Abstract
Conventional wisdom regarding mechanisms of bacterial pathogenesis holds that pathogens arise by external acquisition of distinct virulence factors, whereas determinants shared by pathogens and commensals are considered to be functionally equivalent and have been ignored as genes that could become adapted specifically for virulence. It is shown here, however, that genetic variation in an originally commensal trait, the FimH lectin of type 1 fimbriae, can change the tropism of Escherichia coli, shifting it toward a urovirulent phenotype. Random point mutations in fimH genes that increase binding of the adhesin to mono-mannose residues, structures abundant in the oligosaccharide moieties of urothelial glycoproteins, confer increased virulence in the mouse urinary tract. These mutant FimH variants, however, are characterized by increased sensitivity to soluble inhibitors bathing the oropharyngeal mucosa, the physiological portal of E. coli. This functional trade-off seems to be detrimental for the intestinal ecology of the urovirulent E. coli. Thus, bacterial virulence can be increased by random functional mutations in a commensal trait that are adaptive for a pathologic environment, even at the cost of reduced physiological fitness in the nonpathologic habitat.
In the prevailing view of bacterial pathogenesis, evolutionary adaptation to the pathologic habitat is due to the possession by pathogens of virulence factor genes that are absent from commensal strains (1). Although it is well known that natural variation of allelic genes leads to the expression of distinct protein variants (i.e., protein polymorphism) (2), heteroalleles in a haploid organism are generally considered to be functionally equivalent and, therefore, selectively neutral (3, 4). The exceptions to this rule are highly divergent genes, often of mosaic structure, which are under strong directional selection, as are the genes for resistance to antibiotics and genes maintained by frequency-dependent selection induced by the host immune system (5–8). The transition, however, from commensal to virulent phenotype has been attributed to the acquisition of distinct “virulence genes.” To date, conventional wisdom regarding molecular mechanisms of microbial specialization as pathogens has essentially excluded genes shared by pathogenic and commensal strains from consideration as factors that could become adapted specifically for pathogenicity.
Escherichia coli inhabit the large intestine of humans and other animals as a small but consistent part of the normal microbiota. This primary niche also serves as a reservoir for E. coli that can cause a variety of enteric and extra-enteric diseases (9). One trait that has been considered important for both normal ecology and for pathogenicity is the production of tissue-specific adhesive factors, frequently found in the form of hair-like surface structures called fimbriae. More than 95% of all isolates of E. coli express type 1 fimbriae, which are also called mannose-sensitive or common fimbriae (10, 11). Expression of type 1 fimbriae may not be required for maintaining E. coli in the colon (12, 13), but they do provide E. coli with a significant advantage for the transitory colonization of the oropharyngeal portal that is important for the normal fecal/oral transmission of E. coli among individual hosts (13, 14). In addition to this essential role in commensal ecology, type 1 fimbriae are also important for the pathogenesis of urinary tract infections (UTIs) (15, 16). The d-mannose-sensitive adhesive phenotype of type 1 fimbriae is dependent on the lectin-like activity of a tip-located 30-kDa subunit, FimH (17, 18). Naturally occurring phenotypic variants of the FimH protein have recently been recognized (19, 20). All fimH alleles studied to date encode subunits that mediate high levels of binding to tri-mannose structures (M3; α1–3, α1–6-d-mannotriose), but binding to mono-mannose (M1) residues among the FimH variants can differ up to 15-fold (21). These adhesive variations are due solely to structural differences in FimH that affect receptor specificity of the lectin but do not affect fimbrial morphology or level of fimbriation (20, 21). Thus, all type 1 fimbriae can be functionally subdivided into either low M1-binding (M1L) or high M1-binding (M1H) FimH phenotypes. E. coli exhibiting these two basic phenotypes have been found to predominate in different niches. Most isolates from the large intestine of healthy humans (around 80%) express a distinct M1L phenotype, whereas most isolates from UTIs (more than 70%) express M1H FimH variants (20). The previous studies, however, did not identify selective forces that could cause the differential distribution of phenotypes in the intestinal and uropathogenic E. coli populations.
In this report, it is shown that naturally occurring FimH variations dramatically change the tissue tropism of E. coli and can be a major factor in shifting the bacterial adaptation from commensal to pathologic habitat. Thus, the transition from commensal to virulent phenotype may be mediated not only by acquisition of “virulence genes” but also by selection for genetic variations in a commensal trait that are adaptive to a pathologic environment.
MATERIALS AND METHODS
Construction of Isogenic Strains.
The M1L and M1H phenotype strains used in binding studies (KB91 and KB96, respectively) were created as described elsewhere. Briefly, M1L and M1H fimH genes were cloned by PCR from the intestinal E. coli strain F-18 and UTI isolate MJ#2–2, respectively, subcloned into pACYC184-based plasmids creating pGB17 and pGB19, respectively, and introduced into the fimH-null E. coli strain AAEC191A (pPKL114) (20). Strains KB91 and KB96 were assayed for M1 and M3 binding, utilizing immobilized monomannosylated BSA (M1) and α1–3, α1–6-d-mannotriose BSA (M3) as described previously (21). The M1-binding levels of strain KB91 and strain KB96 were 0.52 ± 0.1 × 106 cfu per well and 4.5 ± 0.52 × 106 cfu per well, respectively, and the M3-binding levels were 6.5 ± 0.50 × 106 cfu per well and 7.2 ± 0.62 × 106 cfu per well, respectively. For mouse UTI experiments, isogenic strains were created using the UTI E. coli strain CI #10. This strain expresses M1H type 1 fimbriae and is negative for genes encoding P-related fimbriae, S/F1C fimbriae, Dr adhesin, aerobactin, groups II and III capsule, hemolysin, cytotoxic necrotizing factor I, and outer membrane protein T. The fimH gene of CI#10 was insertionally inactivated using the pCH103 suicide plasmid, as described previously (22). Southern blots of chromosomal DNA from the fimH-null strain, CI#10–9, indicated that an appropriate double crossover event occurred (data not shown). Strain CI#10–9 was transformed with plasmids pGB17 and pGB19, described above, or with an isogenic plasmid containing a fimH gene missing a 17-bp segment at the 3′-end and encoding a nonfunctional form of the FimH protein. The adhesive phenotype of CI#10–9 complemented with the fimH-bearing plasmids was precisely as predicted for corresponding fimH alleles. Phylogenetic analysis was performed on fimH alleles derived from wild-type fecal and urinary isolates and one recombinant strain described previously (20).
Binding Studies.
Yeast and guinea pig red blood cell (RBC) agglutination assays were performed by mixing equal amounts of serially diluted bacterial suspensions (starting from OD540 nm = 1.0) and a 1% suspension of baking yeast or guinea pig red blood cells in U-bottom microtiter plate wells. Buccal epithelial cell binding and binding inhibition experiments were performed using an inverted adhesion assay, described previously (23). In this assay, bacteria are immobilized in microtiter plate wells, and buccal cells are added to the bacterial substratum, thus eliminating potential interference by bacterial agglutinins. Procedures for purifying uroplaques (i.e., asymmetric unit membranes) from bovine bladder and for testing their ability to bind E. coli in microtiter plate wells were described previously (24). Samples of saliva were obtained and treated as described previously (25).
Experimental UTI.
Female C3H/HeN mice (Harlan Laboratories, Haslett, MI) were used at 8–14 weeks of age. Overnight cultures of the recombinant strains were suspended in 0.01 M PBS (pH 7.0) to a concentration of 5 × 109 per ml, and 0.05 ml were inoculated into bladders of the mice by transurethral catheterization under ketamine/xylazine anesthesia. Mice were sacrificed after 24 h, and the bladders were excised and homogenized. The procedures used were approved by the University of Tennessee, Memphis Animal Care and Use Committee. Numbers of bacteria per bladder were quantitated by viable counts obtained from tissue homogenates. Plasmid stability was 100% over the course of these experiments. The Mann-Whitney u test was used for the calculation of P.
RESULTS
M1H Variants Exhibit Expanded Cell Receptor Specificity.
High M1-reactivity allows M1H variants to interact with a wide variety of receptor glycoproteins, whereas M1L variants require unsubstituted M3 structures, thus limiting their spectrum of binding activities (21). Mannosylated glycoproteins are common components of the surface of various cell types, so it was not surprising to find that the M1H variant mediated adhesion to more cellular substrata than did the M1L variant (Table 1). Whereas the M1L and M1H variants interacted at essentially the same levels with yeast cells and buccal epithelial cells, the M1H variant bound much more effectively to guinea pig erythrocytes and bovine bladder uroplaques. Although yeast and erythrocyte agglutination are the traditional assays used to test type 1 fimbriae, interaction with buccal cells and uroplaques are more important from a physiological perspective, regarding oropharyngeal and bladder colonization by E. coli, respectively.
Table 1.
Adhesive properties of isogenic E. coli strains KB91 and KB96 expressing M1L and M1H FimH variants, respectively
| E. coli strain | FimH variant | M1:M3 ratio | Yeast cell agglutination† | Guinea pig RBC agglutination† | Buccal cell binding‡ | Uroplaque binding§ |
|---|---|---|---|---|---|---|
| KB91 | M1L | 0.08 | 16 | 2 | 185 ± 15 | 0.26 ± 0.05 |
| KB96 | M1H | 0.63 | 16 | 16 | 194 ± 12 | 3.35 ± 0.15 |
Binding in all of these assays was inhibited more than 95% by 1% α-methyl-d-mannopyranoside.
Functionally, strains KB91 and KB96 are completely representative of other M1L and M1H phenotype strains.
Last dilution causing agglutination.
Buccal epithelial cells bound per well, mean ± standard error of mean.
E. coli bound (cfu per well × 106), mean ± standard error of mean.
M1H Variants Provide Selective Advantage for E. coli Colonization of the Urinary Tract.
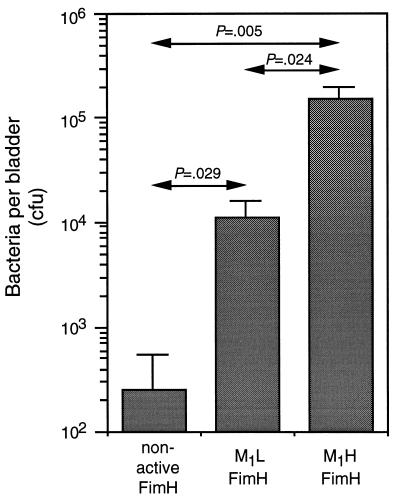
Uroplaques occupy 80% or more of the apical surface of bladder epithelial cells and are considered to be the major adhesion substratum for E. coli in the mammalian bladder (24). We hypothesized that the greater level of binding of the M1H variants to these cellular components confers a selective advantage during bladder colonization. This hypothesis was tested by infecting mice with a set of strains that were isogenic, except for the expressed fimH gene. Twenty-four hours after intravesical inoculation, the recombinant strains bearing either M1L or M1H fimH genes were recovered from bladders in higher numbers than the strain encoding a nonfunctional FimH (Fig. 1), but the colonizing ability of the M1H strain was at least 15-fold higher than that of the M1L strain. Therefore, it can be concluded that in the same genetic background of a uropathogenic isolate, the M1H FimH confers a significant advantage for colonization of the bladder compared with the M1L variant, providing a rationale for the predominance of the M1H FimH phenotype among UTI strains.
Figure 1.
Colonization of mouse bladders by isogenic E. coli expressing nonfunctional FimH, M1L FimH, or M1H FimH subunits. Bars indicate mean cfu per bladder ± SEM. P values indicating level of significance between different groups are indicated.
M1L Phenotype Exhibits a Superior Ability to Resist Inhibitors of Adhesion.
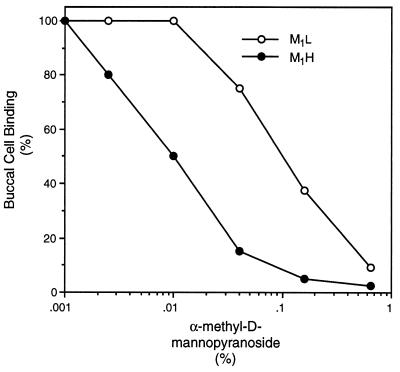
The M1L phenotype is predominant among intestinal E. coli. Because type 1 fimbriae are apparently not required once the bacteria enter this niche (12), the M1L phenotype must provide a selective advantage in the transient colonization of the oropharyngeal portal required for inter-host transmission (13, 14). The number of bacteria bound to mucosal cells is a function of the affinity of the adhesin for the cognate receptor on the epithelial cells and the interference of inhibitors in the body fluids bathing the mucosal surfaces (26). The fact that E. coli expressing either phenotype of FimH interacted with buccal epithelial cells at equivalent levels suggested that any advantage provided by the M1L variant in the oral cavity could be the result of differences of the two variants in their sensitivity to mannose-containing inhibitors. Indeed, at relatively high concentrations of α-methyl-d-mannopyranoside, the binding of both variants to buccal cells is completely inhibited, but at intermediate concentrations of inhibitor, the M1L variant is much less sensitive to inhibition (Fig. 2). The IC50 of the M1L variant was 15-fold higher than that of the M1H variant. Inhibition of yeast cell agglutination followed the same pattern, with the M1L variant being 10-fold more resistant to inhibition.
Figure 2.
Inhibition of interaction of E. coli and buccal cells by α-methyl-d-mannopyranoside.
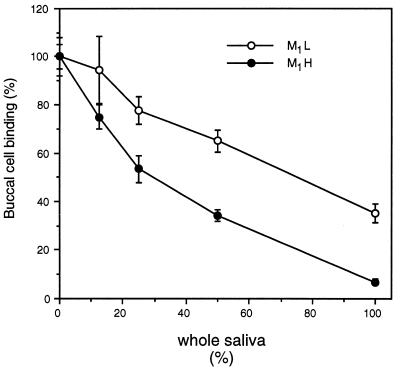
Although the difference in mannose sensitivity of these FimH variants is striking, free mannose is rarely, if ever, found in the natural environment. Mucosal surfaces are, however, bathed with glycoproteins that possess terminally exposed mannose residues (27, 28). It was previously shown that certain surface-immobilized glycoproteins bind both FimH variants equally well, whereas other glycoproteins bind M1H variants much better (21). Model glycoproteins representing these two basic types of mannosylated receptors were tested in solution for their ability to inhibit the interaction of M1L and M1H variants with buccal cells (Table 2). For all four glycoproteins, the concentration required to inhibit the M1H variant by 50% is much lower (3- to 25-fold) than those concentrations required to achieve the same level of inhibition of the M1L variant. Because the majority of inhibitors of E. coli adhesion in the oropharyngeal cavity are likely to be present in saliva, the ability of whole, stimulated human saliva to interfere with the interaction of the E. coli strains with buccal cells was also tested (Fig. 3). Here again, bacteria expressing the M1L variant are much more resistant to inhibition. In the presence of undiluted saliva, bacteria expressing the M1L variant bound buccal cells in 5-fold higher numbers than did the bacteria expressing the M1H variant. The relatively high concentration of saliva needed to inhibit binding of the M1L variant to oropharyngeal cells should confer a higher rate of per os transmission of intestinal strains of E. coli and, overall, impart an increased fitness for commensal ecology.
Table 2.
Effect of soluble glycoproteins on interaction of buccal cells and E. coli strains KB91 (M1L) and KB96 (M1H)
| Inhibitor* | IC50†
|
|
|---|---|---|
| E. coli KB91 (M1L FimH) | E. coli KB96 (M1H FimH) | |
| Yeast mannan | 5.0 ± 0.3 | 0.5 ± 0.2 |
| Bovine intestinal mucin | 1.7 ± 0.1 | 0.6 ± 0.1 |
| Bovine RNase B | 0.9 ± 0.2 | 0.08 ± 0.02 |
| Bovine lactoferrin | 2.1 ± 0.2 | 0.08 ± 0.03 |
The ability of these E. coli strains to bind to the same glycoproteins immobilized on plastic was determined previously (21). The binding of KB91 and KB96 to the glycoproteins were, respectively, 0.5 ± 0.1 and 6.2 ± 0.2 to mannan, 0.4 ± 0.1 and 5.2 ± 0.2 to mucin, 7.5 ± 0.3 and 7.7 ± 0.2 to RNase B, and 6.1 ± 0.1 and 6.0 ± 0.1 to lactoferrin (mean cfu per well × 106, ± standard error of mean).
The numbers given are the concentrations (mean mg per ml, ± standard error of mean) of each glycoprotein required to give 50% inhibition (i.e., IC50).
Figure 3.
Inhibition of the interaction of E. coli and buccal cells by whole, stimulated human saliva.
M1H Variants Have Evolved from M1L Alleles and Are Relatively Short-Lived.
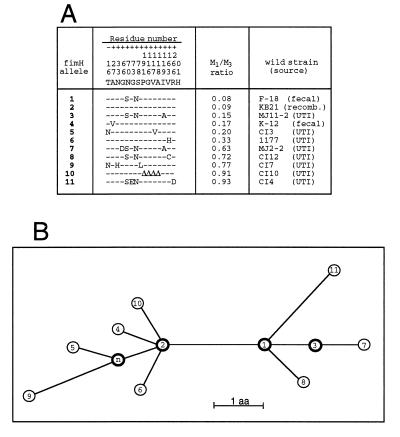
Differential transmission rates for M1L- and M1H-bearing E. coli could be difficult to measure experimentally in vivo, but the population stability of the fimH alleles can be compared based on their evolutionary history. The genealogical relationships of 11 unique fimH alleles that exhibit various M1-binding activities were established (Fig. 4). All interior nodes of the phylogenetic network are represented by FimH subunits with a relatively low M1-binding capability (M1:M3 ratios of 0.08 to 0.15), whereas the terminal nodes are occupied primarily by distinct M1H-phenotype alleles. These data strongly suggest that M1L is the ancestor and M1H is the derived phenotype. A single amino acid substitution is capable of converting an M1L phenotype into an M1H phenotype. The altered residues causing these functional changes were found in different regions of the protein and included both conservative and nonconservative substitutions. Therefore, M1H alleles arise from M1L alleles by various nonsynonymous mutations and do not form distinct genetic lineages (i.e., the mutations are random in nature). Importantly, the position of M1H alleles on the outer nodes and the extremely high sequence similarities show that the M1H variants were derived from the M1L alleles recently and, therefore, are maintained within the population for a relatively short period of time. Such deleterious effects would be inevitable for mutations that decreased the rate of transmission of bacteria within the host population (29). Thus, the phylogenetic network supports the concept that there is impaired fitness of the M1H variant alleles for commensal ecology.
Figure 4.
Phylogenetic analysis of FimH alleles. (A) Amino acid sequences of FimH variants. The alleles are listed based on an increasing M1:M3 binding ratio. The residues listed above the 11 alleles are for the amino acids in original FimH sequence (17) that vary in the other fimH alleles. Only polymorphic residues are shown, and the positions are numbered vertically, from −16 to +201. ▵, deleted residues. (B) Inferred phylogenetic network demonstrating evolutionary relationships of the FimH alleles shown in A. Each node represents a distinct FimH allele, numbered as in A. The allele labeled n represents a hypothetical FimH that differs from allele #2 by the substitution of Asp (N) for Tyr (T) in the leader sequence (residue, −16) and phenotypically should be equivalent to allele #2. Internal nodes are shown in bold. The deduced sequences of the 11 FimH proteins exhibit greater than 99% homology, and the network showing their phylogenetic relationships is fully consistent, without any homoplasty. Branch lengths are scaled to the number of amino acids that differ between alleles, as indicated. The deletion of 4 amino acids in FimH allele #10 is considered to be a single event, equivalent to one amino acid substitution.
DISCUSSION
Based on the analyses reported here, it can be proposed that FimH polymorphism is sustained in the population by diversifying selection, a form of balancing selection where two different genotypes are positively selected in two different environments (30). The M1L variants are common among intestinal E. coli, and M1H variants are common among UTI E. coli as a result of differential adaptation to these different niches. Importantly, adaptation of the M1H variants for the urinary tract is accompanied by a functional trade-off where an increasing ability of FimH adhesins to recognize additional cellular receptors results in a decreasing ability to resist inhibition by mannosylated compounds.
The M1L variants seem to be optimized for adhesion to surfaces naturally bathed with high concentrations of soluble mannosylated compounds, such as the oropharyngeal mucosa. Interestingly, the binding of the M1H variant to buccal cells is inhibited to a greater extent than is the binding of the M1L variant by all types of soluble inhibitors tested. This was true even for soluble RNAse B and lactoferrin, to which both variants bind equally well when the glycoproteins have been immobilized, suggesting that FimH subunits might interact via different mechanisms with surface-bound and soluble forms of a glycoprotein. It is also interesting to note that the inhibitory activity of mucin against these two strains differs less drastically than the activity of other tested glycoproteins. At least a portion of the inhibitory activity of mucin could be due to nonspecific effects of its high viscosity. The same nonspecific effect could be responsible for the relatively small differences in the IC50 of saliva. The molecular details of these phenomena are currently under investigation.
In contrast to the M1L variants, the M1H variants have been selected for an expanded receptor specificity, so they bind well to surfaces of new habitats, particularly where concentrations of soluble mannosylated compounds are relatively low, such as the urinary bladder (31). Information regarding strains used for experimental UTI by other investigators is fully consistent with the hypothesis presented here that the M1H phenotype contributes more to pathogenesis than the M1L phenotype. E. coli strain 1177, recently shown to be a highly successful bladder colonizer (15), exhibits an M1H phenotype (20). In contrast, E. coli 1066 that is clonally related to strain 1177 exhibits M1L phenotype and is a poor colonizer (15). In another study on the use of FimH as a vaccine for cystitis E. coli (16), the E. coli strain used in the experimental UTI model, Nu14, was a very successful colonizer of the murine bladder. This strain exhibits a distinct M1H phenotype (M1:M3 ratio, 0.8). It will be important to determine whether anti-FimH antibodies protect equally against E. coli expressing M1H fimH and those expressing M1L fimH, as the pathogenesis of cystitis caused by low uroplaque-binding M1L variants could be different from that caused by more highly adhesive E. coli bearing the M1H phenotype.
Although M1H variants exhibit increased uropathogenicity, they are likely to be detrimental to transmission through the oral portal due to their increased sensitivity to salivary inhibitors and, therefore, would be less stable in the population than the M1L alleles. However, the M1H FimH alleles obviously can be spread between hosts to at least some extent. Uropathogens are actively disseminated into the environment via contaminated urine in the course of UTI. Also, it was shown previously that at least some uropathogenic E. coli isolates can be sexually transmitted between individuals (32). Possibly, acquisition of other adhesins, such as Dr, S, or P fimbriae, contributes to sustaining uropathogenic strains in the host population (33). Experimental support for each of these suppositions will require further detailed studies.
It must be noted that although the diversification of FimH alleles is obviously a result of within-host evolution of E. coli, it is not yet clear whether the uropathogenic M1H mutants are originally selected from M1L FimH-bearing E. coli occupying the colon or colonizing the urinary tract. It is also not clear whether the mutations of FimH alone could be sufficient for uropathogenicity. However, in this regard it should be noted that of nine virulence determinants analyzed in a recent study, the type 1 fimbrial gene cluster was often the only determinant present in the genetically diverse cystitis isolates tested (34). It was the only determinant common to all isolates, and it was significantly more common than genes encoding, for instance, aerobactin, hemolysin, P fimbriae, or S fimbriae.
It has been previously proposed that more virulent clones of microorganisms emerge from nonvirulent (or less virulent) ancestral strains via within-host diversification (35), possibly due to increased mutation rates (36, 37). Although such studies have been based almost exclusively on theoretical models, considerable sequence diversity has been found in the hypervariable regions of the HIV-1 env gene of virions obtained from different organs of a patient (38). It was hypothesized that genotypic diversification of the original clone transmitted to the individual might allow variants to invade and replicate in new habitats within an infected host but that such variants would not migrate effectively into new hosts. The genetic and functional characterization of the FimH proteins, provided here and in previous publications (19–21), is the first direct example of allelic diversity leading to increased virulence and, at the same time, to decreased commensal fitness of a microorganism.
On the basis of these observations with allelic variants of FimH, a more generally applicable concept can be suggested: a variety of genes original to nonpathogenic microorganisms may be subject to mutations that are adaptive for the transition from commensal to pathologic habitats. Such pathogenicity-adaptive, or pathoadaptive, mutations might be especially important for in-host microevolution of a pathogen during infections that are opportunistic and/or known to proceed from acute to chronic phases. Interestingly, these pathoadaptive mutations, as seems to be the case for FimH, may be detrimental for the original physiological role of the protein and may thus be under negative selection in the commensal niche. It is generally true that mutations affecting the specificity of the original function are most likely to be deleterious or abnormal for the organism in its evolutionarily primary niche (3). The most well known examples of this are the genetic disorders or predispositions to disorders in humans that result from mutations in certain genes, and for this reason allelic variations are becoming a major focus for the Human Genome Project. Now that the chromosomes of several human pathogens have been completely sequenced, attention of bacterial genome projects is turning toward identification of traits specialized for virulence. This search, however, is presently guided by the principle that genes adapted specifically for a pathologic habitat will be absent in nonpathogenic isolates. The concept of pathoadaptive variation of genes shared by commensals and pathogens provides an alternative approach for understanding bacterial virulence and suggests that bacterial genome projects should also be focused on functional analysis of structural variations in common genes.
Acknowledgments
We thank Dr. J. Dale for his encouragement and thoughtful contributions to this work and also Drs. H. Courtney, N. Sharon, and S.-J. Suh for additional important suggestions regarding the preparation of this manuscript. We thank Dr. Betsy Foxman for genotype analysis of E. coli CI #10. These studies were supported in part by research funds from the Department of Veterans’ Affairs.
ABBREVIATIONS
- M1
mono-mannose
- M3
tri-mannose
- M1L
low binding to mono-mannose
- M1H
high binding to mono-mannose
- RBC
red blood cells
- UTI
urinary tract infection
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. X05672).
References
- 1.Falkow S. ASM News. 1997;63:359–365. [Google Scholar]
- 2.Milkman R. Science. 1973;182:1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- 3.Kimura M. The Neutral Theory of Molecular Evolution. Cambridge, U.K.: Cambridge Univ. Press; 1983. [Google Scholar]
- 4.Hartl D L, Dykhuizen D E. In: Population Genetics and Molecular Evolution. Ohta T, Aoki K, editors. Tokyo: Jap. Sci. Soc. Press; 1985. pp. 107–124. [Google Scholar]
- 5.Lomholt H, Poulsen K, Caugant D A, Kilian M. Proc Natl Acad Sci USA. 1992;89:2120–2124. doi: 10.1073/pnas.89.6.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 7.Dean A M. Genetics. 1989;123:441–454. doi: 10.1093/genetics/123.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Nelson K, McWhorter A C, Whittam T S, Selander R K. Proc Natl Acad Sci USA. 1994;91:2552–2556. doi: 10.1073/pnas.91.7.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenstein B I. In: Principles and Practice of Infectious Disease. Mandell G L, Danglers R G, Bennet J E, editors. Vol. 2. New York: Churchill Livingstone; 1989. pp. 1658–1673. [Google Scholar]
- 10.Brinton C C., Jr Nature (London) 1959;183:782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- 11.Ofek I, Doyle R J. Bacterial Adhesion to Cells and Tissues. New York: Chapman and Hall; 1994. pp. 324–334. [Google Scholar]
- 12.McCormick B A, Franklin D P, Laux D C, Cohen P C. Infect Immun. 1989;57:3022–3029. doi: 10.1128/iai.57.10.3022-3029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch C, Orndorff P. Infect Immun. 1990;58:275–278. doi: 10.1128/iai.58.1.275-278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch C, Stocker B, Orndorff P. Mol Microbiol. 1992;6:697–701. doi: 10.1111/j.1365-2958.1992.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Connell H, Agace W, Klemm P, Schembri M, Mårild S, Svanborg C. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, et al. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 17.Klemm P, Christensen G. Mol Gen Genet. 1987;208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 18.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. Proc Natl Acad Sci USA. 1995;92:2081–2088. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokurenko E V, Courtney H S, Ohman D E, Klemm P, Hasty D L. J Bacteriol. 1994;176:748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokurenko E V, Courtney H S, Maslow J, Siitonen A, Hasty D L. J Bacteriol. 1995;177:3680–3686. doi: 10.1128/jb.177.13.3680-3686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokurenko E V, Chesnokova V, Doyle R J, Hasty D L. J Biol Chem. 1997;272:17880–17886. doi: 10.1074/jbc.272.28.17880. [DOI] [PubMed] [Google Scholar]
- 22.Schembri M A, Pallesen L, Connell H, Hasty D L, Klemm P. FEMS Microbiol Lett. 1996;137:257–263. doi: 10.1111/j.1574-6968.1996.tb08115.x. [DOI] [PubMed] [Google Scholar]
- 23.Sokurenko E V, Hasty D L. Methods Enzymol. 1995;253:220–226. doi: 10.1016/s0076-6879(95)53021-5. [DOI] [PubMed] [Google Scholar]
- 24.Wu X-R, Sun T-T, Medina J J. Proc Natl Acad Sci USA. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtney H S, Hasty D L. Infect Immun. 1991;59:1661–1666. doi: 10.1128/iai.59.5.1661-1666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ofek I, Doyle R J. Bacterial Adhesion to Cells and Tissues. New York: Chapman and Hall; 1994. pp. 536–546. [Google Scholar]
- 27.Beachey E H. J Infect Dis. 1981;143:325–330. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 28.Scannapieco F A. Crit Rev Oral Biol Med. 1994;5:203–208. doi: 10.1177/10454411940050030201. [DOI] [PubMed] [Google Scholar]
- 29.Lipsitch M, Nowak M A, Ebert D, May R M. Proc R Soc Lond (B Biol Sci) 1995;260:321–327. doi: 10.1098/rspb.1995.0099. [DOI] [PubMed] [Google Scholar]
- 30.Hedrick P W. Annu Rev Ecol Syst. 1986;17:535–566. [Google Scholar]
- 31.Parkkinen J, Virkola R, Korhonen T K. Infect Immun. 1988;56:2623–2630. doi: 10.1128/iai.56.10.2623-2630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foxman B, Zhang L, Tallman P, Andree B C, Geiger A M, Koopman J S, Gillespie B W, Palin K A, Sobel J D, Rode C K, et al. J Infect Dis. 1997;175:989–992. doi: 10.1086/514007. [DOI] [PubMed] [Google Scholar]
- 33.Levin B R, Svanborg C. Parasitology. 1990;100:S103–S115. doi: 10.1017/s0031182000073054. [DOI] [PubMed] [Google Scholar]
- 34.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 35.Levin B R. Emerg Infect Dis. 1996;2:93–102. doi: 10.3201/eid0202.960203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeClerc J E, Li B, Payne W L, Cebula T A. Science. 1996;274:1208–1211. doi: 10.1126/science.274.5290.1208. [DOI] [PubMed] [Google Scholar]
- 37.Moxon E R, Thaler D S. Nature (London) 1997;387:659–660. doi: 10.1038/42607. [DOI] [PubMed] [Google Scholar]
- 38.Brown A J L, Holmes E C. Annu Rev Ecol Syst. 1994;25:127–165. [Google Scholar]