Abstract
Objective
To investigate gender differences in basic disease characteristics, motor deterioration and nigrostriatal degeneration in Parkinson's disease (PD).
Methods
We studied 253 consecutive PD patients who were not receiving levodopa or dopamine agonists (disease duration ⩽10 years). We investigated the influence of gender and oestrogen status on: (1) age at onset, (2) presenting symptom, (3) severity and progression of motor symptoms (Unified Parkinson's Disease Rating Scale III (UPDRS‐III) scores) and (4) amount and progression of nigrostriatal degeneration ([123I]FP‐CIT single photon emission computed tomography measurements).
Results
Age at onset was 2.1 years later in women (53.4 years) than in men (51.3 years). In women, age at onset correlated positively with parity, age at menopause and fertile life span. Women more often presented with tremor (67%) than men (48%). Overall, patients presenting with tremor had a 3.6 year higher age at onset and a 38% slower UPDRS‐III deterioration. Mean UPDRS‐III scores at disease onset were equal for both genders, as was the rate of deterioration. Women had a 16% higher striatal [123I]FP‐CIT binding than men at symptom onset and throughout the course of PD.
Conclusions
Our results suggest that, in women, the development of symptomatic PD may be delayed by higher physiological striatal dopamine levels, possibly due to the activity of oestrogens. This could explain the epidemiological observations of a lower incidence and higher age at onset in women. Women also presented more often with tremor which, in turn, is associated with milder motor deterioration and striatal degeneration. Taken together, these findings suggest a more benign phenotype in women with PD.
There are several indications of gender differences in Parkinson's disease (PD). Epidemiological studies have shown that both incidence and prevalence of PD are 1.5–2 times higher in men than in women.1,2,3,4,5,6 Furthermore, in 6 out of 8 incidence studies mentioning gender specified age at onset, onset in women was slightly later than in men (by a mean of 2.2 years (range 1–4)).7 After progression into the clinical phase of the disease, women had better Unified Parkinson's Disease Rating Scale (UPDRS) motor scores8 but a greater prevalence of dyskinesias8,9 compared with men (at a disease duration of more than 5 years). Furthermore, men reported several parkinsonian symptoms more frequently than women when asked at a disease duration of 9 years.10 Gender differences in the earlier stage of PD, before initiation of dopamine agonists or levodopa, have not been investigated.
The reported gender differences reflecting distinct time periods—before and after symptom onset—could be related to the different levels of circulating oestrogens in men and women. Several findings indicate that oestrogens may play a role in PD. In animal models of PD, oestrogens had a neuroprotective effect when administered prior to or coinciding with a toxic insult.11,12,13 Secondly, the dopaminergic neurons in the substantia nigra and the striatal dopamine content were more vulnerable to chemical lesioning at dioestrus (low oestrogen) than at pro‐oestrus (high oestrogen).14 However, the possibly beneficial effects of oestrogens suggested by these reports could not be confirmed in humans. Postmenopausal oestrogen use in women was associated with both higher, lower and equal risks of PD.15,16,17 Furthermore, trials of postmenopausal oestrogen supplementation, which started after symptom onset, did not affect parkinsonian symptoms.18,19 However, women who had undergone ovariectomy or hysterectomy had an increased risk of PD.20,21 Thus the precise nature and extent of gender differences and the role of oestrogens in PD remain unclear.
Here we investigated whether and how gender affects both the preclinical and clinical disease stages, reflected by (1) the age of PD onset, (2) the presenting symptom, (3) the severity and progression of motor symptoms assessed with UPDRS derived variables and (4) the amount and progression of nigrostriatal degeneration assessed using [123I]FP‐CIT single photon emission computed tomography (SPECT).
Methods
Subjects
The study population was selected from a prospectively collected and longitudinally observed cohort of all consecutive PD patients who, between 1988 and 2003, visited the movement disorders outpatient clinic of the Radboud University Nijmegen Medical Centre (fig 1). As soon as the UK PD Society Brain Bank criteria22 became generally applied, patients were re‐evaluated to ensure a correct clinical diagnosis of PD according to these criteria. Patients were not included in the longitudinal database in cases of: (a) dementia, (b) physical disabilities resulting from any other disease which could interfere with the rating of PD severity, (c) motor fluctuations at the first visit and (d) non‐PD medication that could interfere with extrapyramidal motor function. Patients were assessed by the same neurologist (MH) who was also the treating physician. In this study, we only included data obtained during the first 10 years after symptom onset and before initiation of levodopa or dopamine agonists in order to compare the natural course of PD between men and women. This yielded a total of 253 eligible patients (62% men; table 1). A minority of patients (<25%) used selegiline, amantadine or orphenadrine, anti‐PD drugs with a very modest effect on motor function. Neither the percentage of men and women using these drugs nor the doses of these drugs differed between the sexes.
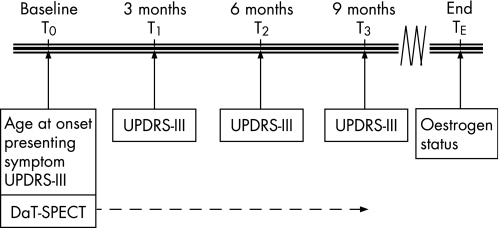
Figure 1 Data collection. Each patient underwent one single photon emission computed tomography (SPECT) scan. The arrow indicates that the disease duration at the moment of scanning varied among patients, ranging from 0.7 to 9.9 years. UPDRS‐III, Unified Parkinson's Disease Rating Scale III.
Table 1 Subject characteristics.
| Men | Women | p Value | |
|---|---|---|---|
| Age at disease onset (y) | 51.3 (25–77) | 53.4 (28–74) | 0.06 |
| Age at time of first sUPDRS‐III (y) | 53.4 (28–79) | 54 (31–75) | NS |
| Age at time of SPECT (y) | 52.8 (35–72) | 54.2 (31–68) | NS |
| Disease duration at time of first sUPDRS‐III (y) | 2.5 (0.4–9.9) | 2.7 (0.2–10.0) | NS |
| Disease duration at time of SPECT (y) | 4.3 (0.7–9.9) | 4.3 (1.6–8.5) | NS |
| No of subjects in age at onset and symptom analysis | 156 | 97 | |
| No of subjects in sUPDRS‐III analysis | 156 | 97 | |
| No of subjects in SPECT analysis | 74 | 36 | NS |
| Proportion (%) of patients taking | |||
| Selegiline | 25.2 | 24.2 | NS |
| Amantadine | 26.6 | 22.1 | NS |
| Orphenadrine | 7.2 | 9.3 | NS |
SPECT, single photon emission computed tomography; sUPDRS‐III, selection of items of the Unified Parkinson's Disease Rating Scale III.
Values are mean (range) unless otherwise stated.
Ethics
Patients were assessed with the UPDRS‐III as part of a standard care programme. [123I]FP‐CIT SPECT was carried out for routine diagnostic procedures or as part of scientific research programmes at our department. In the latter case, informed consent was obtained, as approved by the local ethics committee.
Age at onset and presenting symptom
Age at onset and presenting symptoms were retrieved from the patient's history, taken during the first visit. Age at onset was determined as the age at which the patient had first noticed any parkinsonian motor symptoms.
Motor examination
A total of 253 patients (156 men, 97 women) were longitudinally assessed using a selection of 7 items of the UPDRS part III (sUPDRS‐III: speech, facial expression, tremor at rest right/left, rigidity right/left, rapid alternating hand movements right/left, posture and body bradykinesia), yielding 10 variables that were recorded every 3–6 months during the course of the disease (834 observations, range 1–20 per patient). This selection of UPDRS items results from the fact that we originally used the Webster Scale before the UPDRS became generally accepted.
SPECT imaging
Approximately 3 h after intravenous injection of 200 MBq [123I]FP‐CIT (DaTSCAN; Amersham Health, Eindhoven, the Netherlands), SPECT was performed with a MultiSpect 2 dual headed gamma camera, connected to an ICON computer (Siemens, Hoffman Estates, Illinois, USA). The digital images (128×128 matrix, 64 angles) were reconstructed using a Butterworth filter (0.6 Nyquist, order 7) and reoriented in the canthus–meatus plane. The three consecutive slices with the highest striatal uptake (total thickness 14.6 mm) were selected for quantitative analysis. Fixed size regions of interest (derived from an anatomical brain atlas) were bilaterally drawn over the caudate nucleus, putamen and the occipital cortex (the latter serving as the reference region). Ratios of the [123I]FP‐CIT uptake in the caudate nucleus and occipital cortex and in the putamen and occipital cortex were calculated and normalised to data derived from measurements of Alderson's phantom. Only patients without interfering drugs underwent [123I]FP‐CIT SPECT imaging. Each patient underwent a single SPECT scan. Disease duration at the moment of scanning varied among patients, ranging from 0.7 to 9.9 years (indicated by the arrow in fig 1). This yielded 59 SPECT scan results. Nineteen additional SPECT scans were obtained from the Groningen University Medical Centre, as well as 32 SPECT scans from the Academic Medical Centre, Amsterdam, all meeting the study's inclusion criteria.
Oestrogen status
In 2004, all women who had undergone UPDRS testing were asked to complete a questionnaire concerning their oestrogen status throughout their lives. Oestrogen status included the age at menarche, age at menopause, parity and use of postmenopausal oestrogen replacement therapy. This yielded 77 completed questionnaires.
Data analysis
All analyses were performed by a professional statistician (GFB). Mean age at onset was compared between men and women using the Student's t test. Incidence of presenting symptoms (tremor vs bradykinesia/rigidity) was analysed using logistic regression with the covariates gender and age at onset. SPECT results were log transformed and analysed using linear regression analysis with the covariates gender, age at onset, presenting symptom, disease duration and hospital where the scan was performed. The logarithmic transformation made it possible to adjust for scale differences between the hospitals by including the additional covariate “hospital”. It also removed the skewness in the data and allowed direct estimation of the relative changes (percentage change). The graph was used to indirectly infer initial tracer uptake for the groups, together with the rate of deterioration. The sUPDRS‐III score was calculated as the mean of the scores on the individual motor items. If the scores for more than two items were missing, the overall score was not calculated. As most patients had been tested on multiple occasions, a random effects regression model was used with the levels patient and test. The independent variables included in the analysis were gender, age at onset, presenting symptom and disease duration. Linear regression was used to analyse the effect of age at menarche, age at menopause, duration of the fertile life span and parity at age at onset, presenting symptom, sUPDRS‐III and SPECT measurements. The group of women who had taken oestrogen replacement therapy was too small to use in the statistical analyses. Concerning all research questions, we used exploratory analyses for interactions between the various independent variables and appropriate quadratic terms were included in each model in order to investigate non‐linear trends. In the final analyses, all main effects were kept in the models; interactions and higher order terms were only included if they were statistically significant (p<0.05; two sided). The level of statistical significance of interactions with gender—the main subject of interest—was set at p<0.10 in order not to miss relevant differences with respect to gender due to a lower sensitivity of interaction tests. Ninety‐five per cent confidence intervals (CI) were calculated.
Results
Age at onset
At symptom onset, women were 2.1 (95% CI −0.1 to 4.1) years older than men (women, mean 53.4 years; men, mean 51.3 years; p = 0.06).
Presenting symptom
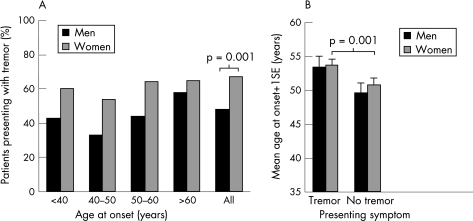
Women presented significantly more often (67%) with tremor at symptom onset than men (48%), irrespective of age at onset (p = 0.001) (fig 2A). The influence of age at onset on tremor incidence (2% increase in tremor presentation for each additional year) did not differ between the sexes. When presenting with tremor as opposed to bradykinesia/rigidity, age at onset was 3.6 years later for both sexes (95% CI 1.5 to 5.7; p = 0.001) (fig 2B).
Figure 2 Presenting symptom. (A) Women presented more often with tremor (84/125 = 67%) than men, across all age groups (107/221 = 48%). (B) When presenting with tremor as opposed to bradykinesia‐rigidity, age at onset was 3.6 years later for both sexes.
sUPDRS‐III
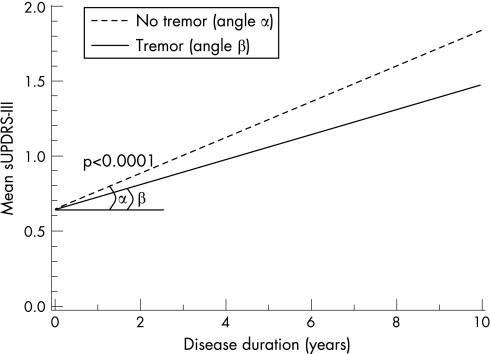
Mean sUPDRS‐III scores did not differ between men and women with PD, at onset or during disease progression (p = 0.55) (fig 3). For both sexes, the scores increased by 0.09 points/year (95% CI 0.08 to 0.10; p<0.0001). Interestingly, tremor dominant patients showed a 38% slower increase in sUPDRS‐III scores (ie, slower deterioration) than bradykinetic‐rigid patients (p<0.0001) (fig 3).
Figure 3 Tremor dominant versus non‐tremor dominant patients. Data shown for patients with age at onset of 50 years. With increasing age at onset, the mean initial sUPDRS (selection of items of the Unified Parkinson's Disease Rating Scale III) increases without changing the rate of deterioration. The regression lines show the estimated increase in mean sUPDRS‐III scores for patients presenting with or without tremor. Patients presenting with tremor showed a 38% lower rate of deterioration (angle β = 0.08 points/year) than patients presenting with bradykinesia/rigidity (α = 0.12 points/year).
SPECT imaging
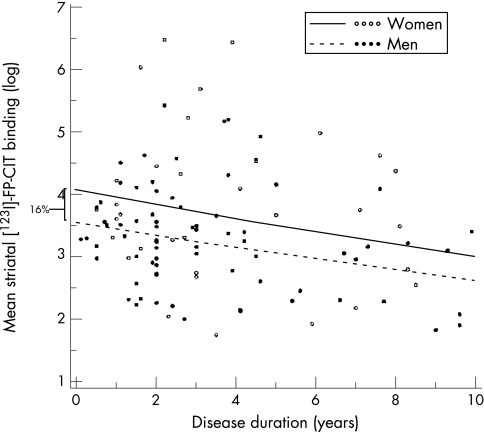
Women had 16% higher [123I]FP‐CIT binding than men at symptom onset (95% CI 3.0 to 32%; p = 0.01) (fig 4). However, the rate of decline in tracer binding (3.1%/year) did not differ between the sexes. Tremor dominant patients tended to have a lower rate of decline than bradykinetic‐rigid patients, although this effect did not reach significance (8.7%; p = 0.1). The fact that the SPECT sample was not fully identical to the sUPDRS‐III sample did not affect these findings; the same results were obtained when analysing only those patients with both SPECT and sUPDRS‐III assessments.
Figure 4 Mean striatal [123I]‐FP‐CIT binding. The lines show the estimated decrease in mean tracer binding for men and women with age at onset of 50 years. A higher age at onset was accompanied by lower initial striatal tracer binding in both men and women. Women had 16% higher [123I]FP‐CIT binding than men. The plotted observed values are those of all patients in the study, irrespective of age at onset.
Oestrogen status
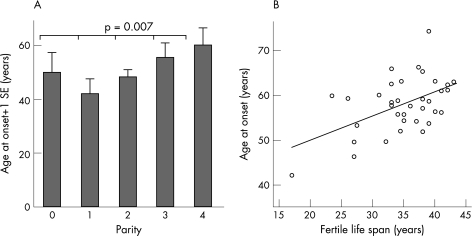
Several oestrogen status items were associated with age at onset. Parity correlated positively with age at onset: the more children a woman had before PD onset, the higher the age at onset (2.7 years later onset per child; 95% CI 0.8 to 4.6; p = 0.007) (fig 5A), the only exception being women without children. These women may have a different basal oestrogen status than women with children. Furthermore, in women with an early PD onset, the choice to have (fewer) children might be influenced by the burden of PD, thereby possibly inverting the relationship between age at onset and parity. To control for this, we repeated the analyses for the subgroup of women with children (n = 67) and for the subgroup of women with an age at onset ⩾40 years (n = 67) and found the same results. Combining both selection criteria (n = 58) did not change these results. Age at menopause correlated with the age at onset when taking those women with symptom onset after menopause (0.5 years later onset per year; 95% CI 0.1 to 0.8; p = 0.009). In these women, the duration of the fertile life span was associated with a 0.5 year later onset per year (95% CI 0.2 to 0.9; p = 0.001) (fig 5B). Without the selection based on the order of menopause and symptom onset, the correlation of age at menopause (p = 0.02) and of duration of the fertile life span (p = 0.05) with age at onset remained significant. Age at menarche did not correlate with age at onset (p = 0.2). No association was found for any of the oestrogen status items with presenting symptom, sUPDRS‐III or SPECT results.
Figure 5 Oestrogen status and age at onset. (A) The more children a woman had before the onset of Parkinson's disease, the higher the age at onset. (B) A longer fertile life span led to a higher age at onset: 0.5 year delay/year.
Discussion
Several findings in this study suggested a more benign PD phenotype in women compared with men. The benefits for women seemed to apply mainly to the events preceding overt PD. Firstly, women tended to be older than men at symptom onset. Secondly, women presented more often than men with a tremor dominant form of PD, which in turn was associated with a slower disease progression. Thirdly, at onset of the disease, women had higher levels of striatal dopamine binding than men. These benefits in the preclinical phase could be related to oestrogen status, because parity (number of children), age at menopause and duration of the fertile life span were all associated with a later age at onset of PD. However, women seemed to have no further advantage over men once PD had become clinically manifest. Thus the rates of disease progression—as indexed by both clinical scores and measurements of striatal dopamine binding—were comparable between men and women with overt symptoms. In the next paragraphs, we discuss these findings in more detail.
Gender differences preceding clinical PD
The 2 year difference in age at PD onset between men and women observed here is comparable with the difference found in most epidemiological studies.7 It suggests that the development of symptomatic PD is slightly delayed in women compared with men. This could be explained by higher initial striatal dopamine levels in women compared with men, as noted in this study. Those higher initial dopamine levels could delay the moment of reaching a critical threshold of striatal dopamine depletion and hence postpone the development of parkinsonian symptoms.
Several observations support this explanation. In our study, at symptom onset and throughout the course of the disease, women had 16% higher striatal [123I]FP‐CIT binding than men. This suggests that the critical threshold of dopamine depletion at which symptoms emerge is 16% higher in women than in men. However, women become symptomatic 2 years later then men. This could be explained by a similar relative loss of dopamine required for symptom onset in men and women, but a higher initial dopamine content in women. Furthermore, healthy women have higher striatal dopamine transporter binding than healthy men.23,24,25 The gender difference in striatal dopamine transporter binding noted in our study thus appears to be physiological and not specifically PD related. This is supported by the fact that healthy women have a lower postsynaptic dopamine D2 receptor affinity than healthy men,26 probably reflecting D2 receptor downregulation induced by high presynaptic striatal dopamine levels. Animal experiments confirm a physiological gender difference: in healthy rats and MPTP lesioned mice, the density of dopamine transporters and striatal dopamine concentrations were higher in females than in males.27,28,29 Secondly, in animal studies, expression of striatal dopamine transporters appeared to be determined by oestrogen28,30 and closely paralleled the level of striatal dopamine.31 Consequently, a situation in which intracerebral oestrogen levels increase (eg, pregnancy) will lead to upregulation of striatal dopamine transporters, in turn resulting in higher striatal dopamine levels.
Although extrapolating animal data to humans requires caution, our data would fit into this concept. We found that parity—the result of pregnancy, a situation of high circulating oestrogen levels—correlated positively with age at onset, a fact not reported previously. However, a relationship between parity and PD irrespective of age at onset has been reported previously: women with PD had reduced parity compared with healthy women.32 Moreover, medical conditions leading to oestrogen depletion (eg, hysterectomy, oophorectomy or short fertile life span) increased the risk of PD20,21,33 while a reduced risk was found for postmenopausal oestrogen use, which increases cumulative oestrogen levels.16,17 Our study showed that a later menopause and a longer fertile life span—also increasing cumulative oestrogen levels—delayed the age at onset. This has not been reported previously.
Gender differences in overt PD
The rate of decline in SPECT binding did not differ between the sexes. Most other studies show rates of decline similar to ours, although not specified for gender.34,35,36,37 The results suggest that once the critical threshold of striatal dopamine depletion has been exceeded, disease progression does not differ between men and women. In other words, oestrogens may exert some form of neuroprotection in the preclinical stage of PD, or may even postpone the beginning of the degenerative process, but fail once symptoms have become clinically apparent. This idea is supported by animal work: oestrogens may protect against nigrostriatal degeneration in animal models of PD when administered prior to the insult11,12,13 but do not exert any beneficial effects when administered after nigrostriatal damage has occurred.12,13 This may help to understand the failure of oestrogen replacement therapy in slowing the progression of overt PD in women. Thus a double blind, placebo controlled study of high dose transdermal 17β‐oestradiol in postmenopausal women with PD showed no effect on motor scores.19 Furthermore, a placebo controlled, randomised, double blind trial in postmenopausal women with PD found no significant effect of orally administered oestradiol on objective and subjective parkinsonian symptoms.18 An additional explanation could be that both orally and transdermally administered oestrogens do not lead to high enough oestrogen concentrations within the blood–brain barrier to exert any effect at all.
When pointing at a variable (ie, oestrogen) as a possible aetiological factor in a disease process, one should take into account that this variable might coexist with or influence another, perhaps unknown, factor which is actually responsible for the observed effect. For example, a long fertile life span may have increased the risk of iron deficiency because of iron loss during menses and pregnancies. Iron, in turn, has been implicated as an important factor in the oxidative processes within the nigrostriatal system although its precise role remains unclear. Consequently, we cannot exclude the fact that the iron status of the women in our study could have contributed to the findings which we associated with oestrogens.
Tremor dominant patients, both men and women, were 3.6 years older at symptom onset than patients presenting with the hypokinetic‐rigid subtype of PD. The tremor dominant patient group also showed a 38% slower increase (ie, slower deterioration) in sUPDRS‐III scores, a finding consistent with previous suggestions of a more benign course for this PD subtype.37,38,39,40 Additionally, [123I]FP‐CIT binding tended to decrease less fast in the tremor dominant patients. Together with a later onset and a more frequent onset with tremor in women, these findings suggest that women are more likely than men to acquire the benign tremor dominant PD subtype.
Methodological issues
This study was not without drawbacks. Firstly, our department is a dedicated referral centre for patients with movement disorders, and this may have affected the basic characteristics of our study population. It would seem unlikely, however, that referral bias confounded our questions regarding the influence of gender on PD. The percentage of men and women with PD in our population was similar to population based numbers. Another issue could be that referrals from a greater distance to our clinic could have represented different (ie, more complicated) PD patients than the ones from nearby. However, we compared the patient characteristics from those referred within the area of Nijmegen with those coming from other geographical regions and did not find any significant differences. Furthermore, we analysed the SPECT results obtained from the three different hospitals located in three distant regions of the Netherlands separately, before pooling them. We found the same results for both separate and combined analyses. Therefore, we feel that the most important referral biases have been excluded. Moreover, the magnitude of this PD cohort enabled us to select many patients with a disease duration of up to 10 years but without exposure to levodopa or dopamine agonists. This created the opportunity to study the unmedicated, natural course of PD.
Secondly, oestrogen status was ascertained retrospectively, which could have introduced recall bias. However, the most important oestrogen status item, parity, is unlikely to be subject to recall bias. Finally, the oestrogen status analyses were based on a limited sample size. Therefore, extrapolating our findings to all women with PD requires caution. Nevertheless, our study may initiate important future research into the hypotheses and explanations we suggested.
Acknowledgements
This work was supported in part by a grant from the Van Alkemade Fonds, the Netherlands.
Abbreviations
PD - Parkinson's disease
SPECT - single photon emission computed tomography
sUPDRS‐III - selection of items of the Unified Parkinson's Disease Rating Scale III
UPDRS‐III - Unified Parkinson's Disease Rating Scale III
Footnotes
Competing interests: None.
References
- 1.Van Den Eeden S K, Tanner C M, Berstein A L.et al Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 20031571015–1022. [DOI] [PubMed] [Google Scholar]
- 2.de Lau L M, Giesbergen P C, de Rijk M C.et al Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 2004631240–1244. [DOI] [PubMed] [Google Scholar]
- 3.Wooten G F, Currie L J, Bovbjerg V E.et al Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry 200475637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag A, Ben‐Shlomo Y, Quinn N P. Cross sectional prevalence survey of idiopathic Parkinson's disease and parkinsonism in London. BMJ 200032121–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claveria L E, Duarte J, Sevillano M D.et al Prevalence of Parkinson's disease in Cantalejo, Spain: a door‐to‐door survey. Mov Disord 200217242–249. [DOI] [PubMed] [Google Scholar]
- 6.Benito‐Leon J, Bermejo‐Pareja F, Rodriguez J.et al Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 200318267–274. [DOI] [PubMed] [Google Scholar]
- 7.Twelves D, Perkins K S, Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord 20031819–31. [DOI] [PubMed] [Google Scholar]
- 8.Lyons K E, Hubble J P, Tröster A I.et al Gender differences in Parkinson's disease. Clin Neuropharmacol 199821118–121. [PubMed] [Google Scholar]
- 9.Zappia M, Annesi G, Nicoletti G.et al Sex differences in clinical and genetic determinants of levodopa peak‐dose dyskinesias in Parkinson disease. Arch Neurol 200562601–605. [DOI] [PubMed] [Google Scholar]
- 10.Scott B, Borgman A, Engler H.et al Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand 200010237–43. [DOI] [PubMed] [Google Scholar]
- 11.Dluzen D. Estrogen decreases corpus striatal neurotoxicity in response to 6‐hydroxydopamine. Brain Res 1997767340–344. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Dluzen D E. Tamoxifen abolishes estrogen's neuroprotective effect upon methamphetamine neurotoxicity of the nigrostriatal dopaminergic system. Neuroscience 2001103385–394. [DOI] [PubMed] [Google Scholar]
- 13.Gajjar T M, Anderson L I, Dluzen D E. Acute effects of estrogen upon methamphetamine induced neurotoxicity of the nigrostriatal dopaminergic system. J Neural Transm 20031101215–1224. [DOI] [PubMed] [Google Scholar]
- 14.Datla K P, Murray H E, Pillai A V.et al Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport 20031447–50. [DOI] [PubMed] [Google Scholar]
- 15.Popat R A, Van Den Eeden S K, Tanner C M.et al Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology 200565383–390. [DOI] [PubMed] [Google Scholar]
- 16.Currie L J, Harrison M B, Trugman J M.et al Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol 200461886–888. [DOI] [PubMed] [Google Scholar]
- 17.Ascherio A, Chen H, Schwarzschild M A.et al Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 200360790–795. [DOI] [PubMed] [Google Scholar]
- 18.Strijks E, Kremer J A, Horstink M W. Effects of female sex steroids on Parkinson's disease in postmenopausal women. Clin Neuropharmacol 19992293–97. [DOI] [PubMed] [Google Scholar]
- 19.Blanchet P J, Fang J, Hyland K.et al Short‐term effects of high‐dose 17[beta]‐estradiol in postmenopausal PD patients: a crossover study. Neurology 19995391–95. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti M D, Maraganore D M, Bower J H.et al Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case‐control study. Mov Disord 200116830–837. [DOI] [PubMed] [Google Scholar]
- 21.Rocca W A, Grossardt B R, Bower J H.et al The Mayo Clinic Cohort study of oophorectomy and aging: results for parkinsonism and Parkinson's disease. Presented at the 57th annual meeting of the American Academy of Neurology, April 9–16, 2005, Miami Beach, Florida, USA
- 22.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staley J K, Krishnan‐Sarin S, Zoghbi S.et al Sex differences in [123I]beta‐CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 200141275–284. [DOI] [PubMed] [Google Scholar]
- 24.Lavalaye J, Booij J, Reneman L.et al Effect of age and gender on dopamine transporter imaging with [123I]FP‐CIT SPET in healthy volunteers. Eur J Nucl Med 200027867–869. [DOI] [PubMed] [Google Scholar]
- 25.Laakso A, Vilkman H, Bergman J.et al Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry 200252759–763. [DOI] [PubMed] [Google Scholar]
- 26.Pohjalainen T, Rinne J O, Nagren K.et al Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998155768–773. [DOI] [PubMed] [Google Scholar]
- 27.Dluzen D E, McDermott J L, Liu B. Estrogen as a neuroprotectant against MPTP‐induced neurotoxicity in C57/B1 mice. Neurotoxicol Teratol 199618603–606. [DOI] [PubMed] [Google Scholar]
- 28.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 19935816–22. [DOI] [PubMed] [Google Scholar]
- 29.Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res 1995692269–272. [DOI] [PubMed] [Google Scholar]
- 30.Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments on ovariectomized rats on brain dopamine uptake sites. J Neurochem 1993601876–1883. [DOI] [PubMed] [Google Scholar]
- 31.Bezard E, Dovero S, Prunier C.et al Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐lesioned macaque model of Parkinson's disease. J Neurosci 2001176853–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martignoni E, Nappi R E, Citterio A.et al Reproductive life milestones in women with Parkinson's disease. Funct Neurol 200318211–217. [PubMed] [Google Scholar]
- 33.Ragonese P, D'Amelio M, Salemi G.et al Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology 2004112010–2014. [DOI] [PubMed] [Google Scholar]
- 34.Staffen W, Mair A, Unterrainer J.et al Measuring the progression of idiopathic Parkinson's disease with [123I] beta‐CIT SPECT. J Neural Transm 2000107543–552. [DOI] [PubMed] [Google Scholar]
- 35.Parkinson Study Group Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 20022871653–1661. [DOI] [PubMed] [Google Scholar]
- 36.Pirker W, Holler I, Gerschlager W.et al Measuring the rate of progression of Parkinson's disease over a 5‐year period with beta‐CIT SPECT. Mov Disord 2003181266–1272. [DOI] [PubMed] [Google Scholar]
- 37.Hilker R, Schweitzer K, Coburger S.et al Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol 200562378–382. [DOI] [PubMed] [Google Scholar]
- 38.Hoehn M M, Yahr M D. Parkinsonism: onset, progression, and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic J, Kapadia A S. Functional decline in Parkinson disease. Arch Neurol 2001581611–1615. [DOI] [PubMed] [Google Scholar]
- 40.Lewis S J, Foltynie T, Blackwell A D.et al Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 200576343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]