Abstract
Background and aim
Inflammation has been extensively implicated in the pathogenesis of Alzheimer's disease (AD). Although there is evidence of a key role for cytokines in neuroinflammation processes, so far the proinflammatory cytokine interleukin (IL)‐18 has not been associated with AD. The aim of this study was to investigate the impact of two polymorphisms of the human IL‐18 gene promoter at positions −607 (C/A) and −137 (G/C) on both susceptibility to and progression of AD.
Results
The results revealed that the genotype distribution of the −607 (C/A) polymorphism was different between patients with AD and control subjects (χ2 = 7.99, df = 2, p = 0.0184). In particular, carriers of the CC genotype were at increased risk of developing AD (OR 2.33; 95% CI 1.29 to 4.22; p = 0.0052). The observed genotypes were in Hardy–Weinberg equilibrium, as for the −607 polymorphism, whereas the −137 polymorphism appeared in Hardy–Weinberg disequilibrium only in the patient group (p = 0.0061). Finally, in a 2 year follow‐up study, the −137 CC genotype was strongly and specifically associated with a faster cognitive decline (F = 4.024; df = 4,192; p = 0.0037 for time by IL‐18 −137 G/C group interaction) with no interaction effect with the apolipoprotein E ε4/non‐ε4 allele presence.
Conclusion
As IL‐18 cytokine promoter gene polymorphisms have been previously described to have functional consequences on IL‐18 expression, it is possible that individuals with a prevalent IL‐18 gene variant have a dysregulated immune response, suggesting that IL‐18 mediated immune mechanisms may play a crucial role in AD.
Alzheimer's disease (AD), the most common form of dementia among elderly people, is a neurodegenerative disorder characterised by progressive formation of amyloid senile plaques, tangles and selective neuronal death in the brain, probably caused by the effect of amyloid beta peptide (Aβ) accumulation.1 The aetiology of AD has not been fully clarified, possibly because multifactorial causes, such as an interaction between ageing, environmental factors and genetic predisposition, have been implicated. In particular, the CNS is susceptible to inflammatory diseases and AD has been largely shown to be linked to chronic inflammatory reactions within the brain.2,3 Indeed, early and consistent activation of microglia cells, the mononuclear phagocytes that participate in mechanisms of innate and adaptive immunity within the brain, have been demonstrated in the cerebral areas of neurodegeneration in patients with AD.4,5 Although activated microglia could have a beneficial role by participating in Aβ clearance because of their ability to phagocyte, they can also produce many toxic factors.6 Growing evidence suggests that chronic microglia mediated immune responses during Aβ deposition can prime an inflammatory destructive cascade and contribute to Aβ plaque formation, thus participating in the aetiopathogenesis of AD.7 Cytokines, such as interleukin (IL)‐1, IL‐6 and tumour necrosis factor α have been clearly involved in this neuroinflammatory process and their production has been found to be increased in patients with AD and impaired in the late stage of the disease.8,9,10,11 Moreover, several studies described an association between cytokine gene polymorphisms and AD,8,9,12,13 suggesting that cytokine variant genes may also be included among the genetic risk factors for AD, although a functional consequence of such polymorphisms has not always been characterised.
Taken together, such observations indicate dysregulation or impairment of the immune response, which may not only reflect an epiphenomenon but may be causally related to the pathology of AD.14 Among the factors capable of modulating the immune response, the cytokine IL‐18 has been shown to have potent immunoregulatory activity on both innate and adaptive responses, and its involvement in ageing and in neurodegenerative brain diseases has also been highlighted recently.15,16 IL‐18, belonging to the IL‐1 cytokine superfamily, is a pleiotropic cytokine, produced by a variety of cell types, including activated microglia and astrocytes.17 Similar to IL‐1β, IL‐18 is synthesised as a biologically inactive precursor molecule which, in order to became active, needs to be cleaved by the intracellular cystein protease caspase‐1, a caspase which is upregulated in brain tissues from subjects at high risk of developing AD and in patients with AD.18 Originally identified as interferon γ inducing factor, IL‐18 has strong proinflammatory activities, based on its ability to induce the synthesis of cell adhesion molecules, nitric oxide, chemokines and other proinflammatory cytokines, such as IL‐1β, tumour necrosis factor α and IL‐8.15,19 At the molecular level, regulation of IL‐18 expression has been studied extensively; the sequences upstream of the human IL‐18 cDNA with promoter activity have been characterised and the functional properties of some of their polymorphic variants described.20
In the present study, the distribution of the human IL‐18 gene promoter polymorphisms at positions −607 (C/A) and −137 (G/C) was analysed in a group of patients with AD and in control subjects, to determine its impact on the risk of developing the disease, in a case control study. In addition, a longitudinal clinical evaluation in the subgroup of patients who completed a 2 year follow‐up period was performed and the influence of the two IL‐18 gene promoter polymorphisms on AD outcome was evaluated.
Materials and methods
Study design and population
Patient selection criteria
Patients with a diagnosis of probable AD were considered appropriate for enrolment. All 339 subjects (66.7% women, mean age at study entry 74.3 (SD 7.5) years, education 6.4 (SD 3.9) years) were unrelated Caucasian patients and were consecutively recruited from memory clinics located in central Italy. These subjects were drug naïve and underwent their first clinical examination for a diagnosis of AD. After the first diagnostic evaluation, patients were treated with acetylcholinesterase inhibitors, according to the guidelines of the Italian Neurological Society and international guidelines for the treatment of AD.21 The nature and purpose of the study were presented and explained to their responsible caregiver and/or legal guardian. Written informed consent was obtained from the patient or the patient's representative and from caregivers prior to beginning detailed screening activities. Approval for the study was obtained from the local ethics committee.
Inclusion criteria were: (1) diagnostic evidence of late onset (age of onset >65 years) probable AD consistent with the National Institute of Neurological and Communicative Diseases and Stroke‐Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria; (2) being healthy and able to walk independently or with a walker or cane; (3) vision and hearing sufficient for compliance with the testing procedures; and (4) laboratory values within normal limits, or considered to be clinically insignificant by the investigator. Exclusion criteria included: (1) lack of a “reliable” caregiver defined as: being able to report to clinic, to ensure compliance with treatment and clinic visits, to contact the patient at least twice weekly, one of which had to be a personal visit; (2) major medical illness, that is, diabetes not stabilised, obstructive pulmonary disease or asthma, haematological/oncological disorders, B12 or folate deficiency, as evidenced by blood concentrations below the lower limit of normal, pernicious anaemia, clinically significant and unstable active gastrointestinal, renal, hepatic, endocrine or cardiovascular system disease, newly treated hypothyroidism, liver function tests (alanine aminotransferase or aspartate aminotransferase) greater than three times the upper normal limit, creatinine concentrations >150 μmol/l; (3) comorbidity of primary psychiatric or neurological disorders (ie, schizophrenia, major depression, stroke, Parkinson's disease, seizure disorder, head injury with loss of consciousness within the past year); (4) known suspected history of alcoholism or drug abuse; (5) CT or MRI evidence of focal parenchymal abnormalities; and (6) scan evidence of neoplasm. Of the total number of enrolled AD cases, three were not genotyped for the −137 locus and another three were not genotyped for the −607 locus, for technical reasons linked to a limited source of blood DNA.
Among the patient group, 108 subjects with mild to moderate AD were followed‐up in a 2 year longitudinal study. We opted for a more stringent selection of the longitudinal subgroup of patients who had a Mini Mental State Examination (MMSE)22 score >10 at baseline. In fact, patients with severe AD are already in the late stage of their illness and their cognitive outcome is not measurable. Within this longitudinal patient group, four died during the follow‐up period and two dropped out because of non‐compliance. Data from 102 patients (68.6% women, mean age at study entry 76.5 (SD 5.8) years, education 4.7 (SD 3.0) years) who completed all of the evaluations over the 2 year period were analysed. Compared with the total patient group, the longitudinal subgroup of patients with AD did not differ significantly for distribution of sex (χ2 = 0.137; df = 1; p = 0.712). However, as expected, based on the selection criteria for the longitudinal patients, who had mild to moderate illness compared with the total group that also included patients with severe disease, other sociodemographic characteristics were different, such as age (t = 2.799; df = 439; p = 0.0054) and educational level (t = −3.910; df = 439; p = 0.0001).
Clinical evaluations
A clinical neurologist used the NINCDS‐ADRDA23 criteria to assess the diagnosis of AD. The clinical psychologist interviewed patients on day 0 (baseline) using the MMSE. The same psychologist administered the MMSE after 1 and 2 years of follow‐up. The MMSE is a commonly used neurocognitive test which measures, using 16 items, orientation, language, verbal memory, attention, visuospatial function and mental control, with scores ranging from 30 (no impairment) to 0 (maximum impairment).
Control subjects
A total of 139 subjects were included as controls (55.4% women, mean age 69.4 (SD 6.4) years, education 7.3 (SD 3.6) years). The control subjects were not related to each another or to the patients with AD; in particular, we selected only those subjects who were the same age as the patients (range 66–96 years), had a MMSE score ⩾24 and did not satisfy the NINCDS‐ADRDA criteria for a diagnosis of AD, as clinically assessed and confirmed by the memory tests of the Mental Deterioration Battery.24 The cognitive level of our control subjects was defined as that described by Measso et al for the Italian population.25 In our study, subjects enrolled as controls were Caucasian, did not show any neurological signs or symptoms at a clinical neurological examination and were recruited from the general population from the same geographical region as the patients. Of the total number of 139 control subjects enrolled, only 131 subjects were genotyped for the −137 locus for technical reasons linked to a limited source of blood DNA.
Genotyping
Genomic DNA was purified from 200 μl of human whole blood using the MagNA Pure LC DNA Isolation Kit I (Roche Diagnostics, Mannheim, Germany) in an automated extractor from the kit's manufacturer (MagNA Pure LC). The polymorphisms at positions −607 and −137 in the promoter of the IL‐18 gene were genotyped by sequence specific PCR, as previously described.20 For the −607 (C/A) specific PCR, a common reverse primer 5′‐TAA CCT CAT TCA GGA CTT CC‐3′ and two sequence specific forward primers 5′‐GTT GCA GAA AGT GTA AAA ATT ATT AC‐3′ and 5′‐GTT GCA GAA AGT GTA AAA ATT ATT AA‐3′ were used. A control forward primer 5′‐CTT TGC TAT CAT TCC AGG AA‐3′ was used to amplify a 301 bp fragment covering the polymorphic site as an internal positive amplification control. All reactions were carried out in a Perkin‐Elmer 9600 Thermocycler (Applied Biosystems, Foster City, California, USA). Samples were initially denatured at 94°C for 2 min, followed by seven cycles of 94°C for 20 s, 64°C for 40 s and 72°C for 40 s, and 25 cycles at 94°C for 20 s, 57°C for 40 s, 72°C for 40 s and a final elongation step at 72°C for 5 min. For −137 genotyping, a common reverse primer 5′‐AGG AGG GCA AAA TGC ACT GG‐3′ and two sequence specific forward primers 5′‐CCC CAA CTT TTA CGG AAG AAA AG‐3′ and 5′‐CCC CAA CTT TTA CGG AAG AAA AC‐3′ were used. A control forward primer 5′‐CCA ATA GGA CTG ATT AT T CCG CA‐3′ was used to amplify a 446 bp fragment covering the polymorphic site to serve as an internal positive amplification control. After 2 min of denaturation at 94°C, PCR was performed by five cycles of 94°C for 20 s, 68°C for 1 min and 25 cycles of 94°C for 20 s, 62°C for 40 s, 72°C for 40 s and 72°C for 5 min. All PCR products were visualised by 2% agarose gel electrophoresis stained by ethidium bromide.
Apolipoprotein E (ApoE) genotyping was performed by real‐time PCR on a Light‐Cycler Instrument (Roche Diagnostics) using the Light‐Cycler ApoE Mutation Detection Kit, commercially available from Roche Diagnostics.
Statistical analyses
We analysed our case control data with Stata 9SE (StataCorp, 2005), looking for case control associations with single nucleotide polymorphisms. Before performing the inferential test, we controlled for deviation from Hardy–Weinberg equilibrium. Comparisons of categorical variables were made using the χ2 test. Differences in continuous variables among different genotypes were analysed by factorial analyses of variance (ANOVA). MMSE scores during the 2 years of follow‐up were analysed using a repeated measures ANOVA with the three IL‐18 −137 (G/C) genotypes and ApoE ε4 versus non‐ε4 genotypes as between subjects factors and rating scale scores during time as a within subjects factor with three levels. The same analysis was also used for IL‐18 −607 (C/A) genotypes.
Results
The distribution of ApoE variants in patients with AD and in control subjects is shown in table 1. The distribution of IL‐18 −607 and −137 genotypes in patients with AD and control subjects is shown in table 2. Analysis of IL‐18 promoter polymorphisms in the total group of patients and in controls revealed statistically significant differences in genotype distributions for the locus −607 (χ2 = 7.99; df = 2; p = 0.0184): a higher frequency of −607 CC carriers was observed in patients with AD compared with control subjects who showed a more frequent distribution of AA genotypes at this locus. Interestingly, heterozygous subjects were equally distributed among the two groups. Furthermore, a logistic regression analysis examining the relationship between the presence of the three −607 C/A genotypes and the risk of developing AD revealed that CC homozygotes were at increased risk of developing AD (odds ratio (OR) 2.33; 95% CI 1.29 to 4.22; p = 0.0052). No significant association was found between IL‐18 polymorphism at position −137 and susceptibility to AD. The observed genotypes at position −607 (C/A) were in Hardy–Weinberg equilibrium, both in the total group of patients and in controls. The −137 G/C polymorphism appeared in Hardy–Weinberg disequilibrium only for the total patient group, in contrast with the controls (estimated disequilibrium for cases D = −0.028, exact p = 0.0061; estimated disequilibrium for controls D = −0.013, exact p = NS). At the same time, in the control subjects and in patients with AD, the presence of the ApoE ε4 polymorphic variant, which is known to be a potent risk factor for developing AD,26 was analysed. As expected, the presence of the ApoE ε4 allele in the total AD patient population was significantly higher than in control subjects (44% vs 15.8%; χ2 = 33.94; df = 1; p<0.0001). However, no significant association among ApoE variant alleles and the studied IL‐18 −607 and −137 gene promoter polymorphisms was found in control subjects (χ2 = 1.634; df = 2; p = 0.442 and χ2 = 2.612; df = 2; p = 0.271, respectively) or in patients with AD (χ2 = 3.646; df = 2; p = 0.162 and χ2 = 4.400; df = 2; p = 0.111, respectively).
Table 1 Frequency of genotypes of apolipoprotein E in control subjects and in patients with Alzheimer's disease (total population and longitudinal subgroup).
| Genotype | Controls (n = 139) | AD total group (n = 339) | AD longitudinal subgroup (n = 102) | χ2; df; p value (AD longitudinal vs AD total) | |
|---|---|---|---|---|---|
| ε2/ε2 | 0.7% (n = 1) | 0% (n = 0) | 0% (n = 0) | ||
| ε2/ε3 | 9.4% (n = 13) | 4.4% (n = 15) | 6.9% (n = 7) | ||
| ε2/ε4 | 1.4% (n = 2) | 2.1% (n = 7) | 1% (n = 1) | ||
| ε3/ε3 | 74.1% (n = 103) | 51.6% (n = 175) | 46.1% (n = 47) | ||
| ε3/ε4 | 14.4% (n = 20) | 34.8% (n = 118) | 38.2% (n = 39) | ||
| ε4/ε4 | 0% (n = 0) | 7.1% (n = 24) | 7.8% (n = 8) | ||
| ε4 vs non‐ε4 | 0.306; 1; 0.5801 | ||||
AD, Alzheimer's disease.
Table 2 Frequency of genotypes of IL‐18 gene promoter polymorphisms in control subjects and in patients with Alzheimer's disease (total population and longitudinal subgroup).
| Locus and genotype | Controls (n = 139) | AD total group (n = 336) | AD longitudinal subgroup (n = 102) | χ2; df; p value (AD longitudinal vs AD total) |
|---|---|---|---|---|
| IL‐18 −607 | ||||
| CC | 27.3% (n = 38) | 36.9% (n = 124) | 36.3% (n = 37) | 5.227; 2; 0.073 |
| CA | 51.1% (n = 71) | 50.6% (n = 170) | 58.8% (n = 60) | |
| AA | 21.6% (n = 30) | 12.5% (n = 42) | 4.9% (n = 5) | |
| Controls (n = 131) | AD total group (n = 336) | AD longitudinal subgroup (n = 102) | χ2; df; p value (AD longitudinal vs AD total) | |
| IL‐18 −137 | ||||
| GG | 49.6% (n = 65) | 53.3% (n = 179) | 49% (n = 50) | 0.780; 2; 0.677 |
| GC | 43.5% (n = 57) | 43.1% (n = 145) | 46.1% (n = 47) | |
| CC | 6.9% (n = 9) | 3.6% (n = 12) | 4.9% (n = 5) |
AD, Alzheimer's disease; IL, interleukin.
Factorial ANOVAs did not detect significant differences in age at onset among patients with AD carrying the different IL‐18 −607 (F = 1.159; df = 2,333; p = 0.315) and −137 (F = 0.443; df = 2,333; p = 0.642) polymorphisms.
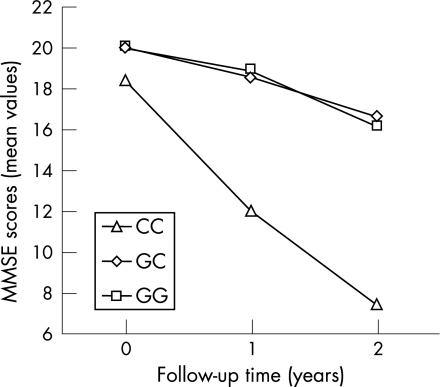
To study the influence of IL‐18 gene promoter polymorphisms on progression of AD, among the total AD group, a subset of 102 patients with mild to moderate AD was selected and followed‐up yearly for 2 years. The distributions of the ApoE variants, IL‐18 −607 and −137 genotypes of the longitudinal AD patient group are reported in tables 1 and 2. They were not significantly different with respect to the whole group of 339 patients with AD. MMSE scores over 2 years of follow‐up are shown in fig 1. A significant change in MMSE total score over time (F = 42.590; df = 2,192; p<0.0001) and a significant time by IL‐18 −137 G/C group interaction (F = 4.024; df = 4,192; p = 0.0037) was observed. No statistically significant time by IL‐18 −137 G/C by ApoE ε4/non‐ε4 group interaction (F = 1.111; df = 4,192; p = 0.353) was found. In particular, the C/C genotype at position −137 was specifically associated with faster cognitive decline, regardless of the presence of the ApoE ε4 allele. Additional statistical factorial ANOVA and χ2 analysis showed no statistically significant differences between the groups of patients with the IL‐18 −137 G/C polymorphisms for age (F = 1.438; df = 2,99; p = 0.242), sex (χ2 = 4.127; df = 2; p = 0.127) or years of education (F = 1.141; df = 2,99; p = 0.324).
Figure 1 Mini Mental State Examination (MMSE) scores of patients with Alzheimer's disease, with different −137 G/C genotypes over 2 years of follow‐up. Carriers of the CC genotype showed a strong decrease in their global cognitive level compared with non‐CC patients.
Discussion
The present study demonstrates for the first time an association between cytokine IL‐18 gene promoter polymorphisms and both susceptibility and clinical outcome of AD. Interestingly, this effect on risk and outcome of AD was not influenced by the presence of different ApoE ε allelic variants. The cytokine IL‐18 is a pleiotropic factor involved in the amplification of inflammatory responses which plays a role in many illnesses with a relevant chronic inflammatory component. In addition, IL‐18 has recently been suggested to be a key player in neuroinflammation and neurodegeneration by exerting a double role, both beneficial and detrimental, in autoimmune, ischaemic, traumatic and infectious disorders of the CNS.16 In the brains of patients with AD, a chronic inflammatory response has been largely demonstrated2,3,11 and it is therefore conceivable that IL‐18 could have a crucial affect on AD neurodegeneration. From a genetic point of view, AD is influenced by multiple susceptibility loci whose combinations contribute to the development of this disorder.27 Accordingly, we have recently shown that genomic polymorphisms in glutathione S‐transferases, a family of enzymes that appear to be critical for protection against oxidative stress, a phenomenon strictly linked to inflammation, are associated with AD susceptibility28 and clinical course.29 Similarly, several polymorphisms in proinflammatory or even anti‐inflammatory cytokine genes have been described as associated with the disease.8,9,12,13 Regarding polymorphisms of the IL‐18 gene, an important recent study analysing the genetic variability of the whole IL‐18 system (IL‐18, the two IL‐18R chains and the IL‐18 binding protein) in patients with cardiovascular diseases demonstrated that certain haplotype variations of the IL‐18 gene consistently influence both circulating levels of IL‐18 and clinical outcome of the disease.30 Moreover, previous studies have analysed the association of −607(C/A) and −137(G/C) IL‐18 gene promoter polymorphisms with immune driven pathologies such as Crohn's disease, diabetes and rheumatoid arthritis. Although the results of these studies are conflicting, in particular regarding diabetes or rheumatoid arthritis,31,32,33,34,35 it is possible that these polymorphisms contribute to the pathogenic mechanisms of some inflammatory diseases. Interestingly, one publication,20 based on a promoter transcription activity assay, reported that two such polymorphisms of the IL‐18 gene promoter have functional consequences, being relevant for nuclear transcriptional factor binding. In fact, nucleotide substitution at position −607 (C→A) is suggested to impair a cAMP responsive element binding site. Furthermore, the C allele at position −137 is hypothesised to modify a H4TF‐1‐binding site.20 The resulting −607A/−137C haplotype has functional consequence by lowering the activity of the IL‐18 gene promoter. In contrast, patients homozygous for genotype −607 CC and −137 GG had increased IL‐18 mRNA levels in comparison with patients with other genotypes.20
In other functional studies, increased transcriptional activity of the wild‐type genotype −137 GG of the human IL‐18 gene has been described,36 and monocytes from subjects with the −137 GG genotype have been shown to produce more IL‐18 than monocytes from subjects with the −137 GC genotype.37 On the basis of such results and in the light of our data, it is conceivable that the −607 CC genotype is associated with higher expression of the IL‐18 gene and that sustained levels of the cytokine can increase susceptibility to AD. In contrast, the CC genotype at position −137 could correspond to a lower activity of the gene, and the tendency to react with lower IL‐18 production following AD onset could cause worsening of the disease course. However, further studies are needed to characterise modulation of IL‐18 expression in AD.
Regarding the −137 G/C polymorphism, a significant and consistent deviation from Hardy–Weinberg equilibrium has been identified in patients with AD, suggesting the presence of selective forces, at least in our population. However, this observation, although not appearing to be supported by any significant association of this polymorphism with an increased risk of developing AD, is in line with the longitudinal data, which show that patients carrying the CC genotype at this position are fast decliners in terms of cognitive performance, thus indicating that homozygosity for this variant allele can be considered as a negative prognostic factor for the disease. Although the group of patients carrying the −137 CC genotype was small (n = 5), the data obtained in the longitudinal study were statistically highly significant and further supported by other personal observations (G Spalletta and P Bossù). However, because of the small sample, the longitudinal results should be considered as the basis for a new hypothesis and should be replicated in further larger studies. Furthermore, it is interesting to note that the human IL‐18 gene maps to 11q22.2–q22.3, a region near the tip of the long arm of chromosome 11, which has previously been reported in linkage studies in AD families.38
Our findings indicate a relevant association between IL‐18 gene polymorphisms and AD, suggesting that this cytokine may play a role in disease pathogenesis. To date, no consistent evidence has been produced relating to IL‐18 in AD neurodegeneration, and conflicting results were obtained for IL‐18 production in patients with AD. In fact, a recent study reported that serum levels of the cytokine did not significantly differ among patients with mild cognitive impairment, severe AD and control subjects39 whereas other authors have described more elevated levels of plasma IL‐18 in AD as well as in patients with vascular dementia, compared with control subjects.40
In conclusion, our study provides new evidence supporting a possible role for the IL‐18 cytokine in AD. Further studies should be performed as they may add a valuable light to the complex mechanisms involving immune dysregulation and leading to the pathogenic pathways of AD.
Abbreviations
Aβ - amyloid beta peptide
AD - Alzheimer's disease
ApoE - apolipoprotein E
IL - interleukin
MMSE - Mini Mental State Examination
NINCDS‐ADRDA - National Institute of Neurological and Communicative Diseases and Stroke‐Alzheimer's Disease and Related Disorders Association
Footnotes
Competing interests: None.
References
- 1.Hardy J, Selkoe D J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road of therapeutics. Science 2002297353–356. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Barger S, Barnum S.et al Inflammation and Alzheimer's disease. Neurobiol Aging 200021383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eikelenboom P, van Gool W A. Neuroinflammatory perspectives on the two faces of Alzheimer's disease. J Neural Transm 2004111281–294. [DOI] [PubMed] [Google Scholar]
- 4.Cagnin A, Brooks D J, Kennedy A M.et al In‐vivo measurement of activated microglia in dementia. Lancet 2001358461–467. [DOI] [PubMed] [Google Scholar]
- 5.Vehmas A K, Kawas C H, Stewart W F.et al Immune reactive cells in senile plaques and cognitive decline in Alzheimer's disease. Neurobiol Aging 200324321–331. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J, Strohmeyer R, Kovelowski C J.et al Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 200240260–269. [DOI] [PubMed] [Google Scholar]
- 7.Blasko I, Stampfer‐Kountchev M.et al How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell 20043169–176. [DOI] [PubMed] [Google Scholar]
- 8.Griffin W S, Mrak R E. Interleukin‐1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol 200272233–238. [PMC free article] [PubMed] [Google Scholar]
- 9.Licastro F, Grimaldi L M, Bonafe M.et al Interleukin‐6 gene alleles affect the risk of Alzheimer's disease and levels of the cytokine in blood and brain. Neurobiol Aging 200324921–926. [DOI] [PubMed] [Google Scholar]
- 10.Perry R T, Collins J S, Wiener H.et al The role of TNF and its receptors in Alzheimer's disease. Neurobiol Aging 200122873–883. [DOI] [PubMed] [Google Scholar]
- 11.Sala G, Galimberti G, Canevari C.et al Peripheral cytokine release in Alzheimer patients: correlation with disease severity. Neurobiol Aging 200324909–914. [DOI] [PubMed] [Google Scholar]
- 12.McGeer E G, McGeer P L. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry 200327741–749. [DOI] [PubMed] [Google Scholar]
- 13.Yucesoy B, Peila R, White L R.et al Association of interleukin‐1 gene polymorphisms with dementia in a community‐based sample: The Honolulu‐Asia Aging Study. Neurobiol Aging 200627211–217. [DOI] [PubMed] [Google Scholar]
- 14.Bossù P, Ciaramella A, Moro M L.et al Alzheimer's disease and immune activation: a translational perspective. Neurosci Res Commun 200435193–201. [Google Scholar]
- 15.Dinarello C A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 200683447S–4555. [DOI] [PubMed] [Google Scholar]
- 16.Felderhoff‐Mueser U, Schmidt O I, Oberholzer A.et al IL‐18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci 200528487–493. [DOI] [PubMed] [Google Scholar]
- 17.Conti B, Park L C, Calingasan N Y.et al Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res 19996746–52. [DOI] [PubMed] [Google Scholar]
- 18.Pompl P N, Yemul S, Xiang Z.et al Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch Neurol 200360369–376. [DOI] [PubMed] [Google Scholar]
- 19.Puren A J. Fantuzzi G, Gu Y, et al. Interleukin‐18 (IFNγ inducing factor) induces IL‐8 and IL‐1β via TNFα production from non‐CD14+ human mononuclear cells. J Clin Investig 1998101711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giedraitis V, He B, Huang W X.et al Cloning and mutation analysis of the human IL‐18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol 2001112146–152. [DOI] [PubMed] [Google Scholar]
- 21.Cummings J L. Alzheimer's disease. N Engl J Med 200435156–67. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 24.Spalletta G, Baldinetti F, Buccione I.et al Cognition and behavior are independent and heterogeneous dimensions in Alzheimer's disease. J Neurol 2004251688–695. [DOI] [PubMed] [Google Scholar]
- 25.Measso G, Cavarzeran F, Zappalà G.et al The mini‐mental state examination: normative study of an Italian random sample. Dev Neuropsychol 1993977–85. [Google Scholar]
- 26.Corder E H, Saunders A M, Strittmatter W J.et al Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993261921–923. [DOI] [PubMed] [Google Scholar]
- 27.Kamboh M I. Molecular genetics of late‐onset Alzheimer's disease. Ann Hum Genet 200468381–404. [DOI] [PubMed] [Google Scholar]
- 28.Bernardini S, Bellincampi L, Ballerini S.et al Glutathione S‐transferase P1 *C allelic variant increases susceptibility for late‐onset Alzheimer disease: association study and relationship with apolipoprotein E epsilon4 allele. Clin Chem 200551944–951. [DOI] [PubMed] [Google Scholar]
- 29.Spalletta G, Bernardini S, Bellincampi L.et al Glutathione S‐transferase P1 and T1 gene polymorphisms predict the longitudinal course and the age at onset of Alzheimer disease. Am J Geriatr Psychiatry (in press) [DOI] [PubMed]
- 30.Tiret L, Godefroy T, Lubos E.et al Genetic analysis of the interleukin‐18 system highlights the role of the interleukin‐18 gene in cardiovascular disease. Circulation 2005112643–650. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K, Fukuda Y, Sashio H.et al IL18 polymorphism is associated with an increased risk of Crohn's disease. J Gastroenterol 200237111–116. [DOI] [PubMed] [Google Scholar]
- 32.Kretowski A, Mironczuk K, Karpinska A.et al Interleukin‐18 promoter polymorphisms in type 1 diabetes. Diabetes 2002513347–3349. [DOI] [PubMed] [Google Scholar]
- 33.Gracie J A, Koyama N, Murdoch J.et al Disease association of two distinct interleukin‐18 promoter polymorphisms in Caucasian rheumatoid arthritis patients. Genes Immun 20056211–216. [DOI] [PubMed] [Google Scholar]
- 34.Szeszko J S, Howson J M, Cooper J D.et al Analysis of polymorphisms of the interleukin‐18 gene in type 1 diabetes and Hardy–Weinberg equilibrium testing. Diabetes 200655559–562. [DOI] [PubMed] [Google Scholar]
- 35.Rueda B, Gonzalez‐Gay M A, Mataran L.et al Interleukin‐18‐promoter polymorphisms are not relevant in rheumatoid arthritis. Tissue Antigens 200565544–548. [DOI] [PubMed] [Google Scholar]
- 36.Liang X H, Cheung W, Heng C K.et al Reduced transcriptional activity in individuals with IL‐18 gene variants detected from functional but not association study. Biochem Biophys Res Commun 2005338736–741. [DOI] [PubMed] [Google Scholar]
- 37.Arimitsu J, Hirano T, Higa S.et al IL‐18 gene polymorphisms affect IL‐18 production capability by monocytes. Biochem Biophys Res Commun 20063421413–1416. [DOI] [PubMed] [Google Scholar]
- 38.Blacker D, Bertram L, Saunders A J.et al Results of a high‐resolution genome screen of 437 Alzheimer's disease families. Hum Mol Genet 20031223–32. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg C, Chromek M, Ahrengart L.et al Soluble interleukin‐1 receptor type II, IL‐18 and caspase‐1 in mild cognitive impairment and severe Alzheimer's disease. Neurochem Int 200546551–557. [DOI] [PubMed] [Google Scholar]
- 40.Malaguarnera L, Motta M, Di Rosa M.et al Interleukin‐18 and transforming growth factor‐beta 1 plasma levels in Alzheimer's disease and vascular dementia. Neuropathology 200626307–312. [DOI] [PubMed] [Google Scholar]