Abstract
Background
Olfactory dysfunction is common in old age, but its basis is uncertain.
Objective
To test the hypothesis that difficulty in identifying odours in old age is related to the accumulation of Alzheimer's disease pathology.
Methods
As part of the Rush Memory and Aging Project, participants completed the 12‐item Brief Smell Identification Test, a standard measure of odour identification. During a mean (standred deviation (SD)) of 2.2 (1.2) years of follow‐up (range 0.2–4.9), 166 people died, with brain autopsies performed on 129 (77.7%) people and neuropathological examinations completed on 77 (mean (SD) age at death 87.5 (5.9) years; median postmortem interval 6.1 h). From a uniform postmortem examination of multiple brain regions, summary measures of plaque and tangle pathology were derived on the basis of silver staining, and those of amyloid β burden, tangle density and Lewy bodies on the basis of immunohistochemistry.
Results
Odour identification performance ranged from 0 to 12 correct (mean (SD) 8.0 (2.6)). In analyses adjusted for age, sex and education, a composite measure of plaques and tangles accounted for >12% of the variation in odour identification. The association remained after controlling for dementia or semantic memory. Density of τ tangles was inversely related to odour identification. A similar effect for amyloid burden was attenuated after controlling for tangles. The association with odour identification was robust for tangles in the entorhinal cortex and CA1/subiculum area of the hippocampus, but not for tangles in other cortical sites. Lewy bodies, identified in 12.5%, were not related to odour identification, probably partly due to to their relative infrequency.
Conclusion
The results suggest that difficulty in identifying familiar odours in old age is partly due to the accumulation of neurofibrillar pathology in central olfactory regions.
Olfactory dysfunction in old age is common, but its neuropathological bases are not securely understood, partly due to the dearth in clinical–pathological research. We are aware of only two such studies,1,2 both of which were restricted to people with clinical dementia, complicating assessment of olfactory functioning and possibly restricting the range of olfactory functioning and neuropathology. Nonetheless, both studies found olfactory impairment to be associated with the presence of cortical Lewy bodies, which is consistent with evidence that olfactory systems in the brain are early sites of Lewy body pathology in Parkinson's disease,3 an early sign of which is olfactory dysfunction.4,5 Neither study observed an association between olfactory impairment and pathology of Alzheimer's disease.1,2 This is surprising as the central olfactory areas are vulnerable to the accumulation of Alzheimer's disease pathology, particularly neurofibrillar tangles,6,7,8 suggesting a need for further research.
In the present study, we examined the relationship between odour identification and Alzheimer's disease pathology using data from the Rush Memory and Aging Project,9 a clinical–pathological study of common chronic conditions of old age. Odour identification was assessed at baseline with a standard test. Among those who subsequently died and underwent brain autopsy, Alzheimer's disease pathology and Lewy bodies were quantified in multiple brain regions, including portions of the central olfactory system. In analyses, we tested the hypotheses that odour identification is inversely related to the extent of Alzheimer's disease pathology and that this effect is primarily due to the accumulation of neurofibrillar pathology in central olfactory regions.
Methods
Participants
Participants in the study were from the Rush Memory and Aging Project, an ongoing longitudinal study of risk factors for common chronic conditions of old age. The study began in 1997, was expanded in 2001, and involves annual clinical evaluations and brain donation at death. It was approved by the institutional review board of the Rush University Medical Center
People were recruited from retirement centres and senior housing facilities in and around Chicago. After a presentation about the project, people rated their interest in participating, and were given information packets and encouraged to discuss it with family and friends. People expressing interest in participation were subsequently contacted by study personnel, who obtained informed consent. Participants agreed to annual evaluations and signed an anatomic gift act donating their brain to Rush investigators at death. To date, >1000 people have enrolled in the study and completed the baseline evaluation. Eligibility for these analyses required completion of the Brief Smell Identification Test10 at baseline and brain autopsy on death during the follow‐up period. Of 166 people who died to date, 129 (77.7%) underwent brain autopsy; of these, the postmortem neuropathological examination has been completed in 77, with the remaining examinations incomplete at the time of these analyses. This group had a mean (standard deviation (SD)) age of 87.5 (5.9) years at death and a mean (SD) of 14.5 (3.3) years of education; 62.3% were women, and 96.1% were white and non‐Hispanic. The annual clinical evaluations included detailed cognitive testing, a complete neurological examination and classification of dementia according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria,11 as described previously.12,13 Dementia was present in 15 people at the time of odour assessment (and in 23 at the time of death). Two cognitive measures were used in analyses: score on the 15‐item Boston Naming Test, a measure of visual confrontation naming, and a composite measure of semantic memory based on the Boston Naming Test, oral fluency and a reading recognition test, with individual tests converted to z scores, and averaged to yield the composite as described elsewhere.12,13,14
Assessment of odour identification
The Brief Smell Identification Test10 was used to assess the ability to recognise familiar odours. For each of 12 items, a microcapsule containing a familiar odour was scratched with a pencil and placed under the nose of the participant, who tried to match the smell with one of four specific choices. The score is the number of odours correctly recognised, with a score of 0.25 assigned to missing responses to a maximum of two; if more than two responses were missing, the entire test was treated as missing, as described previously.15 Performance on the Brief Smell Identification Test has been shown to correspond to the 40‐item University of Pennsylvania Smell Identification Test16 from which it was derived,10,17 and to be associated with risk of dementia18,19 and cognitive decline,15,20 the principal clinical manifestations of Alzheimer's disease pathology.
Assessment of neuropathology
Participants died a mean of 2.2 (1.2) years (range 0.2–4.9 years) after odour identification assessment. The brain was removed in a standard fashion a median of 6.1 h (interquartile range 4.6 h) after death. It was then cut into 1‐cm‐thick coronal slabs, and the slabs from one hemisphere were fixed in 4% paraformaldehyde for 72 h.
The brain regions studied reflect the focus of the Rush Memory and Aging Project on cognitive and motor functioning and Alzheimer's disease. Tissue from five brain regions (midfrontal gyrus, inferior parietal gyrus, middle temporal gyrus, entorhinal cortex and hippocampus (CA1/subiculum)) was cut into 0.5‐cm‐thick blocks, embedded in paraffin wax, and sectioned at 6 µm and stained with modified Bielschowsky silver. For each of the five brain regions of interest, a neuropathologist or trained technician, blinded to all clinical data, separately counted neuritic plaques, diffuse plaques and neurofibrillar tangles in a 1‐mm2 area using a ×10 objective (with ×10 eyepiece) in the site judged to have the most of a given type of pathology. For each type of pathology in each region, the raw count was divided by the SD of all counts of that pathology in that region to yield a standard score. These standard scores were averaged to produce a composite measure of cortical plaques and tangles and summary measures of each type of pathology, as reported previously.21,22
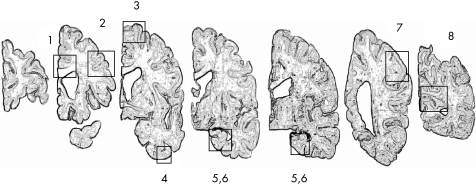
To obtain more systematic and molecularly specific indices of Alzheimer's disease pathology, we used unbiased methods to quantify the percentage area occupied by amyloid‐β‐immunoreactive plaques and the density of paired helical filament (PHF) τ tangles. For each of the following eight brain regions of interest shown in fig 1, fixed tissue was cut into 0.5‐cm‐thick blocks that were embedded in paraffin wax: (1) anterior cingulate cortex (Brodmann area (BA) 24), (2) dorsal lateral prefrontal cortex (BA 46/9), (3) superior frontal cortex (BA 6/8), (4) inferior temporal cortex (BA 20), (5) hippocampus (CA1/subiculum), (6) entorhinal cortex proper (BA 28), (7) angular/supramarginal gyrus (BA 39/40) and (8) primary visual cortex (BA 17). In each of the six neocortical regions, two tissue blocks from adjacent 1‐cm‐thick slabs were embedded in paraffin wax and cut into 20‐µm sections. In the hippocampus, 0.5‐cm tissue blocks were dissected from consecutive 1‐cm‐thick slabs, embedded in paraffin wax, and cut into 20‐µm sections. Up to 24 sections were quantified in each case.
Figure 1 Eight regions of interest dissected from each brain: (1) anterior cingulate cortex (Brodmann area (BA) 24), (2) dorsal lateral prefrontal cortex (BA 46/9), (3) superior frontal cortex (BA 6/8), (4) inferior temporal cortex (BA 20), (5) hippocampus (CA1/subiculum), (6) entorhinal cortex proper (BA 28), (7) angular/supramarginal gyrus (BA 39/40) and (8) primary visual cortex (BA 17).
Amyloid‐β‐ir plaques were labelled with an N terminus directed monoclonal antibody (10D5, courtesy of, Elan Pharmaceuticals, Dublin, Ireland; 1:1000). Immunohistochemistry was carried out as described previously,21,22 using diaminobenzidine as the reporter with 2.5% nickel sulphate to enhance immunoreaction product contrast. An automated immunohistochemical stainer (Biogenex, San Ramon, California, USA) was used to run all sections with identical incubation times in precisely timed runs. PHF tau tangles were labelled with an antibody specific for phosphorylated tau (AT8, Innogenetics, San Ramon, California, USA; 1:1000). Control sections were included in all runs.
A systematic random sampling scheme with a custom algorithm23 was used to capture images of amyloid‐β staining. A region of interest was outlined at low power with StereoInvestigator software V.5 and an Olympus BX‐51 microscope. A grid of predetermined size was then randomly placed by the software programme over the outlined area so that 20–50% of the region was sampled. After camera and illumination calibration, the objective was raised to ×20, and at each sampling site 24‐bit images were obtained with an automatically positioned motorised stage. In each region, the percentage of area occupied by amyloid‐β‐ir pixels was calculated using the aforementioned algorithm,23 and the regional indices were averaged to provide a composite index of amyloid burden, as described in previous research.21,22
Tangle density/mm2 was quantified using a stereological mapping station with StereoInvestigator software V.6 and an Olympus BX‐51 microscope with an attached motorised stage. A given region of interest was delimited at low power, and a grid of predetermined size was placed over it. Then total magnification was raised to ×400, and the programme directed the motorised stage to stop and sample each intersection point on the grid. The operator visualised the fields on the monitor within the superimposed counting frame and counted all objects that did not touch the exclusion lines of the box. About 20–50% of the area within each region of interest was quantified in this way. Tangle density within each region was averaged to yield a composite index of tangle density, as reported previously.21,22
Lewy bodies were identified with antibodies to α‐synuclein, a specific immunohistochemical stain for Lewy bodies. Immunohistochemistry for α‐synuclein was carried out on 20‐μm sections of paraformaldehyde fixed tissue, and after pretreatment with 90% formic acid, with control slides included in each run, as described previously.24 All sections from every case were evaluated by light microscopy for intraneuronal Lewy bodies. In analyses, we treated Lewy bodies as a dichotomous variable as they were present in <15% of the group.
Data analysis
The hypothesised association of Alzheimer's disease pathology with odour identification was assessed in a series of linear regression models, each of which controlled for the potentially confounding effects of age, sex, education and time from smell assessment to death. Odour identification performance was initially regressed on the composite index of plaque and tangle pathology. This model was repeated with a term added for dementia at the time of smell assessment, with dementia cases excluded, controlling for naming or semantic memory at smell assessment, and then with pathological subscores (eg, neurtic plaques) substituted for the composite plaque and tangle index. Next, odour identification score was regressed on composite measures of amyloid deposition and tangle density, firstly in separate models, secondly simultaneously, and finally on measures of tangle density in different brain regions. A final pair of analyses examined whether the presence of Lewy bodies was related to odour identification or modified the effect of neurofibrillar pathology on odour identification. Models were graphically and analytically validated. Programming was done in SAS.
Results
Odour identification scores on the 12‐item test ranged from 0 to 12 correct (8.0 (2.6), skewness −0.9, interquartile range 3.7). Odour identification was inversely related to age (r = −0.33, p = 0.003) but unrelated to sex (t (75) = 0.05, p = 0.957) or education (r = −0.17, p = 0.132), consistent with correlations previously reported in the full cohort. 15
Odour identification and cortical plaques and tangles
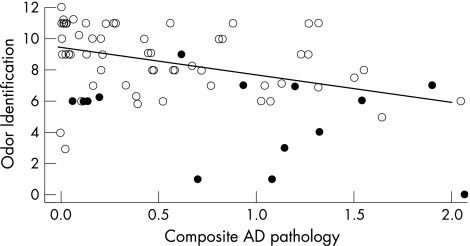
We began analyses with a global index of Alzheimer's disease pathology based on counts of cortical plaques and tangles (0.6 (0.6), range 0–2.1) In an initial linear regression model controlled for age at death, sex, education and time from smell testing until death, there was a decrease of 1.73 points on the odour identification scale for each unit of Alzheimer's disease pathology (SE 0.50, p<0.001). The pathological index accounted for 12.2% of the variance in the ability to recognise familiar odours proximate to death after accounting for the effects of the covariates.
We considered the possibility that dementia might explain the finding given its association with both odour identification and Alzheimer's disease pathology. Examination of the scatterplot of odour identification scores by the composite pathology measure (with the model‐derived regression line added) does not suggest that the subgroup with dementia at the time of odour assessment (n = 15; indicated by solid circles in fig 2) strongly affected the results. This impression was supported in subsequent analyses in which the association of Alzheimer's disease pathology with odour identification remained after controlling for the presence of dementia at the time of smell assessment (estimated coefficient −1.24, SE 0.44, p = 0.006) and after excluding this subgroup (estimated coefficient −1.19, SE 0.51, p = 0.022).
Figure 2 Score on odour identification test plotted by composite measure of Alzheimer's disease pathology, with solid circles indicating dementia at the time of olfactory testing and a linear regression line adjusted for age at death, sex, education and time from smell assessment to death.
As odour identification involves semantic memory,25 we conducted additional analyses controlling for this ability at the time of olfactory testing. The composite measure of Alzheimer's disease pathology continued to be associated with odour identification after controlling for a global measure of semantic memory (estimated coefficient −1.47, SE 0.49, p = 0.003) or a specific measure of naming (15‐item Boston naming test; estimated coefficient −1.48; SE 0.48, p = 0.003).
To test whether the association between Alzheimer's disease pathology and odour identification varied by type of pathology, we repeated the original analysis separately with summary indices of neuritic plaques, diffuse plaques and neurofibrillar tangles. In these analyses, neurofibrillar tangles were robustly related to odour identification (estimated coefficient −1.58, SE 0.45, p<0.001); neuritic plaques had a somewhat weaker association (estimated coefficient −1.01, SE 0.43, p = 0.021) and diffuse plaques had no association (estimated coefficient −0.51, SE 0.35, p = 0.152) with odour identification.
Odour identification, amyloid‐β‐immunoreactive plaques and tau‐immunoreactive tangles
As silver staining is not protein specific, we used immunohistochemical techniques to further investigate the relationship of plaque and tangle pathology with odour identification. To make use of all available data, composite measures of amyloid‐β deposition (mean (SD) 2.56 (3.14), range 0–11.55) and density of paired helical filament τ‐immunoreactive tangles (5.13 (6.92), range 0–38.79) were initially examined. In separate analyses, both amyloid load (estimated coefficient −0.32, SE 0.10, p = 0.002) and tangle density (estimated coefficient −0.16, SE 0.04, p<0.001) were inversely related to odour identification, with amyloid accounting for 11.7% of the variance and tangles accounting for 14.6%. With both amyloid and tangles in the same model, the effect of tangles persisted (estimated coefficient −0.21, SE 0.07, p = 0.005), but the effect of amyloid was reduced by about 70% (estimated coefficient −0.10, SE 0.11, p = 0.388), supporting the idea that tangles mediate the association of amyloid with odour identification, as has been proposed for cognitive function.26
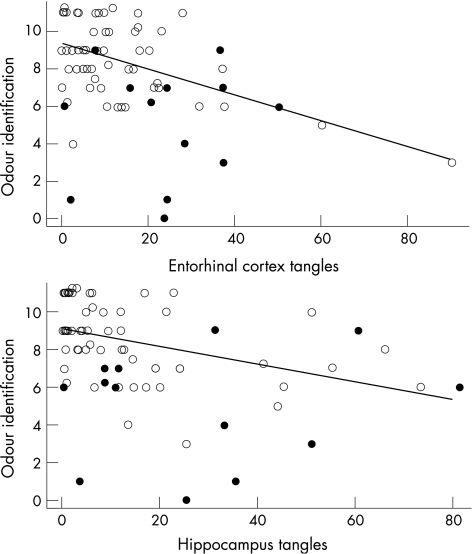
To assess regional variation in the relationship between tangles and odour identification, we separately examined the association of tangle density in eight brain regions with odour identification (table 1). Impaired odour identification was robustly related to increased density of tangles in the entorhinal cortex (upper panel of fig 3) and CA1/subiculum region of the hippocampus (lower panel of fig 3), marginally related to tangles in temporal cortex, and unrelated to tangles in other neocortical areas.
Table 1 Relationship of density of tau‐immunoreactive neurofibrillar tangles in different brain regions with odour identification ability proximate to death*.
| Brain region | Estimated slope | SE | p Value |
|---|---|---|---|
| Hippocampus (CA1, subiculum) | −0.05 | 0.02 | <0.001 |
| Entorhinal cortex | −0.08 | 0.02 | <0.001 |
| Inferior‐temporal cortex | −0.06 | 0.02 | 0.012 |
| Anterior‐cingulate cortex | −0.14 | 0.09 | 0.114 |
| Superior‐frontal cortex | −0.03 | 0.04 | 0.380 |
| Dorsal‐lateral prefrontal cortex | −0.04 | 0.05 | 0.423 |
| Angular/supramarginal‐gyrus cortex | −0.05 | 0.05 | 0.338 |
| Primary‐visual cortex | −0.41 | 0.22 | 0.064 |
*From separate linear regression models adjusted for age at death, sex, education and time from smell assessment to death.
Figure 3 Relationship between odour identification and density of tau‐ immunoreactive neurofibrillar tangles in the entorhinal cortex (upper panel) and CA1/subiculum region of the hippocampus (lower panel), with solid circles indicating dementia at the time of olfactory testing and a linear regression line adjusted for age at death, sex, education and time from smell assessment to death.
Odour identification and α‐synuclein‐immunoreactive Lewy bodies
As Lewy bodies have been associated with olfactory dysfunction in older people with dementia,1,2 we examined the relationship between α‐synuclein‐immunoreactive Lewy bodies (present in 12.5%) and odour identification. Reliable evidence of an association (estimated coefficient −1.02, SE 0.89, p = 0.258) was not found. In a subsequent analysis, we found no interaction between Lewy bodies and tangles (p = 0.814).
Discussion
In a group of community‐dwelling older people who died and underwent brain autopsy, ability to identify familiar odours proximate to death was inversely related to the level of Alzheimer's disease pathology. This association was primarily due to neurofibrillar pathology in the entorhinal cortex and hippocampus. The results suggest that impaired odour identification in old age is due in part to the accumulation of neurofibrillar pathology in central olfactory circuitry.
As noted above, two previous clinical–pathological studies did not observe an association between olfactory function and Alzheimer's disease pathology.1,2 However, these studies differ from the present study in important ways. Firstly, they assessed olfactory detection, whereas odour identification was assessed here. Thus, Alzheimer's disease pathology may affect higher order processes involved in identifying odours, but not lower order processes involved in their detection. Yet, tests of odour detection and odour identification are strongly correlated,27 perhaps because of the critical role of attention and anticipation in apparently simple acts of odour detection,28 and neurofibrillar pathology in Alzheimer's disease is characteristically present throughout the central olfactory system,6 including its first relay, the olfactory bulb,7,29 casting doubt on this explanation. A related issue is that olfactory detection can be difficult to reliably assess,30 and this might have affected previous results, especially as all participants in these studies had dementia when olfaction was assessed, and one study1 relied on a crude bedside test rather than a standard procedure. Secondly, the range of Alzheimer's disease pathology in the previous studies was restricted by limiting analyses to people meeting the pathological criteria for Alzheimer's disease and by reliance on categorical measures of Alzheimer's disease pathology. For example, nearly 90% of participants occupied the second of three points on a modified Braak scale in one study,2 and information on the Braak scale distribution was not provided in the other study.1 The lack of variability in Alzheimer's disease pathology in conjunction with binary measures of olfactory function may have limited the statistical power to detect an association between them.
Another factor differentiating this study from previous clinical–pathological research is that regional indices of neurofibrillar pathology were examined. Although prior research has shown that the level of neurofibrillar pathology in central olfactory regions is moderately correlated with levels of neurofibrillar pathology elsewhere in the brain,29,31,32 the correlations are far from perfect. We found that tangle density within components of the central olfactory system (ie, entorhinal cortex, CA1‐subiculum) was robustly related to odour identification performance, whereas tangle density in areas outside the system was not. This result supports the idea that neurofibrillar pathology is directly contributing to impairment in the ability to identify odours in old age. A previous clinical–pathological study of gait observed a similar regional dissociation in the effect of Alzheimer's disease pathology with gait impairment proximate to death associated with nigral but not cortical tangles.33 Thus, not only the level of neurofibrillar pathology, but also its regional distribution contributes to individual differences in a wide range of neurobehavioural functions in old age.
In neuroimaging studies of older people with Alzheimer's disease, olfactory functioning has been associated with metabolic and volumetric changes in medial temporal lobe.34,35,36 Our results show this association in people without dementia, consistent with studies showing reduced activation of central olfactory areas, including entorhinal cortex and hippocampus, during olfactory processing in older compared with younger people.37,38 Together, these data support the value of olfactory assessment for early detection of Alzheimer's disease.
In contrast with clinical–pathological studies of people with dementia,1,2 Lewy bodies were not related to olfactory impairment in this study of people with and without dementia proximate to death. The effect was in the direction observed in previous research, however, despite Lewy bodies being present in only one eighth of participants. These results are not inconsistent with a contribution of Lewy body pathology to age‐related olfactory dysfunction, therefore, although they imply that the effect may primarily occur after dementia is manifest or depend on brain regions not sampled in this study.
Confidence in these findings is strengthened by several factors. Odour identification was assessed with a standard test. Participants had a wide spectrum of odour identification ability and Alzheimer's disease pathology, enhancing our ability to observe an association between them. There was a high autopsy participation rate and a uniform postmortem examination with blinding to all clinical data, minimising important sources of error. The association of Alzheimer's disease pathology with odour identification persisted after controlling for presence of dementia or level of semantic memory. Alzheimer's disease pathology was assessed in a systematic fashion, and silver staining and immunohistochemistry produced comparable findings, suggesting that the observed association is robust.
This study also has important limitations. Findings are based on a selected cohort of older people, potentially reducing their generalisability. The advantage of studying olfaction in a defined population might be offset, however, by potential bias due to the likely low participation in brain autopsy in a clinical–pathological population‐based study. In addition, although the mean interval from smell assessment to death in this study was less than in previous research,1,2 it still exceeded 2 years, suggesting that these data may underestimate the strength of the association between Alzheimer's disease pathology and the ability to identify odours. Finally, we focused exclusively on odour identification; the association of Alzheimer's disease pathology with other olfactory functions remains to be established.
Acknowledgements
We thank many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin, MPH, and Tracy Hagman for coordinating the study; Zhaotai Cui, MS, for statistical programming; George Dombrowski, MS, and Greg Klein for data management; and Valerie J Young for preparing the manuscript.
Footnotes
Competing interests: None.
References
- 1.McShane R H, Nagy Z, Esiri M M.et al Anosmia in dementia is associated with Lewy bodies rather than Alzheimer's disease pathology. J Neurol Neurosurg Psychiatry 200170739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olichney J M, Murphy C, Hofstetter C R.et al Anosmia is very common in the Lewy body variant of Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005761342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Tredici K D, Rob U.et al Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 200324197–211. [DOI] [PubMed] [Google Scholar]
- 4.Ponsen M M, Stoffers D, Booij J.et al Idiopathic hyposomia as a preclinical sign of Parkinson's disease. Ann Neurol 200456173–181. [DOI] [PubMed] [Google Scholar]
- 5.Sommer U, Hummel T, Cormann K.et al Detection of presymptomatic Parkinson's disease: combining smell tests, transcranial sonography, and SPECT. Mov Disorders 2004191196–1202. [DOI] [PubMed] [Google Scholar]
- 6.Pearson R C A, Esiri M M, Hiorns R W.et al Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer's disease. Proc Natl Acad Sci USA1985824531–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs T, Cairns N J, Lantos P L. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport 200112285–288. [DOI] [PubMed] [Google Scholar]
- 8.Morris J C, Price A L. Pathologic correlates of nondemented aging, mild cognitive impairment, and early‐stage Alzheimer's disease. J Mol Neurosci 200117101–118. [DOI] [PubMed] [Google Scholar]
- 9.Bennett D A, Schneider J A, Buchman A S.et al The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 200525169–175. [DOI] [PubMed] [Google Scholar]
- 10.Doty R L, Marcus A, Lee W W. Development of the 12‐item Cross‐Cultural Smell Identification Test (CC‐SIT). Laryngoscope 1996106353–356. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 12.Wilson R S, Krueger K R, Arnold S E.et al Loneliness and risk of Alzheimer's disease. Arch Gen Psychiatry. In press [DOI] [PubMed]
- 13.Wilson R S, Arnold S E, Schneider J A.et al Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology 200627143–153. [DOI] [PubMed] [Google Scholar]
- 14.Wilson R S, Barnes L L, Krueger K R.et al Early and late cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 200511400–407. [PubMed] [Google Scholar]
- 15.Wilson R S, Arnold S E, Tang Y.et al Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 20062661–67. [DOI] [PubMed] [Google Scholar]
- 16.Doty R L, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav 198432489–502. [DOI] [PubMed] [Google Scholar]
- 17.Doty R L, Frye R E, Agarwal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys 198945381–384. [DOI] [PubMed] [Google Scholar]
- 18.Devanand D P, Michaels‐Marston K S, Liu X.et al Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow‐up. Am J Psychiatry 20001571399–1405. [DOI] [PubMed] [Google Scholar]
- 19.Talbert M H, Liu X, Doty R L.et al A 10‐item smell identification scale related to risk for Alzheimer's disease. Ann Neurol 200558155–160. [DOI] [PubMed] [Google Scholar]
- 20.Graves A B, Bowen J D, Rajaram L.et al Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E ε4 status. Neurology 1999531480–1487. [DOI] [PubMed] [Google Scholar]
- 21.Bennett D A, Schneider J A, Arvanitakis Z.et al Neuropathology of older persons without cognitive impairment from two community‐based studies. Neurology 2006661837–1844. [DOI] [PubMed] [Google Scholar]
- 22.Bennett D A, Schneider J A, Tang Y.et al The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 20065406–412. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell T W, Nissanov J, Han L Y.et al Novel method to quantify neuropil threads in brains from elders with and without cognitive impairment. J Histochem Cytochem 2000481627–1638. [DOI] [PubMed] [Google Scholar]
- 24.Schneider J A, Bienas J L, Gilley D W.et al Improved detection of nigral pathology in Alzheimer's disease. J Histochem Cytochem 20025099–106. [DOI] [PubMed] [Google Scholar]
- 25.Finkel D, Pedersen N L, Larsson M. Olfactory functioning and cognitive abilities: a twin study. J Gerontol Psychol Sci 200156BP226–P233. [DOI] [PubMed] [Google Scholar]
- 26.Bennett D A, Schneider J A, Wilson R S.et al Neurofibrillary tangles mediate the association of amyloid with clinical Alzheimer's disease and level of cognitive function. Arch Neurol 200461378–384. [DOI] [PubMed] [Google Scholar]
- 27.Doty R L, Smith R, McKeown D A.et al Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Percept Psychophys 199456701–707. [DOI] [PubMed] [Google Scholar]
- 28.Zelano C, Bensafi M, Porter J.et al Attentional modulation in human primary olfactory cortex. Nat Neurosci 20058114–120. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi Y, Wszolek Z K, Graff‐Radford N R.et al Tau pathology in the olfactory bulb correlates with Braak state, Lewy body pathology and apolipoprotein epsilon 4. Neuropathol Appl Neuorbiol 200329503–510. [DOI] [PubMed] [Google Scholar]
- 30.Doty R L, McKeown D A, Lee W W.et al A study of the test‐retest reliability of 10 olfactory tests. Chem Senses 199520645–656. [DOI] [PubMed] [Google Scholar]
- 31.Attems J, Lintner F, Jellinger K A. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimer Dis 20057149–157. [DOI] [PubMed] [Google Scholar]
- 32.Christen‐Zaech S, Kraftsik R, Pillevuit O.et al Early olfactory involvement in Alzheimer's disease. Can J Neurol Sci 20033020–25. [DOI] [PubMed] [Google Scholar]
- 33.Schneider J A, Li J L, Li Y.et al Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol 200659166–173. [DOI] [PubMed] [Google Scholar]
- 34.Kesslak J P, Nalcioglu O, Cotman C W. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology 19914151–54. [DOI] [PubMed] [Google Scholar]
- 35.Murphy C, Jernigan T L, Fennema‐Notestine C. Left hippocampal volume loss in Alzheimer's disease is reflected in performance on odor identification: a structural MRI study. J Int Neuropsychol Soc 20039459–471. [DOI] [PubMed] [Google Scholar]
- 36.Buchsbaum M S, Kesslak J P, Lynch G.et al Temporal and hippocampal metabolic rate during an olfactory memory task assessed by position emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies. Arch Gen Psychiatry 199148840–847. [DOI] [PubMed] [Google Scholar]
- 37.Cerf‐Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res 200398639–53. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Eslinger P J, Smith M B.et al Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol Med Sci 200560A510–514. [DOI] [PubMed] [Google Scholar]