Abstract
A 57‐year‐old man with type 2 diabetes mellitus for 10 years showed progressive loss of muscle strength in both legs, pain and muscle atrophy in the femoral region and significant weight loss. On admission, he could not stand alone and used a wheelchair. He also complained of severe pain in the lower extremities. He was diagnosed with proximal diabetic neuropathy (PDN) by characteristic clinical and electrophysiological features. Intravenous immunoglobulin therapy (IVIg 0.4 g/kg×5 days) markedly reduced the severe pain and muscle weakness in the legs. Eventually, pain assessed by the Visual Analogue Scale was relieved by 80% and muscle strength was also well recovered, thereby enabling the patient to walk with a cane. The present case suggests that IVIg therapy may be effective for the relief of pain in PDN.
Proximal diabetic neuropathy (PDN) is a relatively rare form of peripheral neuropathy associated with diabetes mellitus. PDN usually exhibits an acute or subacute course of painful symptoms and muscle weakness with atrophy, often unilateral, in the proximal muscles of the lower extremities.1,2 In many cases, severe weight loss is also seen. Muscle weakness and pain usually spreads to the opposite side and shows persistence.3,4 Recently, findings of vasculitic lesions in nerve biopsies have suggested that PDN is related to an immune mediated mechanism.4,5,6,7 Here we report a patient with PDN, in whom intravenous immunoglobulin therapy (IVIg) treatment dramatically ameliorated severe neuropathic pain and muscle weakness in the lower extremities.
Case report
A 57‐year‐old man suffered from type 2 diabetes mellitus for 10 years and had been receiving medication to lower blood glucose (oral glimepiride). The level of haemoglobin A1c, however, remained above 8%. He did not have retinopathy or nephropathy and had not been given insulin treatments. Pain in the left trunk developed in January 2005, distributed segmentally in the Th 6 to Th 9 area and spread to the right side immediately. Pain in the left leg developed suddenly in July 2005. Initially, it showed a patchy distribution and was localised in the femoral region, but then spread to the whole leg and increased in severity. Muscle weakness developed in the left femoral region in October 2005, and he could not walk alone. At that time, muscle atrophy in the left femoral area was apparent. In November 2005, pain, muscle atrophy and weakness spread to the opposite side so that he could not stand and began to use a wheelchair. He lost 17 kg in body weight (from 82 kg to 65 kg) in 11 months over the course of the disease.
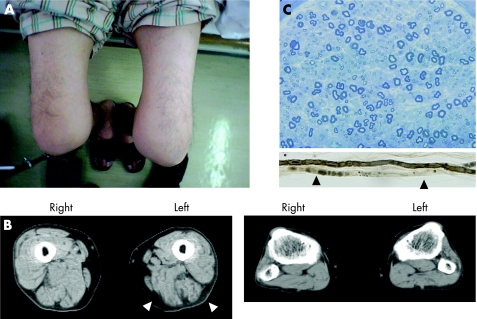
Neurological examination on admission revealed severe muscle weakness predominantly in the proximal muscles of the lower extremities, especially on the left side. Asymmetric muscle atrophy was observed in the left femoral to gluteus muscles (fig 1A). Neither loss of muscle strength nor muscle atrophy was observed in the upper extremities. Burning pain developed spasmodically in the Th 6 to Th 9 segmental region of the trunk, predominantly on the left side. Touch, pain and temperature sensations were moderately decreased in this area. Pain in both legs was severe, burning, spasmodic and showed a patchy distribution localised in the area of the left thigh around the knee joint, and the interior side of the lower leg. Touch, pain and vibratory sensations were moderately decreased in all extremities in a glove and stocking distribution. Deep tendon reflexes were either diminished or absent on both sides of the legs and arms. The Babinski sign was negative.
Figure 1 Clinical features and biopsy results. (A) The patient's lower extremities. The left thigh is visibly atrophic and exhibits redness in its entirety. (B) Muscle atrophy of the thigh (left panel) was confirmed by computed tomography (arrowheads). There was no clear muscle atrophy in the lower legs (right panel). (C) Transverse section of sural nerve specimen stained with toluidine blue; there was a slight decrease in myelinated fibres. Teased fibres showed axonal degeneration (arrowheads). Informed consent was obtained for publication of this figure.
All routine haematological, serological and biochemical examinations were normal except for the elevated haemoglobin A1c at 6.6% following 11 months of dietary treatment. In CSF, protein content was increased at 250 mg/dl (normal range 15–45 mg/dl), whereas the cell count was normal. Cytological assessment of CSF was negative. Motor nerve conduction velocity was 51 m/s in the left median nerve and 53 m/s in the left ulnar nerve, and both the compound muscle action potential amplitudes were within the normal range of 11 mV and 8.2 mV, respectively. In contrast, nerve conduction velocity and compound muscle action potential amplitude of the left posterior tibial nerve, at 34 m/s and 1.3 mV, respectively, were below the normal range. Sensory nerve conduction velocity was 52 m/s in the left median nerve and 47 m/s in the left ulnar nerve, and the sensory nerve action potentials were in the normal range of 5.9 μV and 7.0 μV, respectively. The left sural nerve conduction velocity was 41 m/s, slightly below the normal range, and the sensory nerve action potential was normal (16.6 µV). Electromyograms of the left quadriceps femoris and anterior tibial muscles revealed positive sharp waves at rest. He was not able to contract the muscles of the lower limbs voluntary because of burning pain, and there was no more evidence of denervation on electromyography. The cauda equine was not enhanced with gadolinium on lumbar MRI. Muscle CT revealed muscle atrophy in the left thigh but not in the lower leg (fig 1B). The left sural nerve biopsy specimen showed a slight decrease in fibre density in both large and small myelinated fibres (fig 1C), axonal atrophy and degeneration, and thickening of the capillary basement membrane. Vasculitis or infiltration of inflammatory cells were not seen.
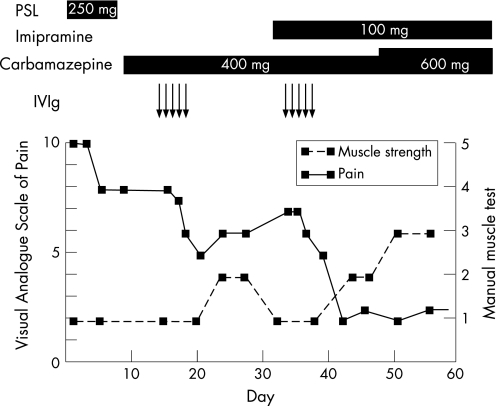
Based on the clinical and electrophysiological findings, we diagnosed the patient with PDN accompanied by diabetic thoracoabdominal neuropathy and distal symmetrical sensory polyneuropathy. Eparestat, mecobalamin, mexiletine and carbamazepine were given with no benefit. Following prednisolone (250 mg) for 3 days, he showed mild clinical improvement. He was then treated with IVIg (0.4 g/kg×5 days) in December 2005 and showed remarkable clinical improvement, particularly in terms of pain and muscle weakness. The level of pain in the area of the left thigh improved from 10 to 5 on the Visual Analogue Scale (VAS).8 Although pain gradually exacerbated in the subsequent 3 weeks from VAS 5 to 7, a repeat course of IVIg dramatically ameliorated the pain (VAS 7 to 2) (fig 2). Muscle strength also improved following IVIg. Both proximal and distal muscles in the right lower leg and the proximal muscle in the left lower leg improved markedly (fig 2). But the distal muscle in the left lower leg improved only slightly, leaving severe disturbance in the dorsal extension of the ankle. After two sessions of IVIg therapy, he could walk with a cane.
Figure 2 Clinical course of Visual Analogue Scale (VAS) and manual muscle test in the left thigh. A 10 cm VAS was anchored by two extremes of pain (left, no pain; right, the worst pain imaginable), and pain ratings were assigned subjectively according to the manner described by Kelly.8 Results of the manual muscle test in the left quadriceps femoris muscle are shown in six stages from 0 to 5. Horizontal bars indicate days during which the given drugs were administered. IVIg, intravenous immunoglobulin therapy; PSL, prednisolone.
Discussion
We treated a patient with PDN who developed severe pain and muscle weakness in both legs, but predominantly on the left side. Two courses of IVIg significantly reduced severe pain and muscle weakness. The clinical course and laboratory findings observed in this patient were concordant with those of typical PDN.1,4 PDN is often accompanied by distal symmetrical sensory polyneuropathy as well as diabetic thoracoabdominal neuropathy, as in this patient.5,9
As multifocal ischaemic changes were found in the nerve biopsies from patients with PDN, some reports have assumed that the primary mechanism of PDN is ischaemia.3,10,11 Indeed, vasculitis of the small vessels in the nerve trunk, brought about by immune mediated mechanisms, has been postulated to contribute to the development of ischaemia. Perivascular infiltration of inflammatory cells, multifocal loss of nerve fibres and haemosiderosis have also been described.
The effectiveness of corticosteroids and γ‐globulin have been reported, supporting the view that an immune process is involved in the pathogenesis of PDN. Although no controlled studies have been performed for therapy in PDN, some papers have reported the effectiveness of IVIg in patients with PDN.5,6,7,12,13 In these reports, details of the clinical course are not shown although it is reported to be effective for not only muscle weakness but also for pain in PDN. In our patient, muscle weakness improved dramatically. Morii et al reported that corticosteroid use dramatically diminished pain.14 As corticosteroid treatment in diabetic patients should be avoided, amelioration of pain by IVIg in our patient was very important.
Neuropathic pain is common but one of the most difficult conditions to ameliorate. Pain in diabetic neuropathies is multifactorial and has heterogeneous causative factors, including microvascular insufficiency associated with atherosclerosis and vasculitis, oxidative/nitrosative stress, defective neurotrophism and autoimmune mediated nerve destruction.15 Tricyclic antidepressants, antiepileptic drugs and analgesics are often used as firstline drugs in the treatment of pain. However, these treatments were not effective in our case. Recently, we reported that IVIg was beneficial for painful sensory neuropathy associated with Sjogren's syndrome, which is thought to be caused by autoimmune mediated nerve and ganglionic destruction.16 New therapies based on pathogenesis can be expected to alleviate symptoms and improve the quality of life. Randomised prospective trials of IVIg will be needed for PDN and could shed light on other painful neuropathies.
Abbreviations
IVIg - intravenous immunoglobulin therapy
PDN - proximal diabetic neuropathy
VAS - Visual Analogue Scale
Footnotes
Competing interests: None.
References
- 1.Barohn R J, Sahenk Z, Warmolts J R.et al The Bruns–Garland syndrome (diabetic amyotrophy). Revisited 100 years later. Arch Neurol 1991481130–1135. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P K. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 199746(Suppl 2)S54–S57. [DOI] [PubMed] [Google Scholar]
- 3.Llewelyn J G, Thomas P K, King R H.et al Epineurial microvasculitis in proximal diabetic neuropathy. J Neurol 1998245159–165. [DOI] [PubMed] [Google Scholar]
- 4.Dyck P J, Windebank A J. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 200225477–491. [DOI] [PubMed] [Google Scholar]
- 5.Said G, Goulon‐Goeau C, Lacroix C.et al Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol 199435559–569. [DOI] [PubMed] [Google Scholar]
- 6.Krendel D A, Costigan D A, Hopkins L C.et al Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol 1995521053–1061. [DOI] [PubMed] [Google Scholar]
- 7.Younger D S, Rosoklija G, Hays A P.et al Diabetic peripheral neuropathy: a clinicopathologic and immunohistochemical analysis of sural nerve biopsies. Muscle Nerve 199619722–727. [DOI] [PubMed] [Google Scholar]
- 8.Kelly A M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 200118205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun S F, Streib E W. Diabetic thoracoabdominal neuropathy: clinical and electrodiagnostic features. Ann Neurol 1981975–79. [DOI] [PubMed] [Google Scholar]
- 10.Dyck P J, Engelstad J, Norell J.et al Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999532113–2121. [DOI] [PubMed] [Google Scholar]
- 11.Kelkar P, Masood M, Parry G J.et al Distinctive pathologic findings in proximal diabetic neuropathy (diabetic amyotrophy). Neurology 20005583–88. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes Filho J A, Nathan B M, Palmert M R.et al Diabetic amyotrophy in an adolescent responsive to intravenous immunoglobulin. Muscle Nerve 200532818–820. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa T, Taguchi T, Tanaka Y.et al Intravenous immunoglobulin therapy for diabetic amyotrophy. Intern Med 200140349–352. [DOI] [PubMed] [Google Scholar]
- 14.Morii T, Fujita H, Toyoshima I.et al Efficacy of immunoglobulin and prednisolone in diabetic amyotrophy. Endocr J 200350831–832. [DOI] [PubMed] [Google Scholar]
- 15.Vinik A I. Advances in diabetes for the millennium: new treatments for diabetic neuropathies. MedGenMed 20046(Suppl 3)13. [PMC free article] [PubMed] [Google Scholar]
- 16.Kizawa M, Mori K, Iijima M.et al Intravenous immunoglobulin treatment in painful sensory neuropathy without sensory ataxia associated with Sjogren's syndrome. J Neurol Neurosurg Psychiatry 200677967–969. [DOI] [PMC free article] [PubMed] [Google Scholar]