Abstract
Background and aim
The risks of recurrent intracerebral haemorrhage (ICH) vary widely (0–24%). Patients with ICH also have risk factors for ischaemic stroke (IS) and a proportion of ICH survivors re‐present with an IS. This dilemma has implications for prophylactic treatment. This study aims to determine the risk of recurrent stroke events (both ICH and IS) following an index bleed and whether ICH recurrence risk varies according to location of index bleed.
Patients and methods
All patients diagnosed with an acute ICH presenting over an 8.5 year period were identified. Each ICH was confirmed by reviewing all of the radiology results and, where necessary, the clinical case notes or post‐mortem data. Recurrent stroke events (ICH and IS) were identified by reappearance of these patients in our stroke database. Coronial post‐mortem results for the same period were also reviewed. Each recurrent event was reviewed to confirm the diagnosis and location of the stroke.
Results
Of the 7686 stroke events recorded, 768 (10%) were ICH. In the follow‐up period, there were 19 recurrent ICH and 17 new IS in the 464 patients who survived beyond the index hospital stay. Recurrence rate for ICH was 2.1/100 in the first year but 1.2/100/year overall. This compares with 1.3/100/year overall for IS. Most recurrences were “lobar–lobar” type.
Conclusion
The cumulative risk of recurrent ICH in this population is similar to that of IS after the first year.
Strokes caused by an intracerebral haemorrhage (ICH) are less common than ischaemic strokes (IS), but they have a much higher early case fatality.1,2 ICH contributes to approximately 10–15% of all strokes in Caucasians, but this proportion is increased in some Asian and South American populations.1,3,4,5,6 This variation in incidence may be due to genetic influences, prevalence and treatment of hypertension and medication use (for example, antiplatelet and anticoagulant drugs) or a combination of these factors.
Given the high mortality, survivors of an ICH are justifiably fearful of another event. They ask, “What are my chances of having a further stroke (ICH), doctor?” Answering this with confidence is challenging. Just as incidence rates are diverse, the reported risk of recurrence following an ICH also varies enormously from 0% to 24% (table 1). This may be explained in part by small sample sizes or short follow‐up periods.7,8,9,15,22 Differences in the mean age and ethnicity of the populations studied, as well as location of the bleed and prevalence of hypertension, are alternative reasons.
Table 1 Risk of recurrence of intracerebral haemorrhage.
| Country | Reference | n | Mean age (y) | Maximum duration of follow‐up (y) | Crude cumulative recurrence rate (%) |
|---|---|---|---|---|---|
| Italy | Fieschi 19887 | 104 | 61 | 1 | 0 |
| USA | Douglas 19828 | 70 | – | (median 2.5) | 0 |
| Denmark | Helwig‐Larsen 19849 | 53 | 54 | 9 | 0 |
| South Korea | Lee 199010 | 518 | 54 | 3 | 2.7 |
| Finland | Fogelholm 199211 | 158 | 68 | 5 | 4 |
| Germany | Buhl 200312 | 968 | 63.7 | 11 | 4.9 |
| Canada | Hill 200013 | 172 | 65 | 11 | 4.9 |
| India | Misra 199514 | 105 | 43.5 | – | 5 |
| Taiwan | Chen 199515 | 892 | 59 | 2 | 5.3 |
| South Korea | Bae 199916 | 989 | 58 | 7 | 5.4 |
| Japan | Maruishi 199617 | 406 | 62.9 | 8 | 5.9 |
| Mexico | Gonzalez‐Duarte 199818 | 359 | 60 | 8.5 | 6.0 |
| France | Neau 199719 | 375 | 64.7 | 10 | 6.4 |
| Japan | Inagawa 200520 | 279 | 63.3 | 7 | 7 |
| Australia | Hankey 199821 | 36 | – | 5 | 8 |
| Japan | Arakawa 199822 | 74 | 60.8 | 5 | 11 |
| Finland | Fogelholm 200523 | 203 | 66.6 | 16 | 11.3 |
| Netherlands | Vermeer 20022 | 243 | – | 10 | 12.1 |
| Italy | Passero 199524 | 112 | 63.7 | (mean 7) | 24 |
The incidence of ICH increases with age1 and it is important to know the recurrence risk for all patients, including the very old. However, the average age of patients in these recurrence studies ranges from 54 to 66 years.10,13,19 This is much lower than expected for our stroke population,25 suggesting some bias against inclusion of older patients with ICH. The predominant location of the index bleed varies from almost all deep to mostly lobar.17,19 Location may alter the chance of recurrence.24 Lobar bleeds have been reported to have a higher recurrence risk in some studies.2,13,19 This may be because of the higher prevalence of cerebral amyloid angiopathy (CAA), ApoE genotype or that anticoagulant related bleeds are more commonly associated with lobar location.26,27,28 Hypertension is a risk factor for both the incidence of ICH and recurrent bleeds,1,29 but in epidemiological studies control of hypertension is difficult to measure, and therefore may contribute to the large variation in risk of recurrence.22 As with incidence data, ethnicity may explain some of the differences.
Some of the risk factors for ischaemic stroke are similar to those for ICH. These include hypertension, male sex, increasing age and possibly diabetes and smoking.30 It is not uncommon for clinicians to have patients who have had both an ischaemic and a haemorrhagic stroke.29,31 This presents a challenge when deciding what the most appropriate secondary prevention strategies are. For example, should aspirin be stopped, started or continued? These dilemmas have been eloquently debated in recent papers,32,33 but cannot be answered confidently without further information. This includes knowing the risks of recurrence of both ICH and ischaemic type strokes, as well as the risk of an ischaemic event in an ICH population (and vice versa).
Therefore, this study has the following aims
to determine the risk of recurrence of an ICH following an index bleed in a predominantly Caucasian population;
to determine whether this recurrence risk varies according to location of the index bleed;
to determine the risk of ischaemic stroke following an index bleed.
Methods
Christchurch city and the surrounding North Canterbury area (denominator population 450 000) are served by a single acute general hospital (includes the regional neurosurgical unit) and two subacute or rehabilitation hospitals. Almost all patients with an acute disabling stroke are admitted to the acute hospital and over 95% receive a CT scan within the first day.34 MRI is available on the acute site, but is not routine as the first investigation for a suspected stroke. Patients are transferred to the subacute hospitals if further inpatient rehabilitation is required.
In New Zealand, patients are allocated a unique national health identifier (NHI) number, which is used for any subsequent health events. This NHI enables tracking of patients between different hospitals (eg, between acute and subacute hospitals for the same index event) and for subsequent hospitalisation or death.
At discharge from hospital, all patients have their diagnoses coded using the International Classification of Diseases‐10 (or ICD 9 previously) classification. These discharge coding data from each of the three hospitals were used to identify all patients who had an acute stroke (I60‐I67 for ICD‐10 and 430–437 for ICD‐9) for the 8.5 year period from 1 January 1996 to 30 June 2004. From these extracted data, a subgroup of all patients diagnosed with an acute ICH (I61 (inclusive of all 161 codes) and I62.9 for ICD 10, or 431 (inclusive of all 431 codes) and 432.9 for ICD 9) was identified. All radiology data (and where necessary the clinical case notes) of each of these ICH patients was reviewed to confirm the diagnosis, location and volume of the bleed. Location was broadly categorised into the following types: lobar, deep, intraventricular, cerebellar and brainstem. Volume was calculated using a grid (for earlier scans)35 and latterly the ABC/2 method.36
In New Zealand, all sudden unexplained deaths are referred to the coroner. The coronial records for the same period were reviewed for any additional patients who may have died because of ICH before reaching hospital. In these cases, the autopsy reports were reviewed to confirm the diagnosis.
Exclusions from the study were subarachnoid haemorrhage, ICH from arteriovenous malformation, tumour, trauma, surgery and/or thrombolysis as well as when the patient usually resided outside of the North Canterbury region.
Recurrence of a stroke event was determined by a subsequent appearance of a person from the original dataset. For each possible recurrence, all relevant neuroimaging (and where necessary the clinical notes) were reviewed to confirm the presence of either a new (distinct from index) ICH or cerebral infarct, as well as the location and volume of bleed for ICH patients. Patients were followed until 31 December 2004 (follow‐up period varied from 6 months to 9 years) or until death. Deaths were ascertained from overlapping sources including: (a) coronial records, (b) Canterbury District Health Board patient management systems and (c) New Zealand Health Information Service. The latter uses the NHI number and records date of death, irrespective of where in the country the death occurred. This source was used as a double check for local data and picked up patients who had subsequently died in the community without re‐entering hospital. Date of death and, where possible, cause of death, were recorded.
Kaplan–Meier survival statistics were used to compare survival rates and recurrence rates. Cox's proportional hazards analysis was used to determine any association with recurrence from age, sex or site of bleed.
The study was approved by the regional Canterbury Ethics Committee (CTR/0407/127).
Results
There were 7686 stroke events recorded during the 8.5 year period, of which 915 were coded as an ICH. A total of 768 patients had the ICH confirmed (10% of all strokes) while 147 patients were excluded. Reasons for exclusion are shown in table 2.
Table 2 Reasons for exclusion from the study.
| Reason for exclusion | n |
|---|---|
| Miscoding | |
| Haemorrhagic transformation of infarct | 36 |
| Ischaemic infarct | 27 |
| Subdural haemorrhage | 1 |
| No stroke identified | 3 |
| Secondary cause for ICH | |
| Malignancy | 22 |
| Arteriovenous malformation | 11 |
| Subarachnoid haemorrhage | 5 |
| Trauma | 5 |
| Thrombocytopenia | 2 |
| Radiotherapy | 1 |
| Miscellaneous reasons | |
| Lives outside of catchment area | 11 |
| Notes or radiology unable to be located | 7 |
| Age <16 years | 1 |
| ICH suspected but not confirmed before death* | |
| Died before neuroimaging or no neuroimaging done and no autopsy done | 15 |
ICH, intracerebral haemorrhage.
*Non‐inclusion of these patients may result in underestimation of the initial ICH incidence, but does not affect recurrence estimates as all of these patients died.
The mean age of ICH patients was 72.4 years (range 16–95), and 33.6% of the sample were 80 years or older. Fifty‐two per cent were female. The group were predominantly Caucasian (92%) with smaller numbers of Asian (2.4%), Maori (2.4%), Pacific Island (2.2%) and Indian (0.5%) which mirrors the ethnicity of the older people in Canterbury.37 The primary imaging was CT scan in 742 patients and MRI in 26. Volume of bleed varied from <1 ml to 300 ml, with a mean of 36 ml.
Almost half the index bleeds were lobar (table 3).
Table 3 Location of index bleed.
| Location of index ICH | No (% of all ICH) | No (%) who died in first 24 h | No (%) who died within 28 days | No (%) surviving until discharge from hospital |
|---|---|---|---|---|
| Lobar* | 373 (49%) | 66 (18%) | 152 (41%) | 218 (58%) |
| Deep | 273 (36%) | 41 (15%) | 98 (36%) | 172 (63%) |
| Intraventricular | 24 (3%) | 4 (17%) | 12 (50%) | 11 (46%) |
| Brainstem | 31 (4%) | 9 (29%) | 17 (55%) | 14 (45%) |
| Cerebellar† | 67 (9%) | 14 (21%) | 29 (43%) | 38 (58%) |
| Total | 768 (100%) | 134 (17.5%) | 308 (40%) | 453 (59%) |
ICH, intracerebral haemorrhage.
*Includes three patients with more than one concurrent ICH.
†Includes one patient with two concurrent cerebellar ICH.
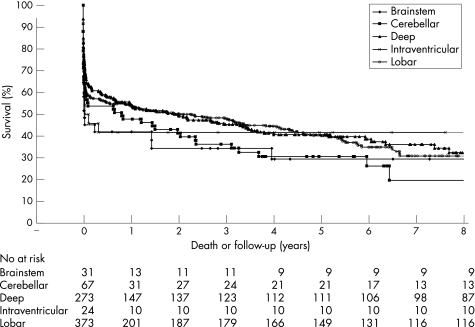
A total of 240 deaths (52% of all deaths) occurred within the first week (fig 1) and 315 deaths occurred in the index hospital admission. The cause of death for those dying in the community (146) was ascertained in 80/146 (55%). Ten of these community deaths were stroke related (7 ICH, 3 IS). The majority of patients, for whom the cause of death is not known, died in long term care hospitals (long term case facilities or nursing homes) (47/62, 76%).
Figure 1 Survival curve for all index intracerebral haemorrhages.
A recurrent ICH occurred in 19 patients (2.5% of all index ICH) with 3 of these patients sustaining a third bleed (all lobar). This equated to a recurrence rate of 1.2/100/year for those who survived beyond their index hospital admission. However, the rate was higher in the first year at 2.1/100. There was a trend towards a lower rate of recurrence for those with deep bleeds (table 4) but these differences according to location of bleed were not statistically significant. Using a proportional hazards model, increased age (p = 0.049), but not sex (p = 0.66) or site of bleed (p = 0.47), was associated with risk of recurrence.
Table 4 Crude recurrence for survivors compared with location of the index bleed.
| Index bleed | Recurrent bleed | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | n | Lobar | Deep | Intraventricular | Cerebellar | Brainstem | Unknown* | Total | Unadjusted % recurrence |
| Lobar | 218 | 8 | 2 | 1 | 11 | 5.0% | |||
| Deep | 172 | 1 | 2 | 1 | 4 | 2.3% | |||
| Intraventricular | 11 | 1 | 1 | 9.1% | |||||
| Cerebellar | 38 | 1 | 1 | 2 | 7.1% | ||||
| Brainstem | 14 | 1 | 1 | 5.3% | |||||
| Total | 453 | 12 | 4 | 1 | 1 | 0 | 1 | 19 | 4.2% |
ICH, intracerebral haemorrhage.
*Unknown location but presumed ICH on the basis of devastating new stroke, reduced level of consciousness and subsequent death, but no new neuroimaging.
Seventeen patients who had an index ICH also had an ischaemic stroke during follow‐up. These infarcts were diagnosed by acute CT head scans in 16 and MRI in one. All were in a different location to the index bleed. The rate of new ischaemic events over the follow‐up period was 1.3/100/year. Unlike ICH recurrence rates, the IS rate was not higher in the first year after the ICH (for patients who survived beyond the index hospital stay). Using a proportional hazards model, neither age, sex or site of bleed was associated with the risk of a subsequent IS.
Two people had both a new infarct and a recurrent bleed after the initial ICH.
Discussion
The main findings of this study are that the risk of ICH recurrence in survivors of an ICH is highest in the first year at 2.1/100/year but after that the overall rate of 1.2/100/year is comparable with the risk of an IS at 1.3/100/year. Increasing age, but not location of the index bleed, is associated with a higher recurrence rate. Almost half of the index bleeds were lobar and the majority of the recurrences were of a “lobar–lobar” type.
There are some considerations in relation to completeness of data collection. The risks of recurrence of IS in our study represent a lower estimate of risk as some events may have been missed. Very early recurrences may have been missed in dying patients who did not have a second CT scan and the deterioration attributed to the index ICH. However, clinical deterioration in all other patients would prompt a repeat scan. Secondly, while there was a reliance on CT rather than MRI scans, we believe that this has not resulted in any significant under counting of recurrence, as CT is sensitive for detecting acute ICH. However, small microbleeds (detectable on MRI) may have been missed.38 Finally, the cause of out‐of‐hospital deaths was unable to be ascertained in 66 people (14% of all deaths). However, all sudden unexplained deaths in our community are referred to the coroner and most ICH patients who survive the initial ictus are symptomatic and so present to the single acute hospital. Disabled patients already residing in an institution were less likely to re‐present to the acute hospital and constituted 75% of those in whom cause of death was not ascertained. Either ICH or IS could have occurred in some of these patients. Some IS may have been missed in patients who presented with neurological signs similar to their index ICH and the CT scan did not show either a new ICH or definite IS. These were not counted as definite IS and so our figures for IS should be regarded as a lower estimate of risk.
Another potential weakness of our study is that medication use at the time of recurrence (eg, antiplatelet agents) and the presence (and control) of hypertension were not known. The adequacy of control of hypertension after an ICH is known to influence risk of recurrence.15,22,29 However, control of hypertension, and other cardiovascular risk factors, in our general community population has recently been shown to be good.39
Balanced against these are significant strengths of the study. The denominator population was well defined and of a reasonable size, a large cohort of ICH patients (n = 768) was followed and the cohort included a large group of older patients (including the “old‐old”). Mean age was considerably older than other reported ICH recurrence series7,10,13,15,18,19,22 and is comparable with the recent Auckland (NZ) Stroke Study.40 Thus this cohort is representative of all patients presenting with ICH, including the very old and those with comorbidities. Unique national identifiers allowed tracing of all recurrent events to be more complete and enabled local survival data to be cross checked against national survival data and there was a long follow‐up period (up to 9 years).
In this New Zealand population, the risk of ICH recurrence in survivors of an ICH is moderate, with a cumulative risk of 1.2/100/year. This is comparable with, or lower than, other predominantly Caucasian populations.2,11,12,13,23,24,31 This is notwithstanding the average age of our cohort (72.4 years) being older than other series (range 54–68 years). One‐third of our cohort was over 80 years old. Age is a recognised risk of both mortality and recurrence risk.1,2,11 Increased age is associated with a higher prevalence of cerebral amyloid angiopathy and lobar bleeds, both of which increase recurrent events.2 Alternatively, an older cohort may have lower risks by reducing initial survival. However, by reporting recurrence in survivors only, this should not be a factor. We believe that the age structure of our study population is more reflective of all strokes and ICH presenting in our community and thus our estimate of risk can be generalised to all stroke survivors.4,40
In Asian series (and possibly South/Central American18), deep bleeds predominate and most recurrences are “deep–deep” type (ie, both index and recurrent bleed were deep).14,16,17,22,27 In contrast, almost half of the index bleeds in this study were lobar which is a similar or higher proportion than previous reports in Caucasian populations.2,7,19,41,42 Furthermore, the majority of the recurrences were “lobar–lobar” type.11,19 Lobar bleeds are thought to be the result of hypertensive disease, anticoagulant use or CAA, either singly or in combination.43 CAA is a recognised risk factor for recurrent haemorrhages in a lobar pattern, with one study showing 30% recurrence rates for biopsy proven CAA.1,27,38 Lobar location of the bleed may increase the unadjusted risk of recurrence but it is not known whether this is independent of CAA or age.2,13,19,24,26 Our findings suggest that it is not independent of these variables. Patients presenting with an index bleed located in the brainstem, cerebellum or ventricles had a recurrence risk equal to or greater than lobar bleeds. While this has not been previously reported, it requires cautious interpretation. The numbers in each index group are much smaller than for lobar and deep bleeds, and the risk calculations are based on one or two recurrent bleeds only. However, it is interesting to note that all but one of these recurrences occurred in a lobar distribution.
In the current study, the IS risk in survivors was comparable to that of ICH recurrence (1.3/100/year and 1.2/100/year, respectively) but lower than the first year recurrence risk (2.1/100). Canadian,13 Dutch2 and Australian studies21 also found that rates of IS and ICH were similar. A Finnish study had similar proportions of ICH and IS, but a much higher absolute, but unadjusted, risk (11.3% and 10.3%, respectively).23 In contrast, other studies suggest that recurrent ICH events are more common than IS by a factor of between 1.4 and 2.9.29,31
The findings of this study have considerable clinical significance. While the risk of a recurrent bleed appears to be higher in the first year after the index ICH, there is an ongoing risk that extends out for many years12,16,18,23 at a rate that is similar to IS. This finding is not surprising, as the risks of hypertension and increasing age persist. Antihypertensive treatment is very effective for both types of stroke29 so ongoing efforts to control high blood pressure in this group are appropriate. Antiplatelet or anticoagulant therapy are appropriate strategies to reduce IS, but are usually contraindicated in ICH survivors.32,33 This seems reasonable in the first year after an ICH. However, as some patients with an index ICH later develop IS, thromboembolic prophylaxis may be appropriate in selected individuals. Aspirin both increases the relative risk of ICH and decreases the relative risk of IS, but the absolute risks in this population are not known.32,33 Therefore, thromboembolic prophylaxis should only be given if the chance of IS is high and after careful consideration of the risks and benefits for the given individual.
Acknowledgements
We are thankful to the staff of the medical records and radiology departments for assisting with searching for the relevant archived documents and radiology. We are also grateful to the NZ Health Information Service for confirmation of deaths. The anonymous reviewers made some very helpful comments, for which we are grateful.
Abbreviations
CAA - cerebral amyloid angiopathy
ICD - International Classification of Diseases
ICH - intracerebral haemorrhage
IS - ischaemic stroke
NHI - national health identifier
Footnotes
Funding: Funding was provided by the Canterbury District Health Board and the Canterbury Health Care of the Elderly Education Trust. This funding supported one of the authors (NFI) as a summer student but played no other role in the study.
Competing interests: None.
References
- 1.Mayer S A, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol 20054662–672. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer S E, Algra A, Franke C L.et al Long term prognosis after recovery from primary intracerebral hemorrhage. Neurology 200259205–209. [DOI] [PubMed] [Google Scholar]
- 3.Bin J, Wang W ‐ Z, Chen H.et al Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke 20063763–68. [DOI] [PubMed] [Google Scholar]
- 4.McNaughton H, Weatherall M, McPherson K.et al The comparability of community outcomes for European and non‐European survivors of stroke in New Zealand. NZ Med J 200211598–100. [PubMed] [Google Scholar]
- 5.Kubo M, Kiyohara Y, Kato I.et al Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community. The Hisayama study. Stroke 2003342349–2354. [DOI] [PubMed] [Google Scholar]
- 6.Lavados P M, Sacks C, Prina L.et al Incidence, 30 day case‐fatality rate, and prognosis of stroke in Iquique, Chile: a 2 year community‐based prospective study PISCIS project. Lancet 20053652206–2215. [DOI] [PubMed] [Google Scholar]
- 7.Fieschi C, Carolei A, Fiorelli M.et al Changing prognosis of primary intracerebral hemorrhage: Results of a clinical and computed tomographic follow up study of 104 patients. Stroke 198819192–195. [DOI] [PubMed] [Google Scholar]
- 8.Douglas M A, Haerer A F. Long term prognosis of hypertensive intracerebral hemorrhage. Stroke 198213488–491. [DOI] [PubMed] [Google Scholar]
- 9.Helwig‐Larsen S, Sommer W, Strange P.et al Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke 1984151045–1048. [DOI] [PubMed] [Google Scholar]
- 10.Lee K S, Bae H G, Yun I G. Recurrent intracerebral haemorrhage due to hypertension. Neurosurgery 199026586–590. [DOI] [PubMed] [Google Scholar]
- 11.Fogelholm R, Nuutila M, Vuorela A ‐ L. Primary intracerebral haemorrhage in the Jyvaskla region, Central Finland, 1985–9: incidence, case fatality and functional outcome. J Neurol Neurosurg Psychiatry 199255546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buhl R, Barth H, Mehdorn H M. Risk of recurrent intracerebral hemorrhages. Neurol Res 200325853–856. [DOI] [PubMed] [Google Scholar]
- 13.Hill M D, Silver F L, Austin P C.et al Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke 200031123–127. [DOI] [PubMed] [Google Scholar]
- 14.Misra U K, Kalita J. Case reports: recurrent hypertensive intracerebral hemorrhage. Am J Med Sci 1995310156–157. [DOI] [PubMed] [Google Scholar]
- 15.Chen S T, Chiang C Y, Hsu C Y.et al Recurrent hypertensive intracerebral hemorrhage. Acta Neurol Scand 199591128–132. [DOI] [PubMed] [Google Scholar]
- 16.Bae H ‐ G, Jeong D, Doh J ‐ W.et al Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis 19999102–108. [DOI] [PubMed] [Google Scholar]
- 17.Maruishi M, Shima T, Okada Y.et al Clinical findings in patients with recurrent intracerebral hemorrhage. Surg Neurol 199644444–449. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez‐Duarte A, Cantu C, Ruiz Sandoval J L.et al Recurrent primary cerebral hemorrhage. Stroke 1998291802–1805. [DOI] [PubMed] [Google Scholar]
- 19.Neau J ‐ P, Ingrand P, Couderq C.et al Recurrent intracerebral hemorrhage. Neurology 199749106–113. [DOI] [PubMed] [Google Scholar]
- 20.Inagawa T. Recurrent primary intracerebral hemorrhage in Izumo City, Japan. Surg Neurol 20056428–36. [DOI] [PubMed] [Google Scholar]
- 21.Hankey G J, Jamrozik K, Broadhurst R J.et al Long term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke 1998292491–2500. [DOI] [PubMed] [Google Scholar]
- 22.Arakawa S, Saku Y, Ibayashi S.et al Blood pressure control and recurrence of hypertensive brain hemorrhage. Stroke 1998291806–1809. [DOI] [PubMed] [Google Scholar]
- 23.Fogelholm R, Murros K, Rissanen A.et al Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2005761534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passero S, Burgalassi L, D'Andrea P.et al Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke 1995261189–1192. [DOI] [PubMed] [Google Scholar]
- 25.Life after stroke: New Zealand guideline for management of stroke. Wellington: Stroke Foundation of New Zealand, 2003
- 26.Woo D, Kaushal R, Chakraborty R.et al Association of apolipoprotein E4 and haplotypes of the apolipoprotein E gene with lobar intracerebral hemorrhage. Stroke 2005361874–1880. [DOI] [PubMed] [Google Scholar]
- 27.Izumihara A, Suzuki M, Ishihara T. Recurrence and extension of lobar hemorrhage related to cerebral amyloid angiopathy: multivariate analysis of clinical risk factors. Surg Neurol 200564160–164. [DOI] [PubMed] [Google Scholar]
- 28.McCarron M O, Nicoll J A R, Ironside J W.et al Cerebral amyloid angiopathy‐related hemorrhage: interaction of APOE 2 with putative clinical risk factors. Stroke 1999301643–1646. [DOI] [PubMed] [Google Scholar]
- 29.Chapman N, Huxley R, Anderson C, Writing Committee for the PROGRESS Collaborative Group et al Effects of a perindopril based blood pressure lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: The Progress trial. Stroke 200435116–121. [DOI] [PubMed] [Google Scholar]
- 30.Juvela S, Kase C S. Advances in intracerebral hemorrhage management. Stroke 200637301–304. [DOI] [PubMed] [Google Scholar]
- 31.Bailey R D, Hart R G, Benavente O.et al Recurrent brain hemorrhage is more common than ischaemic stroke after intracranial hemorrhage. Neurology 200156773–777. [DOI] [PubMed] [Google Scholar]
- 32.Eckman M H, Rosand J, Knudsen K A.et al Can patients be anticoagulated after intracerebral haemorrhage? A decision analysis. Stroke 2003341710–1716. [DOI] [PubMed] [Google Scholar]
- 33.Wani M, Nga E, Navaratnasingham R. Should a patient with primary intracerebral haemorrhage receive antiplatelet or anticoagulant therapy? Br Med J 2005331439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanger H C, Fletcher V, Fink J.et al Improving care for stroke patients: adding an acute stroke unit helps. NZ Med J 20071201250. [PubMed] [Google Scholar]
- 35.Rowe C C, Donnan G A, Bladin P F. Intracerebral haemorrhage: incidence and use of computed tomography. Br Med J 19882971177–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kothari R U, Brott T, Broderick J P.et al The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996271304–1305. [DOI] [PubMed] [Google Scholar]
- 37. Health needs assessment of people aged 65 and over in the Canterbury District Health Board. Canterbury District Health Board. February 2003. http://www.cdhb.govt.nz/communications/publications.htm#health‐needs (accessed 25/5/07)
- 38.Greenberg S M, Eng J A, Ning M.et al Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004351415–1420. [DOI] [PubMed] [Google Scholar]
- 39.Senior H, Anderson C S, Chen M H.et al Management of hypertension in the oldest old: a study in primary care in New Zealand. Age Ageing 200635178–182. [DOI] [PubMed] [Google Scholar]
- 40.Anderson C, Carter K N, Hackett M L.et al Trends in stroke incidence in Auckland, New Zealand, during 1981 to 2003. Stroke 2005362087–2093. [DOI] [PubMed] [Google Scholar]
- 41.Rosenow F, Hojer C, Meyer‐Lohmann C.et al Spontaneous intracerebral hemorrhage: prognostic factors in 896 cases. Acta Neurol Scand 199796174–182. [PubMed] [Google Scholar]
- 42.Radberg J A, Olsson J E, Radberg C T. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke 199122571–576. [DOI] [PubMed] [Google Scholar]
- 43.Steiner T, Rosan J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy. Stroke 200637256–262. [DOI] [PubMed] [Google Scholar]