Diffusion‐weighted imaging (DWI) may detect hyperintense lesions in patients with transient hypoglycaemia‐induced hemiparesis or coma, which are completely reversible after glucose infusion.1,2,3 In vivo animal studies have documented the visualisation of such hypoglycaemia‐induced changes of signal intensity and the reversal by glucose intake in detail.4 However, the time necessary for hyperintense lesions on DWI to disappear after glucose infusion in humans is still unclear. We treated a patient in a hypoglycaemic coma with DWI abnormalities in the splenium of the corpus callosum and the bilateral posterior limbs of the internal capsules, which were drastically improved 2 h after glucose infusion.
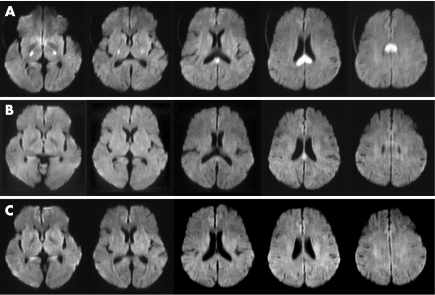
A 54‐year‐old woman was taken to an emergency department after being found in an unresponsive state. The patient had had type 2 diabetes mellitus, hypertension and hyperlipidaemia for 7 years, controlled by oral drugs. On arrival, she was comatose (Glasgow Coma Scale 4; E1, V1, M2). Her pupils exhibited isocoria (2 mm/2 mm) and were reactive to light. Tetraparesis and decerebrate rigidity to pain was evident. Her blood pressure was 91/40 mm Hg and her heart rate was 88 beats/min. She had spontaneous respiration and a normal temperature. Magnetic resonance imaging (MRI) and magnetic resonance angiography were carried out immediately, as we considered the possibility of brain stem infarction. DWI showed hyperintense lesions in the splenium of the corpus callosum and the bilateral posterior limbs of the internal capsules (fig 1A). T2‐weighted and fluid‐attenuated inversion recovery sequences showed no abnormal lesions, and magnetic resonance angiography showed no major abnormalities, including basilar artery occlusion. Laboratory examinations showed no metabolic abnormalities except for severe hypoglycaemia with a blood glucose concentration of 1.8 mmol/l. The patient was immediately given 40 ml of 50% glucose infusion; she recovered consciousness shortly afterwards. The abnormal hyperintense lesions had almost disappeared on DWI obtained 2 h after glucose infusion (fig 1B), and had completely disappeared 2 days later (fig 1C). Blood glucose concentrations after glucose infusion and 2 days later were 13.9 and 6.5 mmol/l, respectively. The final cognitive function was completely normal in her daily life. The patient's hypoglycaemia was due to low dietary intake and irregular medication in the 4 days prior to admission. The duration of hypoglycaemia was not apparent.
Figure 1 (A) Diffusion‐weighted imaging (DWI) on admission showing hyperintense lesions in the splenium of the corpus callosum and the bilateral posterior limbs of the internal capsules. (B) DWI obtained 2 h after glucose infusion showing almost full recovery except for a small part of the splenium of the corpus callosum. (C) DWI obtained 2 days after glucose infusion showing complete regression of the hyperintense lesions.
Follow‐up MRI has been carried out between 8 h and 10 days in previous cases,1,2,3 hence the duration of DWI abnormalities cannot be exactly known. But in reality, the hyperintense lesions on DWI had almost disappeared in only 2 h in our patient. The in vivo animal study of hypoglycaemia found complete apparent diffusion coefficient (ADC) normalisation except in the periventricular regions by 10 min after glucose infusion.4 Glucose deprivation is widely assumed to lead to severe brain energy failure, reduction of cell membrane ionic pump activity and consequent shift of cerebral water from the extracellular space to the intracellular space. The fast recovery of the clinical symptoms and normalisation of imaging abnormalities presumably resulted from the immediate intravenous glucose supplementation. Although, in our patient, follow‐up MRI was carried out in 2 h after glucose infusion and rapid improvement in DWI abnormalities was detected by chance, there is a possibility that the actual duration of DWI abnormalities in previous case reports also might be short, as in our patient.
In previously reported cases, several marked MRI findings were shown. DWI and ADC were more sensitive than fluid‐attenuated inversion recovery imaging to detect the abnormal lesions.2,3 The initial ADC values were moderately decreased, but were fully reversible.2,3 Perfusion‐weighted MRI showed no perfusion deficit2 or a slight increase in relative cerebral blood volume restricted to the lesion seen on DWI.3 Magnetic resonance angiography detected no haemodynamically relevant stenosis or vasospasm.1,2,3 DWI showed changes localised in the splenium,2 the internal capsule1,3 and the corona radiata.1,2 In our patient, the lesions were located in the splenium and the internal capsule.
Typical lesions in more severely affected patients had different localisations including the basal ganglia, the pons, the temporal and occipital cortices, and the hippocampus. Magnetic resonance signal changes in the splenium have been found in various pathological conditions such as alcohol use, infections, hypoglycaemia, trauma, salt abnormalities and seizure.5 DWI often showed other areas of involvement, particularly the posterior limb of the internal capsule.5 T2‐relaxation MRI studies of healthy patients showed heterogeneous water content in the splenium and the posterior limbs of the internal capsule, but tissue myelin water content was relatively higher.6 Effects associated with the splenium and the posterior limb of the internal capsule injury as described above can compromise cellular fluid regulation.5 Our findings may support the hypothesis that the splenium and the posterior limb of the internal capsule can more easily affect cellular fluid mechanics compared with the surrounding tissue in some pathological conditions.5
In conclusion, abnormal hyperintense lesions on DWI in hypoglycaemic coma may disappear rapidly after glucose infusion.
Footnotes
Competing interests: None.
Informed consent was obtained for publication of the patient's details described in this report.
References
- 1.Aoki T, Sato T, Hasegawa K.et al Reversible hyperintensity lesion on diffusion‐weighted MRI in hypoglycemic coma. Neurology 200463392–393. [DOI] [PubMed] [Google Scholar]
- 2.Bottcher J, Kunze A, Kurrat C.et al Localized reversible reduction of apparent diffusion coefficient in transient hypoglycemia‐induced hemiparesis. Stroke 200536e20–e22. [DOI] [PubMed] [Google Scholar]
- 3.Cordonnier C, Oppenheim C, Lamy C.et al Serial diffusion and perfusion‐weighted MR in transient hypoglycemia. Neurology 200565175. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa Y, Formato J E, Latour L L.et al Severe transient hypoglycemia causes reversible change in the apparent diffusion coefficient of water. Stroke 1996271648–1656. [DOI] [PubMed] [Google Scholar]
- 5.Doherty M J, Jayadev S, Watson N F.et al Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol 200562433–437. [DOI] [PubMed] [Google Scholar]
- 6.Whittall K P, MacKay A L, Graeb D A.et al In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med 19973734–43. [DOI] [PubMed] [Google Scholar]