Abstract
Background and aims
To investigate the hypothesis that patients with a hemisphere stroke may perceive their longitudinal body axis (LBA) rotated in the frontal plane. This error in an egocentric frame of reference could be detrimental to posture, as tilted LBA would imply an unequal distribution of body mass about the true vertical.
Method
26 healthy subjects matched in age with 18 patients living with stroke participated in the study. The 18 patients were tested on average 80 days after a first left (n = 8) or right (n = 10) hemisphere stroke. Participants perceived their LBA by adjustments of the orientation of a luminous rod pivoting around a dorsonavel axis to the subjective direction of LBA. Participants were studied in the supine position to dissociate somaesthetic cues from graviceptive cues.
Results
Patients with stroke perceived their LBA rotated to the contralesional side in comparison with controls (p = 0.004). For all controls and 10 patients with stroke, the perceived LBA was very close to true LBA (mean (SD) 0.24° (1.31°)). For eight patients with stroke (six right stroke, two left stroke), the perceived LBA was rotated from true body orientation in the direction opposite to the lesioned side (range 3–9.5°, mean 5.2°). These eight patients provided similar estimates by tactile manipulation of the rod (without vision). The rotation of perceived LBA was more pronounced for right‐hemisphere strokes. The magnitudes of perceptual rotations correlated with sensory loss, signs of spatial neglect and the degree of postural and gait disability.
Conclusion
This is the first study showing that certain patients with a hemisphere stroke perceive their LBA rotated to the contralesional side. The consequences for perceptuomotor coordination have implications for their postural disorders.
Patients with cortical stroke may experience forms of spatial disorientation that can affect perception of the spatial organisation of the axes and planes of the body. The resulting distortions of the “egocentric frame of reference” may contribute to the impairment of sensorimotor coordination. A particularly important egocentric reference for the organisation of movement and balance is the longitudinal body axis (LBA)1 and colinear sagittal plane.2 Studies on patients with cortical lesions, particularly affecting the parietal lobe, have shown an ipsilesional shift in their perception of the sagittal plane.3,4,5,6,7,8 When attempting to point “straight ahead”, their indication deviates to the side of the lesion. This ipsilesional deviation has been interpreted as a result of a rotation of the egocentric frame of reference9,10 or as a result of a lateral shift of the egocentric frame of reference.11,12 The phenomena of lateral postural inclination,13 lateral pushing14 and visual vertical tilt15,16,17,18 in the frontal plane exhibited by some patients with stroke affecting the cerebral hemispheres suggest that cortical lesions may also cause patients to perceive that the LBA tilted from its true orientation. This is the hypothesis of this study. If it were true, this phenomenon would have clear consequences for balance, as tilted LBA and sagittal plane imply an unequal distribution of body mass about the true vertical.
Perception of orientation of the sagittal plane is constructed from multisensory components, including visual, auditory, somatosensory and vestibular inputs, and also from information relating eye, head and trunk positions.19 However, the somatosensory basis of the sagittal plane alone has been shown to be sufficient and robust, as the supine observer is able to indicate his or her LBA and sagittal plane accurately in the absence of vision, even when otolithic and other graviceptive cues are minimised.20,21,22,23,24
The main objective of this study to assess the LBA in isolation from gravitoinertial and otolithic cues has not yet been investigated in patients with stroke. Our second aim was to relate any rotation of LBA perception to clinical deficits and the site of the lesion, with the idea that tilted perceived LBA, if present, could be associated with clinical deficits (such as somatosensory loss or spatial neglect) or lesion location (such as the parietal cortex) involved in disorders of body‐sheme construction.25 Our third aim was to analyse the relationship between potential rotation of LBA perception and postural disability.
Methods
We were interested in the possibility of a rotation in perception of the LBA after stroke. The clinical relevance of such a rotation could be shown by the fact that it should occur with a marked frequency, as with other features of spatial neglect shown by the following power calculation. It has been reported that 37% (ie, approximately one third 26) of patients with hemisphere stroke experience lateral deviation in perception of the sagittal plane. As perception of the LBA is a key element in constructing the orientation of the sagittal plane, we hypothesised that misperception of the orientation of the LBA could occur in patients with a similar frequency. With this expected frequency of occurrence in patients, the number of patients required to establish a significant rotation in the perception of the midline in comparison with controls would be 18 (per group) at p<0.05, 80% power t test (in fact, in this target group of 18 patients, we found abnormal perception of the long axis in 8 (44%) patients).
Accordingly, 26 controls (14 men and 12 women, mean (standard deviation (SD)) age 54.3 (6) years) and 18 consecutive patients with stroke (13 men and 5 women, mean (SD) age 58.9 (14) years) gave informed consent to the study in accordance with the guidelines of the local ethics committee. Patients with neuropathy, psychiatric disorders or problems of comprehension due to aphasia or dementia were excluded, as were those whose statuses were unstable or whose stroke encroached on both hemispheres. Apraxia was not a criterion of exclusion. All patients with stroke had been undergoing rehabilitation. Patients with stroke and controls did not differ in either age (t test, p = 0.148) or sex (Fisher's exact probability p = 0.18). The mean time interval since onset of stroke was 80 (SD 49) days. All controls were right handed on the basis of the Edinburgh Inventory.27 All but two patients with stroke were right handed (P12 and P17).
All patients had had a single‐hemispheric stroke in the territory of the middle cerebral artery. Ten had a right‐hemisphere stroke and 8 a left‐hemisphere stroke, 11 an ischaemic stroke and 7 a haemorrhagic stroke. To avoid an overestimation of lesion size in haemorrhagic strokes, lesion location and size were analysed using magnetic resonance imaging or computed tomography scans carried out about 2 months after onset of stroke. Areas of abnormal signal were drawn on eight horizontal brain sections given by the Talairach and Tournoux atlas,28 by VC, who was blinded to the LBA results. For each patient with stroke, the lesion location was determined using landmarks given by the atlas.28 To analyse the possible concordance between lesion location and rotation in LBA perception, a schematic view of stroke areas of patients with abnormal LBA perception was constructed by superimposing their brain sections. Images of left strokes were reversed to be combined with those of right strokes, and the superimposed images of the following brain structures were analysed on the basis of landmarks given by the Talairach and Tournoux atlas28: thalamus, internal capsule, striatum, corona radiata, frontal cortex, the Rolandic's cortex, parietal cortex, temporal cortex and the occipital cortex. Lesion size was estimated by counting the number of structures encroached on by the lesion.29 According to the median value of these scores, patients with stroke were classified as having a small lesion (score <4) or a large lesion (score ⩾4).
Clinical features
Clinical examination included collection of data on motor weakness, spasticity, sensory loss, visual field defect, spatial neglect, and postural and walking abilities. Table 1 shows the clinical features of patients with stroke.
Table 1 Clinical characteristics of patients with stroke.
| Patient | Sex/age (years) | Aetiology | Lesion side | Lesion location | Bells test (omissions) | Line bisection (mm) | Behavioural neglect | Hypoaesthesia (pressure sensitivity) | Spasticity | Weakness | Visual field defect | SCP | PASS | Gait independence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P 1 | M/60 | I | Rt | F/R/CR/P | 26 | 5 | 7 | 4.2 | 2.5 | 21.5 | No | 3 | 26 | 2 |
| P 2 | M/72 | I | Rt | F/R/CR/S | 9 | 17.5 | 11 | 4.46 | 0 | 11 | Yes | 4.5 | 17 | 1 |
| P 3 | M/75 | I | Rt | F/R/CR/P | 0 | 7.5 | 6 | 4.40 | 8.5 | 4 | No | 1.5 | 29 | 2 |
| P 4 | F/65 | I | Rt | F/CR/P | 19 | 58 | 10 | 4.82 | 0 | 10 | No | 3.25 | 6 | 0 |
| P 5 | M/68 | I | Lt | CR/S/IC/Th | 9 | −5 | 1 | 4.08 | 6 | 8 | No | 2.25 | 19 | 1 |
| P 6 | M/56 | H | Rt | F/R/P/T/CR/S/IC/Th | 3 | 5 | 12.8 | 4.84 | 12.5 | 6.5 | No | 2 | 25 | 1 |
| P 7 | M/67 | I | Rt | S/IC/Th | 17 | 12.5 | 10 | 5.26 | 2.5 | 5.5 | Yes | 5.5 | 9 | 0 |
| P 8 | M/50 | H | Lt | CR/S/IC/Th | 4 | 3 | 2 | 3.46 | 5.5 | 11 | No | 0.75 | 28 | 1 |
| P 9 | F/26 | H | Rt | CR/S/IC | 1 | −0.5 | 1 | 4 | 1 | 35 | No | 0.25 | 35 | 5 |
| P 10 | F/68 | I | Lt | CR/S/IC | 14 | −7.5 | 7 | 2.64 | 2 | 6 | No | 2 | 12 | 0 |
| P 11 | M/57 | H | Rt | IC/Th | 4 | −3.5 | 6.25 | 3.59 | 3 | 11 | No | 0.75 | 29 | 2 |
| P 12 | M/55 | H | Lt | CR/S/IC/Th | 1 | −0.5 | 2 | 3.1 | 1.5 | 14.5 | No | 0.25 | 33 | 5 |
| P 13 | M/72 | I | Lt | CR/IC/Th | 5 | 2.5 | 0 | 4.08 | 0 | 40 | No | 0 | 34 | 5 |
| P 14 | M/52 | I | Lt | F/R/P/T/CR/S/IC | 0 | −4 | 1 | 3.96 | 3 | 25.5 | No | 0 | 35 | 5 |
| P 15 | M/27 | I | Lt | CR/S /IC/Th | 0 | 1 | 0 | 2.83 | 1 | 24 | No | 1 | 34 | 4 |
| P 16 | F/61 | I | Lt | T/O/IC | 1 | −7 | 0 | 2.79 | 7.5 | 26.5 | Yes | 0 | 34 | 4 |
| P 17 | M/57 | H | Rt | CR/S /Th | 1 | −0.5 | 0 | 3.25 | 0 | 15 | No | 2 | 27 | 2 |
| P 18 | F/73 | H | Rt | CR/S/IC | 15 | 0 | 3 | 3.96 | 0.5 | 26.5 | No | 1 | 31 | 5 |
CR, corona radiata; F, frontal cortex; H, haematoma; I, ischaemia; IC, internal capsule; Lt, left; N: normal; O, occipital cortex; P, parietal cortex; PASS, Postural Assessment Scale for Stroke; R, Rolandic cortex; Rt, right; S, striatum; SCP, Scale for Contraversive Pushing; T, temporal cortex; Th, thalamus.
Motor weakness was assessed by a standardised examination of muscle strength adapted for patients with central neurological disorders.30 The final weakness score was then adjusted to range from 0 to 40 (normal strength). Spasticity was assessed using the Ashworth Scale.31 Ten muscle groups were tested and the final score was adjusted to range from 0 to 40 (extremely severe and diffused spasticity). As has been proposed previously,13,32 the somatosensory threshold was assessed by an investigation of pressure sensitivity using a Semmes–Weinstein aesthesiometer.33 Pressure sensitivity was tested both at the pulp of the big toe and in the area of the second metacarpophalangeal joint. We used a set of 20 calibrated nylon filaments equal in length but varying in diameter, each implanted at one end of a plastic rod. The force applied to the skin, needed for a given patient to perceive the stimulus, was subjected to log transformation to obtain a 20‐point linear scale (values increasing with the hypoaesthesia).29 Values obtained in the upper and lower limbs were then averaged. The visual field was tested by Goldman perimetry for hemianopia or quadrantanopia. Spatial neglect was assessed using the Bells test34, the ability to bisect two 200‐mm length lines,35 and a behavioural scale.26 A sign transformation was carried out on line bisections so that, by convention, a positive value indicated an ipsilesional deviation. The behavioural scale consisted of 10 four‐point scale items related to neglect in everyday life. The total score ranged from 0 to 30 (severe neglect).
Postural abilities were assessed using the Postural Assessment Scale for Stroke ranging from 0 to 36 (good postural control),32 which is the best ordinal scale for quantifying postural disorders within the 3 months after onset of stroke.36 Some patients with a hemisphere lesion display contralesional lateropulsion with or without resistance to passive correction (pushing). Lateropulsion and pushing were assessed using the Scale for Contraversive Pushing37 ranging from 0 to 6 (severe lateropulsion and pushing). Pushing behaviour was diagnosed according to Karnath's criteria.37 Two patients with stroke (P2 and P7) were diagnosed as showing pushing at the time of the experiment. Gait independence was assessed using the Lindmark and Hamrin Motor Assessment Scale38 ranging from 0 to 6 (independent gait).
Procedure
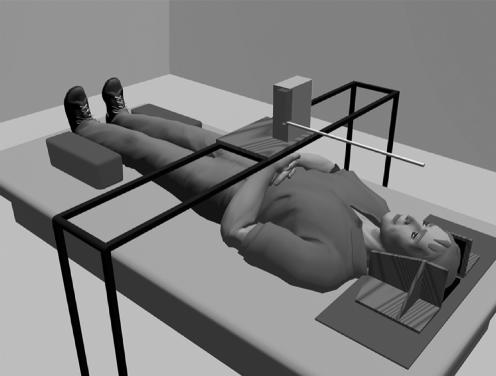
For LBA measurements, subjects lay in supine position in complete darkness, with head, trunk and legs aligned by the experimenter and laterally restrained by pressure pads. A motorised luminous rod (50 cm in length and 1 cm in diameter) that rotated in the horizontal plane (subject's frontal plane) about one end was mounted at approximately 27 cm above the subject with its centre of rotation aligned with the subject's umbilicus (fig 1). The subjects performed 10 adjustments. To avoid possible cuing between trials, no feedback was given to the subjects, who were required to close their eyes. Owing to the possibility of motor impersistence, the faces of patients with stroke were also covered to make sure that they did not see the light between trials. Before starting the experiment, each subject was familiarised with the task by two training trials. There was no time limit for the experiment. The initial position of the rod was set randomly between |0|° and |30|° of the LBA as frequently to the left as to the right. The subject orally indicated how the luminous rod should be adjusted to align with their LBA. Instructions specified that the LBA was an imaginary straight line passing through the midpoints between eyes and shoulders to the midpoint between the feet. The error (in degrees) between the objective LBA and the subject's adjustment was measured by a potentiometer with a spatial resolution of 0.1°. For each subject, we considered the orientation direction as the algebraic mean of 10 repetitions and SD as the within‐subject SD of these repeated measurements. In controls, rotation towards the left shoulder was assigned a negative value and rotation to the right was positive. In patients with stroke, the sign of the orientation of body axis perception was transformed with respect to the lesion's side, a positive value indicating an ipsilesional rotation. Given the novelty of LBA measurements in a clinical perspective, a test–retest procedure was carried out for 10 patients tested on two occasions 1 day apart. The intrarater reliability was high, as attested by the Spearman's r = 0.91 (p<0.001).
Figure 1 Experimental set‐up.
The range of normality of LBA orientation in controls was calculated as the mean (2 SD) for both orientation and SD of the LBA estimations. Ranges of normality were −2.86° to 2.38° for the orientation and 0.41° to 2.48° for SD.
Patients with stroke who showed an obvious rotation in the visual determination of their subjective LBA were also required to manually adjust the rod with eyes closed to the represented direction of their LBA. This haptic determination of the LBA provided indirect indications that rotations of the luminous rod were not due to eye position. Indeed, Bronstein et al39 showed that after ocular torsion, only the visual vertical was biased, whereas the haptic vertical was accurately perceived.
Statistical analysis
The LBA estimations were normally distributed for patients with stroke and for controls as the SDs after log transformation. Therefore, groups were compared using parametric statistics: t tests. A two‐way analysis of variance was used to test the influence of lesion size (small v large) and side (left v right) on LBA perception. When required, groups (of unequal variances) were compared using Welch's t test.40 Data are given as mean (SD). The magnitude of each observed effect was estimated by “calibrated deviation”, as recommended by Corroyer and Rouanet.41 Three magnitudes were considered: small (0–0.50), medium (0.50–1) and large (>1). To account for the influence of lesion size as a possible confounding factor, the relationships between clinical deficits and perceived LBA perception were tested using partial Spearman's non‐parametric correlations, with lesion size as a covariable.
Results
Estimation of the LBA in controls and patients with stroke
Body axis orientation
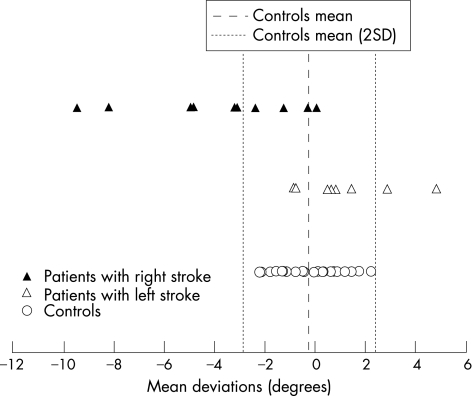
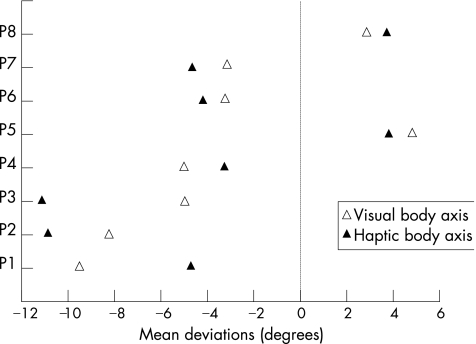
The mean LBA perception of patients with stroke (after sign transformation, −2.62° (SD 2.93°)) was different (Welch's t test, p = 0.004) from that of controls (−0.24° (SD 1.31°)), and this was a large‐magnitude effect (calibrated deviation = 1; fig 2). Of the 18 patients with stroke, 8 presented abnormal contralesional rotation of their subjective LBA (the rotation ranged from 3° to 9.5°; mean = 5.2°). None of the eight subjects with LBA rotation was left handed. In addition to the visual determination of the LBA, these eight patients with stroke were also required to perform a haptic (tactile) subjective LBA (fig 3). Visual and haptic adjustments for these eight patients with stroke were strongly correlated (r = 0.81; p = 0.014), indicating that visual rotations of the subjective LBA probably did not result from ocular torsion.
Figure 2 Mean direction of the body axis perception for 26 controls and 18 patients with hemisphere stroke (before sign transformation). Negative values correspond to a rotation in direction of the subjects' left shoulder. The amplitude of rotation is scaled on the x axis.
Figure 3 Mean orientation of visual and haptic determinations of the body axis for patients with stroke showing an abnormal visual body axis perception (the identification of patients with stroke is similar to that given in table 1).
SDs of body axis perception
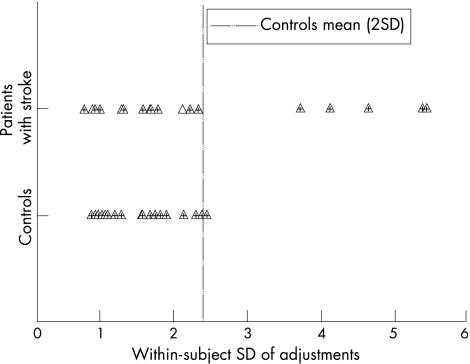
Patients with stroke showed higher SDs than controls (mean 2.36° (SD 1.44°) v 1.56° (SD 0.51°); Welch's t test, p = 0.017; fig 4), and this was a medium‐magnitude effect (calibrated deviation = 0.94). Of the 18 patients with stroke, 5 presented abnormally large SDs (+2 SDs from the mean of controls). The magnitudes of SDs and orientation error were uncorrelated (r = 0.30; p = 0.21), and four of the five patients with stroke who had abnormal SDs also had abnormal orientation estimates.
Figure 4 Standard deviations (SD) in the body axis perception. SD is scaled on the x axis.
Relationship between lesion size and location and LBA perception
A two‐way analysis of variance was carried out to test the influence of lesion size (small v large) and side (left v right) on LBA perception. Rotation in LBA perception was greater in patients with right‐hemisphere stroke than in those with left‐hemisphere stroke (F1,14 = 8.19; p = 0.01; η2 = 0.37, power (α = 0.5) = 0.76), and greater in patients with a large lesion than in those with a small one (F1,14 = 5.18; p = 0.04; η2 = 0.27, power (α = 0.5) = 0.56). The interaction between these two factors was not significant (F1,14 = 3.56; p = 0.08; η2 = 0.23, power (α = 0.5) = 0.42). Among the eight patients with stroke showing abnormal LBA contralesional rotation, six had right‐hemisphere stroke and two had left‐hemisphere stroke. In these patients, the lesion was mainly centred around the Rolandic cortex (frontoparietal cortex), the adjacent corona radiata and the striatum.
Relationship between clinical deficits and LBA perception
The rotation in LBA perception was found to correlate with sensory loss (r = −0.67; p = 0.003), severity of spatial neglect assessed by the number of omissions in the Bells test (r = −0.56; p = 0.018), the ipsilesional rotation in line bisection (r = −0.50; p = 0.004) and the Behavioural Neglect Scale (r = −0.61; p = 0.009).
By contrast, no significant relationship was found between the rotation of LBA perception and motor weakness (r = 0.34; p = 0.18), spasticity (r = −0.11; p = 0.69) or visual field defect (Fisher's exact probability p = 0.41). These analyses clearly showed that rotation of the perceived LBA was strongly associated with contralesional hypoaesthesia and spatial neglect, whereas motor weakness, spasticity and the existence of a visual field defect had no influence on LBA perception.
Relationship between LBA perception and postural disorders
The greater the rotation of the perceived LBA orientation, the more severe the postural (r = 0.49; p = 0.04) and gait (r = 0.57; p = 0.02) disabilities, and the more pronounced the lateropulsion and pushing (r = −0.66; p = 0.004). Moreover, the more uncertain the LBA perception, the higher the Scale for Contraversive Pushing (r = 0.73; p = 0.001), whereas no relationship was found between SDs of LBA perception and gait independency (r = −0.44; p = 0.075).
Discussion
This study shows that: (1) patients with a hemisphere stroke may perceive their LBA substantially (a marked magnitude effect) rotated towards the contralesional side in comparison with controls; (2) measurements of LBA perception are reproducible (low within‐subject SD and repeat‐measurement stability), which confirms that perception of the LBA is a valid concept20,21,22,24; (3) magnitudes of perceptual rotations correlate with sensory loss, spatial neglect signs, and postural and gait disabilities; and (4) the rotation of perceived LBA is more pronounced in patients with a right‐hemisphere stroke.
Mechanisms
The relatively small sample size leads us to be cautious about the correlates between the rotation in LBA rotation and the clinical characteristics of patients with stroke. However, this study gives a first indication about the associations between rotation in LBA perception and spatial neglect as well as hypoaesthesia. The absence of any correlation between LBA rotation and motor weakness or spasticity is compatible with a sensory and cognitive interpretation of LBA rotation. These findings are congruent with the nature of the task involving body perception and representation. The perception of body geometry in an immobile, supine subject, in darkness, mainly uses touch‐pressure and joint‐position senses. We can assume that normal LBA perception requires right–left‐sided symmetrical somaesthetic information, which is compromised in some patients with stroke with spatial neglect and hypoaesthesia, and this may explain the strong correlation we found between LBA rotation, spatial neglect and hypoaesthesia. To align the rod with the LBA, subjects must construct a longitudinal axis aligned to his or her body perception. Spatial neglect is also known to be associated with changes in body representation, which may participate in the strong association between LBA rotation and spatial neglect. We showed that lesions in subjects who perceived their LBA rotated were centred around the Rolandic cortex (partly corresponding to the superior parietal cortex) and around the striatum, two structures known to code for spatial information related to the body.25
Postural disorder and LBA perception
Strong associations were also found between rotations in LBA perception and postural and gait disorders. Although contralesional lateropulsion severity was strongly related to the rotation magnitude of the perceived LBA, it was clear for two reasons that rotation of the LBA alone was not directly responsible for the lateropulsion and vice versa. Indeed, the lateropulsion sometimes observed in hemisphere strokes in clinical practice consists of contraversive pushing, but the contralesional rotation of the subjective LBA observed in this study should theoretically lead to ipsiversive lateropulsion. Patients with rotated LBA could perceive their LBA rotated to the contralesional side when they are upright, and correct this erroneous perception by ipsiversive lateropulsions. Moreover, even though the two pushers tested in our study had abnormal rotation of their subjective LBA, the rotation was comparable in magnitude to that of patients with stroke who showed contralesional rotation of their subjective LBA. Therefore, the rotated subjective LBA cannot be the cause of the contralesional lateropulsion or of the pushing. Postural disorders are primary disabilities in patients with stroke and are caused by complex mechanisms. In addition to misorientation with respect to gravity (lateropulsion, pushing), postural disability after stroke is also due to distortions in various coordinating systems, motor weakness, sensory deficits, impaired postural coordination and stabilisation.39 Even though the rotation in the perceived LBA cannot explain lateropulsion or pushing, which are interpreted as a misorientation with respect to gravity,37,42 tilt rotation of the LBA probably has dramatic postural consequences in standing patients with stroke.
LBA and visual vertical
Notably, contralesional rotations of LBA perception found in our study were congruent with the literature on verticality perception after hemispheric stroke. Indeed, the perception of vertical (haptic, visual or postural) has also been proved to be contralesionally rotated,15,16,17,18 principally in patients with spatial neglect. The contralesional rotation of the LBA perception and vertical estimation observed in patients with stroke are congruent but should not be regarded as similar phenomena, because of LBA is independent the vertical in our study (subjects are supine). Our pilot study emphasises the need to investigate both subjective LBA and the subjective vertical in patients with hemisphere stroke.
Study limitations
Our findings were probably not induced by a methodological bias. The paradigm requires calibration of the true LBA, which is operator dependent and thus remains relatively subjective. To minimise this possible source of error, the calibration process was always carefully carried out by two operators (JB and VC or JB and DP). Perception of line orientation may also be due to ocular torsion and deviations of the eye from the primary position (during exploration), termed “pseudo‐torsion”.43,44 Although ocular torsion may occur spontaneously after hemisphere lesions in a small number of patients,15 our results probably could not be induced by ocular torsion as the LBA was tilted for both visual and haptic modalities, implying a misperception at a high level of sensory integration.15,39 The slight ocular torsion (pseudo‐torsion) induced by visual exploration is insufficient to account for the magnitudes of LBA rotation observed in our patients . In any case, if relevant to the task at hand, this effect should also have been found in controls.
Owing to the relatively small sample size and the pilot nature of the study, we cannot draw any conclusions about the prevalence of the LBA rotation after stroke. Studies on a larger sample size based on quantitative analysis of lesion location are needed to investigate further the prevalence of LBA misperception after stroke, as well as its anatomical correlates.
Finally, this study is the first step towards the understanding the striking the behaviour sometimes observed in patients with acute or subacute stroke when lying in bed. The sound leg actively pushes the paretic leg, making the LBA diagonally oriented with respect to the bed. We speculate that this may be a way of aligning a tilted representation of the LBA to the bed direction.
Acknowledgements
We thank John Golding for his contribution to the statistical analysis and Anne‐Sophie Gissot for her help.
Abbreviations
LBA - longitudinal body axis
Footnotes
Funding: This work was partly supported by “la foundation de l'avenir”.
Competing interests: None.
References
- 1.Howard I P, Hu G. Visually induced reorientation illusions. Perception 200130583–600. [DOI] [PubMed] [Google Scholar]
- 2.Jeannerod M.The neural and behaviour organisation of goal‐directed movement. Oxford: Oxford University Press, 1988
- 3.Chokron S, Imbert M. Variations of the egocentric reference among normal subjects and a patient with unilateral neglect. Neuropsychologia 199533703–711. [DOI] [PubMed] [Google Scholar]
- 4.Karnath H O, Schenkel P, Fischer B. Trunk orientation as the determining factor of the ‘contralateral' deficit in the neglect syndrome and as the physical anchor of the internal representation of body orientation in space. Brain 19911141997–2014. [DOI] [PubMed] [Google Scholar]
- 5.Richard C, Honore J, Bernati T.et al Straight‐ahead pointing correlates with long‐line bisection in neglect patients. Cortex 20044075–83. [DOI] [PubMed] [Google Scholar]
- 6.Heilman K M, Bowers D, Watson R T. Performance on hemispatial pointing task by patients with neglect syndrome. Neurology 198333661–664. [DOI] [PubMed] [Google Scholar]
- 7.Ferber S, Karnath H O. Parietal and occipital lobe contributions to perception of straight ahead orientation. J Neurol Neurosurg Psychiatry 199967572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perenin M T, Karnath H O. In: ed. Optic ataxia and unilateral neglect: clinical evidence for dissociation spatial functions in posterior parietal cortex. Heidelberg: Springer‐Verlag, 1997
- 9.Karnath H ‐ O, Ferber S. Is space representation distorted in neglect? Neuropsychologia 1999377–15. [DOI] [PubMed] [Google Scholar]
- 10.Jeannerod M, Biguer B. Référence égocentrique et espace représenté. Rev Neurol (Paris) 1989145635–639. [PubMed] [Google Scholar]
- 11.Vallar G, Guariglia C, Nico D.et al Spatial hemineglect in back space. Brain 1995118467–472. [DOI] [PubMed] [Google Scholar]
- 12.Richard C, Rousseaux M, Saj M.et al Straight ahead in spatial neglect. Evidence that space is shifted, not rotated. Neurology 2004632136–2138. [DOI] [PubMed] [Google Scholar]
- 13.Pérennou D A, Amblard B, Leblond C.et al Biased postural vertical in humans with hemispheric cerebral lesions. Neurosci Lett 199825275–78. [DOI] [PubMed] [Google Scholar]
- 14.Karnath H O, Ferber S, Dichgans J. The origin of contraversive pushing: evidence for a second graviceptive system in humans. Neurology 2000551298–1304. [DOI] [PubMed] [Google Scholar]
- 15.Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol 199435403–412. [DOI] [PubMed] [Google Scholar]
- 16.Kerkhoff G, Zoelch C. Disorders of visuospatial orientation in the frontal plane in patients with visual neglect following right or left parietal lesions. Exp Brain Res 1998122108–120. [DOI] [PubMed] [Google Scholar]
- 17.Saj A, Honore J, Bernati T.et al Subjective visual vertical in pitch and roll in right hemispheric stroke. Stroke 200536588–591. [DOI] [PubMed] [Google Scholar]
- 18.Yelnik A P, Lebreton F O, Bonan I V.et al Perception of verticality after recent cerebral hemispheric stroke. Stroke 2002332247–2253. [DOI] [PubMed] [Google Scholar]
- 19.Andersen R A. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc London B Biol Sci 19973521421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Templeton W B. The role of gravitational cues in the judgement of visual orientation. Percept Psychophys 197314451–457. [Google Scholar]
- 21.Goodenough D R, Oltman P K, Sigman E.et al The rod‐and‐frame illusion in erect and supine observers. Percept Psychophys 198129365–370. [DOI] [PubMed] [Google Scholar]
- 22.Parker D E, Poston R L, Gulledge L. Spatial orientation: visual‐vestibular‐somatic interaction. Percept Psychophys 198333138–146. [DOI] [PubMed] [Google Scholar]
- 23.Pizzamiglio L, Vallar G, Doricchi F. Gravitational inputs modulate visuospatial neglect. Exp Brain Res 1997117341–345. [DOI] [PubMed] [Google Scholar]
- 24.Spidalieri G, Sgolastra R. Psychophysical properties of the trunk midline. J Neurophysiol 199778545–549. [DOI] [PubMed] [Google Scholar]
- 25. In: Thier P, Karnath H O. eds. Parietal lobe contributions to orientation in 3D space. Heidelberg: Springer, 1997
- 26.Azouvi P, Marchal F, Samuel C.et al Functional consequences and awareness of unilateral neglect: study of an evaluation scale. Neuropsychol Rehabil 19966133–150. [Google Scholar]
- 27.Oldfield R C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971997–113. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P.Co‐planar stereotaxic atlas of the human brain 3‐dimensional proportional system: an approach to cerebral imaging. Stuttgart: Thieme, 1988
- 29.Pérennou D A, Leblond C, Amblard B.et al The polymodal sensory cortex is crucial for controlling lateral postural stability: evidence from stroke patients. Brain Res Bull 200053359–365. [DOI] [PubMed] [Google Scholar]
- 30.Held J, Pierrot‐Desselligny E, Bussel B.et al Devenir des hémiplégies vasculaires par atteinte sylvienne en fonction du côté de la lésion. Ann Réadapt Méd Phys 197518592–604. [Google Scholar]
- 31.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964192540–542. [PubMed] [Google Scholar]
- 32.Benaim C, Pérennou D A, Villy J.et al Validation of a standardized assessment of postural control in stroke patients: the postural assessment scale for stroke patients (PASS). Stroke 1999301862–1868. [DOI] [PubMed] [Google Scholar]
- 33.Semmes J, Weinstein S, Ghent L.et alSomatosensory changes after penetrating brain wounds in man. Cambridge: Harvard University, 1960
- 34.Gauthier L, Dehaut F, Joanette Y. The Bells test for visual neglect: a quantitative and qualitative test for visual neglect. Int J Neuropsychol 19891149–54. [Google Scholar]
- 35.Harvey M, Milner A D, Roberts R C. Differential effects of line length on bisection judgements in hemispatial neglect. Cortex 199531711–722. [DOI] [PubMed] [Google Scholar]
- 36.Mao H F, Hsueh I P, Tang P F.et al Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke 2002331022–1027. [DOI] [PubMed] [Google Scholar]
- 37.Karnath H O, Ferber S, Dichgans J. The origin of contraversive pushing: evidence for a second graviceptive system in humans. Neurology 2000551298–1304. [DOI] [PubMed] [Google Scholar]
- 38.Lindmark B, Hamrin E. Evaluation of functional capacity after stroke as a basis for intervention: presentation of a modified chart for motor capacity assessment and its reliability. Scand J Rehabil Med 198820103–109. [PubMed] [Google Scholar]
- 39.Bronstein A M, Pérennou D A, Guerraz M.et al Dissociation of visual and haptic vertical in two patients with vestibular nuclear lesions. Neurology 2003611260–1262. [DOI] [PubMed] [Google Scholar]
- 40.Welch B. The generalization of student's problem when several different population variances are involved. Biometrika 19473428–35. [DOI] [PubMed] [Google Scholar]
- 41.Corroyer D, Rouanet H. Sur l'importance des effets et ses indicateurs dans l'analyse statistique des données. L'Année Psychol 199497607–624. [Google Scholar]
- 42.Pérennou D A, Bronstein A M. Balance disorders and vertigo after stroke: assessment and rehabilitation. In: Bogousslavsky J, Barnes M, Dobkin B, eds. Recovery after stroke. Cambridge: Cambridge University Press, 2005320–398.
- 43.Ygge J, Zee D S. Control of vertical eye alignment in three‐dimensional space. Vision Res 1995353169–3181. [DOI] [PubMed] [Google Scholar]
- 44.Straumann D, Zee D S, Solomon D.et al Validity of Listing's law during fixations, saccades, smooth pursuit eye movements, and blinks. Exp Brain Res 1996112135–146. [DOI] [PubMed] [Google Scholar]