both methods were used in an interdisciplinary context.
Patients and methods
Patients and treatment criteria
Starting in June 1999, all patients presenting with an intracranial aneurysm at the Neurocentre of Johann Wolfgang Goethe, University Frankfurt/Main (Departments of Neurosurgery, Neurology and Neuroradiology) were prospectively entered into a SPSS database (SPSS Institute, Chicago, Illinois, USA). From this database, all patients with UIA were retrieved who were treated by surgery or endovascular procedures. In patients with multiple aneurysms and previous subarachnoid haemorrhage (SAH), the symptomatic aneurysm was identified by distribution of blood on CT at admission and aneurysm configuration on angiography, resulting in acute treatment of the aneurysm. The remaining non‐ruptured aneurysm(s) was included as UIA.
Between June 1999 and May 2005, 691 patients were entered into the database. Of these, 173 (25.0%) patients were identified as harbouring 206 UIA. In 118 (68.2%) of these 173 patients, treatment was performed. In 55 patients (73 UIA) who did not receive treatment for UIA, the reasons for conservative management were poor clinical condition or death after a previous SAH from a ruptured aneurysm, small aneurysms with follow‐up observation as the treatment of choice or refused treatment.
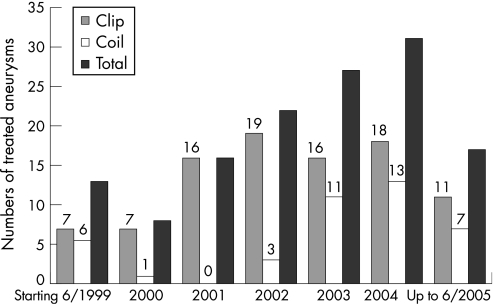
The total number of repaired UIA increased during the observational period because of the establishment of a dedicated cerebrovascular centre with both treatment modalities available. The low number of endovascular occluded UIA in 2000 and 2001 was because of the introduction of a new angiography suite with three dimensional rotational angiography. During 2002, 2003 and 2004, an increased number of UIA were treated by endovascular coil obliteration, while the number of microsurgical clip obliterated aneurysms remained almost unchanged (fig 1).
Figure 1 Absolute numbers of treated aneurysms either by clip or coil obliteration, starting in June 1999, up to May 2005. Of note is that in the years 1999 and 2005, only 6 months of data were analysed.
All patients underwent pretreatment four vessel digital subtraction angiography (DSA), including three dimensional reconstruction starting in October 2001. For treatment assignment (either clipping or coiling), no standard selection criteria were used, but each aneurysm was jointly discussed by the cerebrovascular interdisciplinary team consisting of an experienced cerebrovascular neurosurgeon and neuroradiologist. However, the results of the prospective ISUIA study served as a framework for the decision about optimum management. Thus patients without previous SAH and anterior circulation aneurysms smaller than 7 mm with a regular configuration (no tubular shape, no blebs) and the absence of familial and genetic risk factors were treated by surveillance with magnetic resonance angiography or conventional angiography and only treated if any change in size or morphology of the aneurysm was detected. Other aneurysms were considered as indicating treatment.
Patients with previous SAH from a ruptured aneurysm and additional aneurysm(s) were recommended treatment of UIA after rehabilitation. Treatment was also suggested for patients with symptomatic aneurysms. This treatment algorithm follows the recommendations for treatment of UIA of the German Society of Neurosurgery.10 Each treatment decision was performed on the basis of individual aneurysm criteria (size, morphology, configuration, location, parent vessel or branching vessel involvement in the aneurysm base) and patient criteria (age, life expectancy, comorbidity and patients' preferred treatment modality). Basic criteria for primary surgical treatment assignment were patients less than 50 years of age harbouring anterior circulation aneurysms of <12 mm in size. Only if complete endovascular obliteration was expected without the need for any assisted reconstruction technique (balloon, stent) was endovascular treatment the preferred technique. Patients older than 50 years with posterior circulation aneurysms were primarily assigned to endovascular treatment. In general, surgery was considered if endovascular techniques were expected to be associated with additional risks exceeding the surgical risks and vice versa.
In cases of multiple aneurysms, surgery was performed if the aneurysm which needed to be treated could not be obliterated by endovascular techniques. In these patients, all surgically accessible aneurysms on the ipsilateral side and sometimes on the contralateral side were clipped. However, before clipping of multiple aneurysms, the surgeon always knew which aneurysm was potentially coilable and so he could refrain from clipping if surgical inspection revealed an increased risk or higher risk compared with coiling. Treatment options were discussed with the patients and final treatment assignment was in favour of the treatment modality thought to be associated with the lowest periprocedural risk and the highest rate of anticipated long term stable occlusion for that aneurysm in a given patient.
Periprocedural management
All patients gave written consent to be included in the database. All surgical and endovascular treatment procedures were performed under general anaesthesia. In surgical candidates, all aneurysms of the anterior circulation and posterior communicating artery were approached by either pterional or frontolateral craniotomy, and clipping was performed in a standardised microsurgical procedure. One patient had an internal carotid artery aneurysm and a superior cerebellar artery aneurysm, both of which were clipped via a left pterional craniotomy. One posterior inferior cerebellar artery aneurysm was operated on via a suboccipital lateral approach and one basilar artery trunk aneurysm between the origin of the posterior cerebral artery and the superior cerebellar artery was clipped via an orbito‐zygomatic approach after endovascular treatment had failed. In all patients with surgical repair of UIA, intraoperative monitoring was performed using standard somatosensory evoked potential and additional motor evoked potential monitoring. Beginning in 2002, operative microscope integrated indocyanine green video angiography was used routinely to exclude stenosis of the parent or branching vessels as a result of aneurysm clip or residual aneurysm filling.11,12,13
For endovascular obliteration, we selected patients with suitable aneurysm morphology indicating that dense coil packing as a prerequisite for a good long term result was feasible. Further criteria in favour of endovascular treatment were age greater than 60 years and/or increased surgical risks. Balloon remodelling or stent assisted coiling was used in cases of wide neck aneurysms. During the endovascular procedure, a bolus of heparin 50–100 U/kg was given to achieve an activated clotting time of 240 s. Prior to stent assisted coiling, antiplatelet drugs (aspirin 100 mg and clopidogrel 75 mg) were administered 3 days before treatment and continued for 3 months afterwards. Patients with a wide neck aneurysm treated without a stent received aspirin 100 mg on the day before the intervention, which was continued for 4 weeks afterwards.
All patients were monitored postoperatively in the intensive care unit. Routine cranial computed tomography (cCT) scans were performed 24–48 h after treatment for standardised documentation of treatment related complications. All cCT scans were reviewed by an independent neuroradiologist and scrutinised for any signs of postoperative bleeding, cerebral ischaemia or brain oedema. Additional emergency cCT scans were performed in cases of neurological worsening or overt intraprocedural complications. Data on periprocedural complications were entered in the database immediately at the time of occurrence by a physician not directly responsible for treatment.
Success of aneurysm obliteration was assessed at the end of the endovascular procedures and within 10 days after surgery by control DSA. Obliteration was scored as complete (no residual filling of the aneurysm), residual neck (obliteration with minor aneurysm neck) or residual aneurysm (residual fundus filling) during control angiography. All patients who received endovascular treatment were followed by DSA after 6 months and by 1.5 or 3 T magnetic resonance angiography after 2 years. Patients with surgically treated aneurysms and a residual neck or wrapped aneurysm underwent repeated DSA after 6–12 months to assess any regrowth of the aneurysm.
Follow‐up was assessed according to the modified Rankin Scale (mRS) 6 months after treatment in all patients. None was lost to follow‐up. Favourable outcome was defined as an mRS score of 0, 1 or 2. Morbidity related to treatment was defined as an mRS score of 3, 4 or 5 (moderate to severe neurological disability) at 6 months if the outcome was related to treatment of the UIA. Deficits clearly related to a coexisting disorder or previous SAH were not attributed to aneurysm treatment. Thus patients were also evaluated with regard to whether they had an increase or decrease in their mRS post treatment. Evidence of cerebral infarction, haemorrhage or any other complication related to treatment was recorded at the time of occurrence and entered into the database.
Statistical evaluation
The Student's t test was used to compare patient age and aneurysm size in the surgical and endovascular treated groups. Differences in sex, previous SAH, frequency of postoperative residual aneurysm neck and need for repeated treatment were calculated using Fisher's exact or the χ2 test. The significance level was p<0.05.
Results
Reasons for performing an angiography leading to a diagnosis of an UIA are listed in table 1.
Table 1 Reasons for performing an angiography leading to a diagnosis of an unruptured intracranial aneurysm.
| Treatment | p Value | ||
|---|---|---|---|
| Open surgery (n = 81) | Endovascular (n = 37) | ||
| Headache (%) | 20 (24.7) | 8 (20.5) | 0.82 |
| Previous SAH from other aneurysm (%) | 15 (18.5) | 4 (10.3) | 0.4 |
| Ischaemia, including transient ischaemic attack (%) | 9 (11.1) | 3 (7.7) | 0.75 |
| Undefined spells | 7 (8.6) | 6 (15.4) | 0.34 |
| MRI | 6 (7.4) | 6 (15.4) | 0.19 |
| Seizures | 4 (4.9) | 0 (0.0) | 0.31 |
| Visual disturbance (%) | 3 (3.7) | 4 (10.3) | 0.2 |
| Cranial nerve deficits (%) | 0 (0.0) | 2 (5.1) | 0.09 |
| Brain tumour (%) | 2 (2.5) | 0 (0.0) | 1 |
| CNS degenerative diseases (%) | 0 (0.0) | 1 (2.6) | 0.3 |
| Computed tomography | 1 (1.2) | 0 (0.0) | 1.0 |
| Familiar history | 1 (1.2) | 0 (0.0) | 1.0 |
| Carotid artery stenosis | 1 (1.2) | 0 (0.0) | 1.0 |
| Other | 12 (14.8) | 3 (7.7) | 0.38 |
SAH, subarachnoid haemorrhage.
Unspecific headaches were the most common symptom leading to further diagnostic work up. Previous SAH occurred in 15 (18.5%) of the surgical patients and in 4 (10.8%) of the endovascular patients (p = 0.4, Fisher's exact test).
Intention to treat was in favour of surgical repair in 91 UIA and endovascular treatment in 42 UIA. In 3 (7.1%) patients, endovascular treatment was technically not feasible and had to be abandoned. Therefore, definite treatment was carried out in 94 UIA (81 patients) by microsurgical clipping and in 39 UIA (37 patients) by endovascular obliteration.
After treatment, no patient suffered a SAH. No patient died as a result of treatment of an UIA, resulting in a mortality rate of 0%. Table 2 summarises the baseline characteristics of the patients with respect to clip or coil treatment. Age (p = 0.76; t test) and sex (p = 0.2; Fisher's exact test) did not differ significantly in the surgical and endovascular group. Interestingly, surgically treated patients had significantly more multiple aneurysms than endovascularly treated patients (p<0.05, Fisher's exact test).
Table 2 Baseline characteristics of the patients with respect to clip or coil treatment.
| Treatment | p Value | ||
|---|---|---|---|
| Open surgery (n = 81) | Endovascular (n = 37) | ||
| Age (y) (mean (SD)) | 47.6 (9.3) | 48.2 (11.1) | 0.76 |
| Females (%) | 59 (72.8) | 27 (72.3) | 0.2 |
| No of patients with multiple aneurysm (%) | 34 (42.0) | 9 (24.3) | 0.03 |
| Total number of UIA | 94 | 39 | |
| Maximum diameter of the aneurysm (mm) | 7.98 (6.4) | 9.18 (8.0) | 0.4 |
| Size of the aneurysms in mm (%) | |||
| <7 | 41 (43.6) | 18 (46.1) | 0.84 |
| 7–12 | 44 (46.8) | 15 (38.5) | 0.44 |
| 13–24 | 6 (6.4) | 2 (5.1) | 1.0 |
| >25 | 3 (3.2) | 4 (10.3) | 0.19 |
UIA, unruptured intracranial aneurysms.
The mean diameter of the aneurysm in the group of endovascularly treated patients was 9.18 (8.0) mm (range 3–35) compared with 7.98 (6.4) mm (range 2–44) in surgically treated patients (p = 0.4; t test). Location and treatment of the aneurysms are presented in table 3.
Table 3 Location and treatment of the aneurysms.
| Treatment | p Value | ||
|---|---|---|---|
| Open surgery (n = 94) | Endovascular (n = 39) | ||
| Cavernous part of carotid artery (transitional, %) | 0 (0.0) | 2 (5.1) | 0.08 |
| Internal carotid artery paraclinoidal (%) | 8 (8.5) | 5 (12.8) | 0.52 |
| Posterior communicating artery (%) | 17 (18.1) | 6 (15.4) | 0.85 |
| Internal carotid artery bifurcation | 11 (11.7) | 9 (23.1) | 0.11 |
| Anterior communicating and anterior cerebral artery (%) | 20 (21.3) | 9 (23.1) | 0.82 |
| Middle cerebral artery (%) | 35 (37.2) | 0 (0) | <0.01 |
| Tip of basilar artery (%) | 0 (0.0) | 5 (12.8) | <0.01 |
| Vertebrobasilar system, other than basilar tip (%) | 3 (3.2) | 3 (7.7) | 0.36 |
All transitional aneurysms in the cavernous part of the carotid artery and at the basilar tip were treated endovasculary. In this series, all middle cerebral artery (MCA) aneurysms (p<0.01, Fishers exact test) were treated by microsurgical clipping. A higher percentage of carotid bifurcation aneurysms (NS) and more aneurysms of the posterior circulation were treated by endovascular procedures (for basilar tip aneurysm, p<0.01, Fisher's exact test). Paraclinoidal aneurysms, posterior communicating artery aneurysms and aneurysms arising from either the A1 or anterior communicating artery were obliterated by microsurgical clipping or coil embolisation almost at the same rate (NS).
All aneurysms were successfully repaired. Complete obliteration after clipping was found in 93.6% (88 of 94 UIA). Four (4.3%) complex aneurysms (some were partially calcified, some had branching vessels within the aneurysm base) were treated by reconstructing the base of the aneurysm. A small residual neck had to be left to avoid parent vessel stenosis or occlusion of branching vessels in these cases. Two small posterior communicating artery aneurysms with fetal type posterior communicating artery were wrapped to maintain patency of the posterior communicating artery. In both cases the origin of the artery was located close to the aneurysm dome. Both patients were followed by DSA and no regrowth or changes in morphology appeared during follow‐up. Endovascular treatment consisted of intrasaccular coil placement alone (n = 28), intrasaccular coil placement after stent implantation (n = 5), coiling with balloon in the remodelling technique (n = 3) or parent vessel occlusion (n = 3). No significant association between reconstructive technique and thromboembolic complications (p = 0.08, Fisher's exact test) or brain infarction (p = 0.4, Fisher's exact test) was found. Twenty‐six of 39 endovascularly treated aneurysms (66.7%) were completely obliterated. Thirteen UIA (33.3%) showed small residual neck remnants, but no residual filling of the endovascularly treated aneurysm was seen. None of the endovascularly repaired aneurysms ruptured during the procedure. The number of aneurysms with small neck remnants was significantly higher after endovascular treatment (13 of 39) compared with surgical clipping (4 with residual neck, 2 wrapped out of 94 UIA; p<0.01 Fisher's exact test) (table 4).
Table 4 Repair of the aneurysms.
| Treatment | p Value | ||
|---|---|---|---|
| Open surgery (n = 94) | Endovascular (n = 39) | ||
| Complete obliteration (%) | 88 (93.6) | 26 (66.7) | |
| Small residual aneurysm neck (%) | 4 (4.3) | 13 (33.3) | <0.01* |
| Wrapping | 2 (2.1) | ||
| Stable during follow‐up or spontaneous obliteration (%) | 6 (100) | 8 (61.5)** | |
| Retreatment | 0 | 5 | <0.01 |
| Initial incomplete obliteration | 3 | ||
| Initial complete obliteration | 2 | ||
*For statistical calculation both wrapped aneurysm were also included as residual aneurysms.
**Two patients with initial incomplete obliteration had a complete occlusion of the aneurysm neck due to thrombosis during follow‐up angiography.
DSA controls 6 months after coiling showed coil compaction in 5 of 39 patients, which all underwent re‐coiling. Three of these patients had initial incomplete obliteration and two had angiographically documented small residual necks. In three other patients with a small neck, spontaneous obliteration of the aneurysm neck was seen during follow‐up angiography.
Overall complication rate was similar after surgical and endovascular repair of UIA (17 of 94 (18%) and 8 of 39 (20.5%)) (table 5).
Table 5 Complication rates.
| Treatment | p Value | ||
|---|---|---|---|
| Open surgery (n = 94) | Endovascular (n = 39) | ||
| Total (%) | 17 (18.0) | 8 (20.5) | 0.8 |
| Thromboembolic complication (%) | 0 (0) | 6 (15.4) | <0.01 |
| Cerebral infarction (%) | 8 (8.5) | 3 (7.7) | 1.0 |
| With clinical symptoms due to brain infarction (%) | 4 (4.2) | 3 (7.7) | 0.41 |
| Intracerebral haemorrhage (%) | 5 (5.3) | 0 (0.0) | 0.32 |
| With clinical symptoms due to haemorrhage (%) | 2 (2.1) | 0 (0.0) | 1.0 |
| Other (%) | 4 (4.3) | 2 (5.1) | 1.0 |
| Myocardial infarction (%) | 1 | 0 (0.0) | 1.0 |
| Pneumonia (%) | 1 | 0 | 1.0 |
| CSF fistula (%) | 1 | 0 | 1.0 |
| Visual field defect (%) | 1 | 0 | 1.0 |
| Groin haematoma (%) | 0 | 1 | 0.28 |
| Iliac artery dissection (%) | 0 | 1 | 0.28 |
Symptomatic complications leading to acute neurological impairment were found after 6 (6.4%) surgical and 3 (7.7%) endovascular procedures (p = 0.72; Fisher's exact test). A detailed analysis of complications revealed that after endovascular repair of UIA, six thromboembolic complications occurred, which could be managed by intra‐arterial administration of GP IIb‐IIIa inhibitors. A new deficit after coiling was observed in three patients. After surgical repair, a new hypodensity on CT scan (including very small lesions) was detected in eight patients, which was symptomatic in four. Five patients suffered from intracerebral haemorrhage. In three of these patients the haemorrhage was small with only minimal space occupation, but in the remaining two patients the intracerebral haemorrhages led to a new impairment postoperatively. In none of the cases of intracerebral haemorrhage was surgical evacuation necessary. All other complications were managed without further sequelae for the patients.
Assessment of overall clinical outcome after 6 months proved favourable (mRS 0–2) in 97.7% of all 133 aneurysms (97.9% after surgery and 97.4% after endovascular treatment). An adverse outcome defined as an mRS score >2 occurred in a total of 3 (2.3%) patients (1 postoperative intracerebral haemorrhage and 2 ischemia). Table 6 shows the overall treatment related outcome according to the mRS 6 months after treatment of an UIA. Two surgically treated patients with a postoperative mRS of 3 had a preoperative mRS of 4 which was caused by a previous SAH in one patient and a stroke from thrombi eventually derived from a partially thrombosed large MCA aneurysm in the other patient. Both patients improved compared with the preoperative status after treatment of the UIA and a continued course of rehabilitation, and were therefore not included as unfavourable outcome. In these patients, the postoperative mRS of 3 was not related to adverse treatment effects of the UIA and therefore both patients were not scored as having an unfavourable treatment related outcome.
Table 6 Overall treatment related outcome according to the modified Rankin Scale 6 months after treatment of an unruptured intracranial aneurysm.
| Modified Rankin Scale | Treatment | Total (n = 133) (%) | |
|---|---|---|---|
| Open surgery (n = 94)* | Endovascular (n = 39) | ||
| 0 = No symptoms (%) | 35 (37.2) | 16 (41.0) | |
| 1 = No significant disability (%) | 38 (40.4) | 16 (41.0) | 96.2* |
| 2 = Slight disability (%) | 17 (18.1) | 6 (15.4) | |
| 3 = Moderate disability (%) | 0 (0.0)* | 1 (2.6) | |
| 4 = Moderate severe disability (%) | 2 (2.1) | 0 (0.0) | 2.3* |
| 5 = Severe disability (%) | 0 (0.0) | 0 (0.0) | |
| 6 = Death (%) | 0 (0.0) | 0 (0.0) | |
*Two surgically treated patients with postoperative mRS of 3 had a preoperative mRS of 4, which was caused by previous SAH and improved compared with the preoperative status and therefore not included as unfavourable outcome (see fig 2).
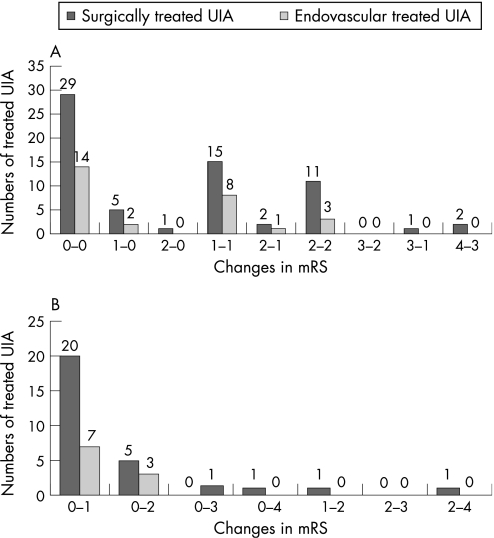
The relative changes in mRS associated with treatment are demonstrated in fig 2. After repair of 133 UIA, 70.2% (66 UIA) of surgically clipped and 71.1% (28 UIA) of endovascularly treated aneurysms improved or remained unchanged in their clinical status, as proved by the mRS score at 6 months after treatment (p = 0.1; Fisher's exact test).
Figure 2 Detailed outcome of patients after surgical and endovascular repair of an unruptured intracranial aneurysm, according to the modified Rankin Scale (mRS), 6 month after treatment. (A) All unruptured intracranial aneurysms (UIA) in which the clinical status of the patient remained unchanged or improved after treatment, showing their pre‐ and postoperative mRS scores. (B) All UIA in patients with clinical deterioration after treatment, which was in the majority of cases mild deterioration, as reflected by the high number which changed from mRS 0 to mRS 1.
Discussion
The International Study of Unruptured Intracranial Aneurysm (ISUIA)1,2 addressed two major issues. Firstly, the natural history of UIA, and secondly, the risks associated with their repair. Therefore, the ISUIA has not only had a great impact on the decision about whether or not to treat an UIA, but could also influence the assignment of patients to either open surgical or endovascular treatment. Furthermore, the results of the International Subarachnoid Aneurysm Trial (ISAT)14,15 indicated that, in selected small aneurysms with defined necks in good grade patients, endovascular treatment of ruptured intracranial aneurysm is superior to microsurgery. There is a tendency to use these results as an argument in treatment decisions of unruptured aneurysms, which could lead to a more liberal endovascular treatment policy for patients with UIA, causing a shift from surgery to endovascular treatment. The aim of this study was to prospectively analyse treatment related morbidity and outcome of patients after repair of UIA in a single centre with special emphasis on an interdisciplinary treatment algorithm.
Analysis of the ISUIA data1,2 and other studies concerning the rupture rate of intracranial aneurysms16 has led to an ongoing discussion about the treatment indication, in particular for small UIA.17,18,19,20 Thus even after ISUIA, treatment recommendations in an individual patient (whether or not to repair an UIA, what treatment modality) remain a clinical dilemma. Therefore, the treatment recommendation has to be tailored to the individual aneurysm in a given patient, with consideration of relevant patients and aneurysm specific factors. In general, the individual risk associated with repair of an UIA has to be balanced against the risk of rupture, with devastating consequences in terms of outcome. Our treatment algorithm therefore follows an interdisciplinary consensus for the management of UIA.10 According to these criteria, approximately 48% of treated UIA were smaller than 7 mm in this series, although the risk of bleeding based on the ISUIA data seems to be low. New surgical techniques, such as indocyanine green angiography11,12,13 combined with continuous intraoperative electrophysiological monitoring and new endovascular methods,5,21,22,23 may contribute to increased safety and efficacy of UIA repair as well as the concentration of such elective procedures in dedicated cerebrovascular centres. For both endovascular24 and surgical25 repair of UIA, it has been shown that high volume case load hospitals had better results and fewer complications compared with hospitals that handled comparatively fewer UIA.
In phase 1 of ISUIA,1 the overall mortality rate at 1 year associated with surgical repair was 3.8% and 1% after endovascular treatment. Compared with phase 1, the results of phase 2 of ISUIA2 showed a lower mortality rate of 2.3% (45 of 1917 patients) for surgical repair. Mortality rate after endovascular repair was higher (3.1% (14 of 451 patients)). Analysing the patients who died and those with an unfavourable outcome (mRS 3–6) in ISUIA, the rate was 10.0% (ISUIA phase 1) and 6.4% (ISUIA phase 2) for surgical and 4.1% and 6.0% for endovascular treatment, respectively. In our series, the mortality rate was 0%, and treatment related adverse outcome, defined as mRS 3–6, was 2.1% (2 of 94) after clipping and 2.6% (1 of 39) after endovascular treatment, which therefore compares favourably with ISUIA and matches well the results of other series.3,6,7,26,27,28,29,30,31,32,33 However, our data were derived from a single centre, involving a relatively small number of patients, and therefore it is difficult to extrapolate these results. This is a clear limitation of the study.
Comprising all and even minimal periprocedural complications—with and without clinical manifestations—the overall complication rate associated with surgical was 18% and 20% for endovascular repair of a UIA. However, only 2 (2.1%) patients had a permanent deficit, resulting in an unfavourable outcome after surgical clipping. This matches well with other surgical series reporting morbidity rates of 4–7%.3,6,26,29,30,31,33,34,35,36,37 Periprocedural thromboembolic events (6 of 38) were the most common complications associated with endovascular repair. However, intra‐arterial administration of GpIIb/IIIa platelet receptor antagonists resulted in successful thrombolysis and prevented neurological deficits in at least three patients. Therefore, even if thromboembolic complications occur during endovascular procedures, newer pharmacological compounds, such as GpIIb/IIIa platelet receptor antagonists, are very effective in preventing permanent cerebral ischaemic deficits. Interestingly, some studies which used routine postinterventional diffusion weighted imaging demonstrated a surprisingly high number (42%) of postprocedural lesions on diffusion weighted imaging, with most less than 2 mm in diameter.38 Again, as discussed for the 0% mortality rate, in terms of the low overall clinically relevant morbidity rate, it must be emphasised that these data derive from a single institution with a limited number of patients and therefore it is difficult to directly compare our results with those of large multicentre studies.
Niskanen et al showed that intraprocedural complications were similar during surgical repair (9 of 105 patients) and endovascular embolisation (6 of 53 patients), but postprocedural complications occurred more frequently in surgically treated patients.39 In our series, intraprocedural complications were also similar with the two procedures (13 of 94 surgically repaired (13.8%) and 6 of 39 (15.4%) endovascularly treated UIA). However, in contrast with the study of Niskanen et al,39 in our series, postprocedural complications were similar for both treatment modalities (4 of 94 surgically and 2 of 39 endovascularly repaired UIA).
The success of the UIA obliteration rate was not analysed in both phases of ISUIA, for neither surgical nor endovascular UIA repair. Several other studies have reported successful (complete or almost complete (>95%)) obliteration with endovascular techniques (70–90%).3,5,7,27 In the series of Wanke et al,7 89.5% of UIA were either completely obliterated or showed more than 95% occlusion after the endovascular procedure and only 4 UIA were incompletely (<95%) obliterated. However, endovascular repair of UIA can be limited in very broad based aneurysms or when branching vessels are incorporated into the base of the aneurysm. In a large series of endovascularly treated UIA, a failure rate of 5.7% (14 of 247 UIA)27 was reported and was up to 29.3% in a smaller series3 after attempted coil embolisation. After interdisciplinary discussion and treatment assignment, endovascular repair was technically not feasible in only 3 UIA (7.7%) and surgical repair had to be performed. All other UIA could either be obliterated completely (66.6%) or showed only small residual necks (33.3%).
The rate of complete surgical obliteration was 93.6% (88 of 94 UIA), and in 4 UIA a small residual neck had to be left to prevent stenosis of the parent vessels. All of the four aneurysms with residual remnants were very large or giant aneurysms. Some were partially calcified and/or had parent or branching vessel involvement in the aneurysm base. In two small posterior communicating artery aneurysms with fetal type posterior communicating artery and origin of the artery within the aneurysm dome, clipping was impossible and wrapping was performed to prevent bleeding and to maintain patency of the posterior communicating artery. Follow‐up by conventional angiography showed no morphological changes in these patients. Of note is that all MCA aneurysms were repaired by surgical clip in this series. This was the preferred treatment modality at our centre with an anticipated more stable aneurysm obliteration. Therefore, our policy corresponds to other studies, which showed that surgery is superior over endovascular treatment for MCA aneurysms in patients with an unfavourable angioanatomy.8,9
Without randomisation, any current management protocol where neurosurgeons and interventional neuroradiologists decide on a case‐by‐case basis about the individual optimum treatment causes selection bias. There is certainly a higher likelihood that complex aneurysms with incorporation of branching vessels into the aneurysm neck or fundus or large broad based aneurysms will be more often treated by surgical clipping. This precludes direct comparison of the results of clipping and coiling. Our results demonstrate that despite this selection bias, surgical repair of UIA is achievable with a low rate of unfavourable outcomes. On the other hand, endovascular treatment is successful with the right patient selection and procedure related morbidity is low.
Conclusion
Unruptured intracranial aneurysms can be obliterated by surgery or endovascular treatment in the majority of patients, with a low percentage of unfavourable outcomes. This has to be taken into consideration when counselling patients with UIA. In this series, outcome was not dependent on treatment and showed highly satisfactory results after both surgical clipping and endovascular repair. However, the rate of recanalisation of UIA is higher after endovascular obliteration, necessitating re‐treatment. Complex partially calcified aneurysms, including parent or branching vessels in the aneurysm morphology, might be candidates for surgery, and even if small residual necks have to be left to avoid ischaemic deficits, these necks are stable during follow‐up. The number of endovascularly repaired aneurysms has increased over the past years. Treatment of patients with UIA should always be discussed by experienced vascular neurosurgeons and interventional neuroradiologists to decide, in an interdisciplinary manner, whether or not treatment should be offered and what treatment modality is the most appropriate for the individual patient. This is a prerequisite for a good outcome in patients.
Acknowledgment
The authors acknowledge the help of Marina Eberhardt (preparing the manuscript and the figures) and Anne Sicking (data acquisition).
Abbreviations
cCT - cranial computed tomography
DSA - digital subtraction angiography
ISUIA - International Study of Unruptured Intracranial Aneurysm
MCA - middle cerebral artery
mRS - modified Rankin Scale
SAH - subarachnoid haemorrhage
UIA - unruptured intracranial aneurysms
Footnotes
Competing interests: None.
References
- 1. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 19983391725–1733. [DOI] [PubMed] [Google Scholar]
- 2.Wiebers D O, Whisnant J P, Huston J I I I.et al Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003362103–110. [DOI] [PubMed] [Google Scholar]
- 3.Raftopoulos C, Goffette P, Vaz G.et al Surgical clipping may lead to better results than coil embolization: results from a series of 101 consecutive unruptured intracranial aneurysms. Neurosurgery 2003521280–1287. [DOI] [PubMed] [Google Scholar]
- 4.Lanterna L A, Tredici G, Dimitrov B D.et al Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case‐fatality, morbidity, and effectiveness in preventing bleeding—a systematic review of the literature. Neurosurgery 200455767–775. [DOI] [PubMed] [Google Scholar]
- 5.Pouratian N, Oskouian R J, Jr, Jensen M E.et al Endovascular management of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry 200677572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon R A, Fink M E, Pile‐Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg 199480440–446. [DOI] [PubMed] [Google Scholar]
- 7.Wanke I, Doerfler A, Dietrich U.et al Endovascular treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 200223756–761. [PMC free article] [PubMed] [Google Scholar]
- 8.Regli L, Uske A, de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg 1999901025–1030. [DOI] [PubMed] [Google Scholar]
- 9.Regli L, Dehdashti A R, Uske A.et al Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl 20028241–46. [DOI] [PubMed] [Google Scholar]
- 10.Raabe A, Seifert V, Schmiedek P.et al Management nichtrupturierter intrakranieller Aneurysmen. Deutsches Ärzteblatt 2003100256–262. [Google Scholar]
- 11.Raabe A, Beck J, Gerlach R.et al Near‐infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 200352132–139. [DOI] [PubMed] [Google Scholar]
- 12.Raabe A, Nakaji P, Beck J.et al Prospective evaluation of surgical microscope‐integrated intraoperative near‐infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 2005103982–989. [DOI] [PubMed] [Google Scholar]
- 13.Raabe A, Beck J, Seifert V. Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near‐infrared video technology. Zentralbl Neurochir 2005661–6. [DOI] [PubMed] [Google Scholar]
- 14.Molyneux A J, Kerr R S, Yu L M.et al International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005366809–817. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux A, Kerr R, Stratton I.et al International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 20023601267–1274. [DOI] [PubMed] [Google Scholar]
- 16.Morita A, Fujiwara S, Hashi K.et al Risk of rupture associated with intact cerebral aneurysms in the Japanese population: a systematic review of the literature from Japan. J Neurosurg 2005102601–606. [DOI] [PubMed] [Google Scholar]
- 17.Ausman J I. Comments on the unruptured aneurysm study from Japan; does this study clarify what to do? J Neurosurg 2005102593–595. [DOI] [PubMed] [Google Scholar]
- 18.Wiebers D O. Patients with small, asymptomatic, unruptured intracranial aneurysms and no history of subarachnoid hemorrhage should generally be treated conservatively: for. Stroke 200536408–409. [DOI] [PubMed] [Google Scholar]
- 19.Weir B. Patients with small, asymptomatic, unruptured intracranial aneurysms and no history of subarachnoid hemorrhage should be treated conservatively: against. Stroke 200536410–411. [DOI] [PubMed] [Google Scholar]
- 20.Ausman J I. The Unruptured Intracranial Aneurysm Study‐II: a critique of the second study. Surg Neurol 20046291–94. [DOI] [PubMed] [Google Scholar]
- 21.Weber W, Siekmann R, Kis B.et al Treatment and follow‐up of 22 unruptured wide‐necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. AJNR Am J Neuroradiol 2005261909–1915. [PMC free article] [PubMed] [Google Scholar]
- 22.Malek A M, Halbach V V, Phatouros C C.et al Balloon‐assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 2000461397–1406. [DOI] [PubMed] [Google Scholar]
- 23.Vallee J N, Pierot L, Mont'alverne F.et al Unruptured intracranial aneurysms treated by three‐dimensional coil embolization: evaluation of the postoperative aneurysm occlusion volume. Neuroradiology 200547438–445. [DOI] [PubMed] [Google Scholar]
- 24.Hoh B L, Rabinov J D, Pryor J C.et al In‐hospital morbidity and mortality after endovascular treatment of unruptured intracranial aneurysms in the United States, 1996–2000: effect of hospital and physician volume. AJNR Am J Neuroradiol 2003241409–1420. [PMC free article] [PubMed] [Google Scholar]
- 25.Barker F G, Amin‐Hanjani S, Butler W E.et al In‐hospital mortality and morbidity after surgical treatment of unruptured intracranial aneurysms in the United States, 1996–2000: the effect of hospital and surgeon volume. Neurosurgery 200352995–1007. [PubMed] [Google Scholar]
- 26.Moroi J, Hadeishi H, Suzuki A.et al Morbidity and mortality from surgical treatment of unruptured cerebral aneurysms at Research Institute for Brain and Blood Vessels‐Akita. Neurosurgery 200556224–231. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez N, Murayama Y, Nien Y L.et al Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. AJNR Am J Neuroradiol 200425577–583. [PMC free article] [PubMed] [Google Scholar]
- 28.Goddard A J, Annesley‐Williams D, Gholkar A. Endovascular management of unruptured intracranial aneurysms: does outcome justify treatment? J Neurol Neurosurg Psychiatry 200272485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orz Y I, Hongo K, Tanaka Y.et al Risks of surgery for patients with unruptured intracranial aneurysms. Surg Neurol 20005321–27. [DOI] [PubMed] [Google Scholar]
- 30.Ogilvy C S, Carter B S. Stratification of outcome for surgically treated unruptured intracranial aneurysms. Neurosurgery 20035282–87. [DOI] [PubMed] [Google Scholar]
- 31.King J T, Jr, Berlin J A, Flamm E S. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta‐analysis. J Neurosurg 199481837–842. [DOI] [PubMed] [Google Scholar]
- 32.Taha M M, Nakahara I, Higashi T.et al Endovascular embolization vs surgical clipping in treatment of cerebral aneurysms: morbidity and mortality with short‐term outcome. Surg Neurol 200666277–284. [DOI] [PubMed] [Google Scholar]
- 33.Raaymakers T W, Rinkel G J, Limburg M.et al Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta‐analysis. Stroke 1998291531–1538. [DOI] [PubMed] [Google Scholar]
- 34.Hempelmann R G, Barth H, Buhl R.et al Clinical outcome after surgery of intracranial unruptured aneurysms: results of a series between 1991 and 2001. Acta Neurochir Suppl 20028251–54. [DOI] [PubMed] [Google Scholar]
- 35.Tsukahara T, Murakami N, Sakurai Y.et al Treatment of unruptured cerebral aneurysms; a multi‐center study at Japanese national hospitals. Acta Neurochir Suppl 20059477–85. [DOI] [PubMed] [Google Scholar]
- 36.Wirth F P, Laws E R, Jr, Piepgras D.et al Surgical treatment of incidental intracranial aneurysms. Neurosurgery 198312507–511. [DOI] [PubMed] [Google Scholar]
- 37.Deruty R, Pelissou‐Guyotat I, Mottolese C.et al Management of unruptured cerebral aneurysms. Neurol Res 19961839–44. [DOI] [PubMed] [Google Scholar]
- 38.Grunwald I Q, Papanagiotou P, Politi M.et al Endovascular treatment of unruptured intracranial aneurysms: occurrence of thromboembolic events. Neurosurgery 200658612–618. [DOI] [PubMed] [Google Scholar]
- 39.Niskanen M, Koivisto T, Rinne J.et al Complications and postoperative care in patients undergoing treatment for unruptured intracranial aneurysms. J Neurosurg Anesthesiol 200517100–105. [DOI] [PubMed] [Google Scholar]