Abstract
Background
Well characterised antineuronal antibodies (ANAbs) have been shown to be highly specific markers of neurological syndromes with a paraneoplastic aetiology. Previous reports indicate that pure motor neuron disease (MND) is rarely of paraneoplastic origin.
Objective
To screen systematically for the prevalence of well characterised paraneoplastic ANAbs in a large collective of patients with pure MND.
Methods
In a cohort of 145 patients with MND, the frequency of ANAbs was estimated by ELISA, employing recombinant antigens (HuD, Yo, Ri, CV2/CRMP5, Ma2 and amphiphysin).
Results
None of the sera revealed high antineuronal antigen reactivity. Very low reactivity was detected in only five sera, in all probability representing background activity.
Conclusion
According to these data, routine analysis for ANAbs in patients with isolated MND is not mandatory.
Paraneoplastic neurological syndromes (PNS) are remote effects of cancer, often associated with autoantibodies which cross react with antigens expressed by both the underlying tumour and neuronal tissue. These antibodies are believed to reflect an autoimmune process.
Amyotrophic lateral sclerosis (ALS) is defined as a progressive primary degeneration of upper and lower motor neurons in the absence of other disease processes,1 but its pathogenesis is a matter of controversy.
The idea that isolated motor neuron disease (MND) represents a manifestation of PNS is controversial. Some studies report concurrence of cancer and MND2; in addition, improvement of neurological symptoms after treatment of cancer has been seen,3,4 suggesting that some rare cases of motor neuron syndrome may be considered as paraneoplastic disorders. However, epidemiological surveys did not reveal an increased incidence of cancer in patients with ALS.5,6
Recently, an international consensus statement on PNS recommended that, in the absence of a detected tumour, only so called well characterised onconeuronal antibodies (anti‐HuD, Yo, Ri, CV2/CRMP5, Ma2 and amphiphysin) should be used to classify the associated neurological disorder as definite PNS.7 However, there is only limited information8,9,10,11 on the prevalence of well characterised antineuronal antibodies (ANAbs) in patients with MND. Some anecdotal reports describe paraneoplastic motor neuron syndromes associated with anti‐Yo12 and, more often, with anti‐Hu antibodies.13,14,15 However, a large sample of patients with isolated MND has not been systematically screened for the prevalence of ANAbs.
In this study, we assessed the frequency of well characterised ANAbs among patients with pure MND, in order to detect possible PNS mimicking idiopathic MND.
Patients and methods
Between January 1995 and October 2005, 222 patients with isolated MND were retrospectively evaluated at the Neurological Department, University of Freiburg, Germany. Sera obtained at the time of the primary diagnosis and clinical data from case records were available from 145 patients. The diagnosis of ALS was based on the revised El Escorial criteria.1 Patients with isolated upper motor neuron involvement had no other identifiable cause for degeneration of the corticospinal tracts than MND.1 Diagnosis of isolated lower motor neuron (LMN) degeneration was made according to clinical and EMG examination, together with the absence of electrophysiological or neuroimaging evidence of other disease processes.1
Additionally, three patients with MND and accompanying cancer were included. In one patient, breast cancer was detected 2 years before the diagnosis of MND, with isolated affection of LMN. Two patients suffered from plasmocytoma and affection of the LMN and ALS, respectively. Malignancy was present within 2 years of diagnosis. Nerve conduction studies were normal.
Twenty‐one sera, positive for ANAbs of various specificities, from patients with definite PNS, according to established criteria,7 and sera from 45 healthy subjects (mean age 21–25 years) and 45 sera from patients with normal pressure hydrocephalus served as controls. Sera were stored at −80°C prior to investigation.
Detection of ANAbs in the sera was performed by ELISA using standard protocols.16 Briefly, 96 well flat bottomed ELISA plates (Falcon, Heidelberg, Germany) were coated for 24 h at 4°C with 100 μl/well of recombinant antigens (HuD, Ma2 0.8 μg/ml; Yo 0.4 μg/ml; CV2/CRMP5, Ri, amphiphysin 0.2 μl/ml). Sera were diluted 1:1000 for detection of anti‐amphiphysin, anti‐CV2/CRMP5 and anti‐Ri antibodies, 1:500 (anti‐Ma2 antibodies, anti‐HuD antibodies) or 1:2000 for detection of anti‐Yo antibodies. Bound antibodies were detected by peroxidase conjugated goat antihuman IgG antibodies (Dianova, Hamburg, Germany), diluted 1:5000. As substrate solution, orthophenylendiamine 0.4 mg/ml (Dako, Hamburg, Germany) was added. Optical density (OD) was read at 410 nm in an ELISA reader (Dynatech MR 4000, Denkendorf, Germany). The diagnostic cut‐off OD reading was set as 4 SDs above the mean of 45 control sera from 23 healthy subjects, and 22 sera from patients with normal pressure hydrocephalus, while the remaining sera served as controls of the cut‐off value. Serial dilutions (from 1:500 to 1:128 000) were performed on all sera above the diagnostic cut‐off of each antigen. A commercial immunoblot using recombinant antigens (Ravo Diagnostika, Freiburg, Germany) and immunohistochemistry (Euroimmun, Luebeck, Germany) were used as confirmatory tests.
To assess possible intrathecal specific antibody synthesis in MND patients with low antineuronal antigen reactivity in serum, we analysed CSF/serum pairs previously adjusted to equal amounts of total IgG (2.5 mg/l) using established ELISAs. Specific antibody index values for each antigen were calculated as the ratio of CSF/serum quotients for specific antibodies and total IgG, according to the method proposed by Reiber and Peter.17 An antibody index >1.3 was considered as evidence of intrathecal specific antibody synthesis. Statistical significance was determined by the Student's t test and χ2 analysis.
The local ethics committee approved the study.
Results
Demographic data of the patients and controls are given in table 1.
Table 1 Demographic and clinical characteristics of the patients and controls.
| Group/subgroups | n | Age (y) (mean (SD)) | Sex (M/F) |
|---|---|---|---|
| Motor neuron disease | |||
| UMN | 5 | 62.0 (7.1) | 4/1 |
| LMN | 43 | 60.1 (10.5) | 30/13 |
| ALS | 97 | 61.1 (11.5) | 62/35 |
| Total | 145 | 60.8 (11.2) | 97/48 |
| Controls | |||
| NPH | 45 | 66.4 (18.0) | 25/20 |
ALS, amyotrophic lateral sclerosis; LMN, lower motor neuron; NPH, normal pressure hydrocephalus; UMN, upper motor neuron.
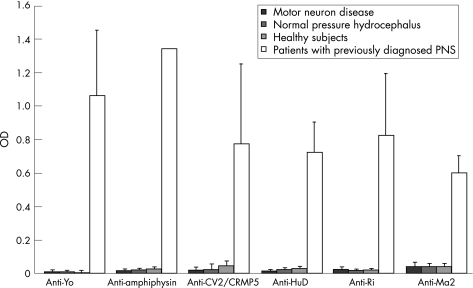
All sera from 21 patients with previously diagnosed unambiguous PNS revealed a clear positive result (fig 1) in the established ELISAs. Serial dilutions showed titres between 1:16 000 and 1:128 000, indicating high antineuronal antigen reactivity. Only 2 of 45 sera from healthy controls and patients with normal pressure hydrocephalus showed very weak ODs above the diagnostic cut‐off.
Figure 1 Antineuronal antigen reactivity of sera, expressed as optical density (OD) and SD: motor neuron disease (n = 145), normal pressure hydrocephalus (n = 23), healthy subjects (n = 22) and definite paraneoplastic neurological syndromes (PNS), according to established criteria7 (n = 21). Diagnostic cut‐off values: antiamphiphysin 0.099, CV2/CRMP5 0.156, HuD 0.077, Ma2 0.177, Ri 0.065 and Yo 0.049.
None of the sera from MND patients showed high antineuronal antigen reactivity (fig 1); only five sera revealed a very weak reaction (two anti‐Ri, two anti‐Yo, one anti‐Yo and one anti‐Ri; NS). However, confirmation tests of these sera using recombinant immunoblot and immunohistochemistry were negative. Furthermore, serial dilutions of all sera with a weak reaction above the diagnostic cut‐off revealed titres not exceeding 1:2000. Calculating the specific antibody index, we did not find specific intrathecal antibody synthesis in these subjects (ODCSF below the diagnostic cut‐off).
No antibodies directed against HuD, amphiphysin, CV2 or Ma2‐antigens were detected in any MND serum sample.
Patients with accompanying cancer were clearly negative for ANAbs.
Discussion
This study analysed the frequency of six well characterised ANAbs in a large sample of patients with isolated MND. Six ELISAs using highly purified recombinant antigens were established. Overall, we could not detect high antineuronal antigen reactivity in any of the 145 sera from MND patients. In view of the vast antineuronal antigen reactivity seen in patients with definite PNS, the very weak reactivity above the diagnostic cut‐off of five sera from MND patients probably reflects background activity of the ELISA rather than a correlation with probable PNS. This suggestion is further confirmed by the fact that none of these patients revealed a tumour after standard clinical investigation. In addition, weak reactions were also seen in two healthy controls. Confirmatory tests of these sera using immunoblot, immunohistochemistry and additional analysis for intrathecal ANAb synthesis were negative. The vast majority of the controls showed very low ODs in all recombinant ELISAs. All patients with PNS had ODs several times above the cut‐off value. Taken together, the established ELISAs suggest a high diagnostic sensitivity and specificity in our sample.
While epidemiological surveys5,6 failed to show a correlation between cancer and increased incidence of MND, some reports3,4 described improvement in neurological symptoms following treatment of malignant disease. Furthermore, lymphoproliferative syndromes18 seem to be linked to subacute motor neuronopathy, and some rare cases of primary lateral sclerosis have been reported in association with breast cancer.14 These reports suggest that some very rare cases of MND could be considered as a paraneoplastic syndrome.
We did not find antineuronal reactivity in the sera from three MND patients with accompanying cancer (breast cancer, plasmocytoma). However, we cannot rule out a possible paraneoplastic origin of MND in these cases as some PNS may occur without ANAbs.
There is only limited evidence of an association between pure MND and well characterised ANAbs. In anti‐Hu associated paraneoplastic encephalomyelitis, up to 20% of patients suffer from motor weakness as a prominent feature and appear to have LMN involvement, but these patients almost always had signs that other areas of the nervous system were involved.19 Dalmau and colleagues19 confirmed that none of these patients developed pure motor neuron syndrome mimicking ALS. Only a few reports12,13,14 have provided evidence of a paraneoplastic origin of motor neuron syndromes associated with highly specific ANAbs. To our knowledge, there are only two case reports of pure MND and cancer associated with classical ANAbs.12,13
A few studies, with a limited number of cases,8,9,10,11,20 systematically examined patients with isolated MND for the prevalence of ANAbs, and revealed either negative results or only atypical ANAbs. However, while a neurological disorder associated with classical ANAbs strongly indicates PNS, the significance of partially characterised or atypical ANAbs observed outside the context of classical PNS is not yet clear.7
Our data, from a large sample of patients with MND, were based on ELISA analysis using six highly specific recombinant antigens, and do not support an elevated prevalence of ANAbs in isolated MND, suggestive of a paraneoplastic syndrome.
In conclusion, the absence of classical ANAbs in our study suggests that routine analysis for these paraneoplastic markers in patients with pure MND is not mandatory. According to our results, a paraneoplastic aetiology of MND in association with well characterised paraneoplastic ANAbs is not likely, but these data do not exclude a possible association between MND and cancer in very rare cases. However, testing for ANAbs in MND patients should be performed in clinically atypical cases.
Abbreviations
ALS - amyotrophic lateral sclerosis
ANAbs - antineuronal antibodies
LMN - lower motor neuron
MND - motor neuron disease
OD - optical density
PNS - paraneoplastic neurological syndromes
Footnotes
Ravo Diagnostika GmbH provided the recombinant antigens for this study.
Competing interests: S Rauer is a founding executive board member of Ravo Diagnostika GmbH, Freiburg, Germany.
References
- 1.Brooks B R, Miller R G, Swash M.et al El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 20001293–299. [DOI] [PubMed] [Google Scholar]
- 2.Brain K, Croft P B, Wilkinson M. Motor neuron disease as a manifestation of neoplasm. Brain 196588479–500. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell D M, Olczak S A. Remission of a syndrome indistinguishable from motor neurone disease after resection of bronchial carcinoma. BMJ 19792176–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans B K, Fagan C, Arnold T.et al Paraneoplastic motor neuron disease and renal cell carcinoma: improvement after nephrectomy. Neurology 199040960–962. [DOI] [PubMed] [Google Scholar]
- 5.Chio A, Brignolio F, Meineri P.et al Motor neuron disease and malignancies: results of a population‐based study. J Neurol 1988235374–375. [DOI] [PubMed] [Google Scholar]
- 6.Zisfein J, Caroscio J T. No association of amyotrophic lateral sclerosis with cancer. Mt Sinai J Med 198855159–161. [PubMed] [Google Scholar]
- 7.Graus F, Delattre J Y, Antoine J C.et al Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004751135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown R H, Jr, Johnson D, Ogonowski M.et al Antineural antibodies in the serum of patients with amyotrophic lateral sclerosis. Neurology 198737152–155. [DOI] [PubMed] [Google Scholar]
- 9.Kiernan J A, Hudson A J. Anti‐neurone antibodies are not characteristic of amyotrophic lateral sclerosis. Neuroreport 19934427–430. [DOI] [PubMed] [Google Scholar]
- 10.Vigliani M C, Polo P, Chio A.et al Patients with amyotrophic lateral sclerosis and cancer do not differ clinically from patients with sporadic amyotrophic lateral sclerosis. J Neurol 2000247778–782. [DOI] [PubMed] [Google Scholar]
- 11.Vianello M, Vitaliani R, Pezzani R.et al The spectrum of antineuronal autoantibodies in a series of neurological patients. J Neurol Sci 200422029–36. [DOI] [PubMed] [Google Scholar]
- 12.Khwaja S, Sripathi N, Ahmad B K.et al Paraneoplastic motor neuron disease with type 1 Purkinje cell antibodies. Muscle Nerve 199812943–945. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Berger J R, Snodgrass S.et al Motor neuron disease: a paraneoplastic process associated with anti‐hu antibody and small‐cell lung carcinoma. Ann Neurol 199640112–116. [DOI] [PubMed] [Google Scholar]
- 14.Forsyth P A, Dalmau J, Graus F.et al Motor neuron syndromes in cancer patients. Ann Neurol 199741722–730. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa M, Nishie M, Kurahashi K.et al Anti‐Hu associated paraneoplastic sensory neuronopathy with upper motor neurone involvement. J Neurol Neurosurg Psychiatry 2004751051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauer S, Kaiser R. Enzyme linked immunosorbent assay using recombinant HuD‐autoantigen for serodiagnosis of paraneoplastic neurological syndromes. Acta Neurol Scand 2001103248–254. [PubMed] [Google Scholar]
- 17.Reiber H, Peter J B. Cerebrospinal fluid analysis: disease‐related data patterns and evaluation programs. J Neurol Sci 2000184101–122. [DOI] [PubMed] [Google Scholar]
- 18.Gordon P H, Rowland L P, Younger D S.et al Lymphoproliferative syndromes and motor neuron disease: an update. Neurology 1997481671–1678. [DOI] [PubMed] [Google Scholar]
- 19.Dalmau J, Graus F, Rosenblum M K.et al Anti‐Hu associated paraneoplastic encephalomyelitis/sensory neuronopathy: a clinical study of 71 patients. Medicine (Baltimore) 19927159–72. [DOI] [PubMed] [Google Scholar]
- 20.Greiner A, Schmausser B, Petzold K.et al Neuronal targets of serum and cerebrospinal fluid autoantibodies in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 19969167–71. [DOI] [PubMed] [Google Scholar]