Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease with severe cervical cord damage due to degeneration of the corticospinal tracts and loss of lower motor neurones. Diffusion tensor magnetic resonance imaging (DT MRI) allows the measurement of quantities reflecting the size (such as mean diffusivity) and orientation (such as fractional anisotropy) of water‐filled spaces in biological tissues.
Methods
Mean diffusivity and fractional anisotropy histograms from the cervical cord of patients with ALS were obtained to: (1) quantify the extent of tissue damage in this critical central nervous system region; and (2) investigate the magnitude of the correlation of cervical cord DT MRI metrics with patients' disability and tissue damage along the brain portion of the corticospinal tracts. Cervical cord and brain DT MRI scans were obtained from 28 patients with ALS and 20 age‐matched and sex‐matched controls. Cord mean diffusivity and fractional anisotropy histograms were produced and the cord cross‐sectional area was measured. Average mean diffusivity and fractional anisotropy along the brain portion of the corticospinal tracts were also measured.
Results
Compared with controls, patients with ALS had significantly lower mean fractional anisotropy (p = 0.002) and cord cross‐sectional area (p<0.001). Mean diffusivity histogram‐derived metrics did not differ between the two groups. A strong correlation was found between mean cord fractional anisotropy and the ALS Functional Rating Score (r = 0.74, p<0.001). Mean cord and brain fractional anisotropy values correlated moderately (r = 0.37, p = 0.05).
Conclusions
Cervical cord DT MRI in patients with ALS allows the extent of cord damage to be graded. The conventional and DT MRI changes found are compatible with the presence of neuroaxonal loss and reactive gliosis, with a heterogeneous distribution of the pathological process between the brain and the cord. The correlation found between cord fractional anisotropy and disability suggests that DT MRI may be a useful adjunctive tool to monitor the evolution of ALS.
Amyotrophic lateral sclerosis (ALS) is the most common adult‐onset motor neurone disease, characterised by a progressive and simultaneous degeneration of upper and lower motor neurones.1,2 In its typical form, the disease begins either in one limb or with a combination of bulbar and corticobulbar symptoms, and continues with progressive weakness of the bulbar, limb, thoracic and abdominal musculature.1,2 By using a variety of conventional magnetic resonance imaging (MRI) sequences, several studies3,4,5,6,7,8,9,10,11,12,13,14,15 have shown changes in signal intensity along the brain portion of the corticospinal tracts, particularly in the posterior limb of the internal capsule and cerebral peduncles, varying between 25% and 80%. Reduced magnetisation transfer ratios in the internal capsule8,11 and N‐acetylaspartate levels in the motor cortex13,16,17 of patients with ALS have also been observed. However, none of these studies has reported a correlation between such magnetic resonance abnormalities and the degree of disability.8,11,13,16,17
Diffusion‐tensor magnetic resonance imaging (DT MRI) enables the random diffusional motion of water molecules to be measured and thus provides quantitative indices of the structural and orientational features of the central nervous system (CNS).18 DT MRI has been used to assess quantitatively the tissue damage of the brain portion of the corticospinal tracts in ALS,12,19,20,21,22,23 and all studies have shown increased mean diffusivity (indicating a loss of structural barriers limiting the motion of water molecules) and decreased fractional anisotropy (indicating a loss of tissue organisation). However, brain DT MRI studies also resulted in heterogeneous clinicopathological correlations, as some authors found a moderate correlation between brain DT MRI metrics and the severity of disability,12,21,23 but others did not.19 In the past few years, DT MRI has also been used successfully to grade the extent of cervical cord damage associated with demyelinating conditions.24,25,26
Considering that the cervical cord in ALS is one of the most affected portions of the CNS (owing to the combined presence of neuronal loss in the anterior horns of the grey matter and degeneration of the corticospinal tracts), we obtained mean diffusivity and fractional anisotropy histograms of the cervical cord from patients with ALS with the following aims: (1) to quantify the extent of tissue damage in this critical CNS region; and (2) to investigate the magnitude of the correlation of cervical cord DT MRI metrics with patients' disability and tissue damage along the brain portion of the corticospinal tracts.
Patients and methods
In all, 28 patients (16 men and 12 women, mean age 55 (range 27–73) years) with probable or definite ALS27 were recruited consecutively. All patients had a sporadic form of the disease. The median disease duration was 26 (range 6–58) months. Each patient was examined clinically, and a questionnaire for the ALS Functional Rating Scale (ALSFRS) was used to assess disease severity.28 The mean functional score in our patients group was 27 (range 7–38) years. A total of 20 age‐matched and sex‐matched healthy people (11 men and 9 women, mean age 53 (range 28–73) years) served as controls. All the participants gave written informed consent before entry into the study. The study was approved by the local ethics committee.
MRI scans were obtained using a machine operating at 1.5 T (Magnetom Avanto, Siemens, Erlangen, Germany), with a maximum gradient strength of 33 mT/m and a slew rate of 125 mT/m/ms. Using standard matrix head and neck coils, the following pulse sequences were acquired from all participants.
Cervical cord: (a) dual‐echo turbo spin echo (TSE; TR = 2000 ms; TE = 30/145 ms; flip angle = 150°; echo train length (ETL) = 23; field of view (FOV) = 300×300 mm; matrix size = 320×320; 7 sagittal contiguous slices with a thickness of 4 mm); (b) sagittal T1‐weighted three‐dimensional magnetisation‐prepared rapid acquisition gradient echo (TR = 11.6 ms; TE = 4.2 ms; flip angle = 15°; FOV = 300×300 mm; matrix size = 230×230; slice thickness = 0.9 mm); and (c) single‐shot spin SE echo planar imaging (EPI; TR = 2900 ms; TE = 84 ms; flip angle = 90°; FOV = 240×90 mm; matrix size = 128×48; nominal pixel size = 1.87×1.87 mm; interecho spacing = 0.77 ms). Five sagittal slices with a slice thickness of 4 mm and a slice gap of 1.2 mm were acquired. Usually, the three central slices of the slab covered the cord. For each section, diffusion‐weighting gradient pulses were applied in 12 non‐collinear orientations. An additional set of images without diffusion weighting was also obtained. Diffusion measurements were optimised by using only two b factors (b = 0 and 900 s/mm2), as described previously.29 Two acquisitions for each set of diffusion data were performed and averaged after magnitude reconstruction to improve the signal‐to‐noise ratio. Three saturation bands were used, positioned in the anterior part of the neck and transversely at the edges of the FOV in the anterior–posterior direction.
Brain: (a) dual‐echo TSE (TR = 3460 ms; TE = 27/109 ms; flip angle = 150°; ETL = 5; FOV = 250×250 mm; matrix size = 512×512; 35 contiguous axial slices with a thickness of 4 mm). The slices were positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum; (bn) T2‐weighted TSE (TR = 3460 ms; TE = 109 ms; flip angle = 150°; ETL = 13; number of averages = 2; FOV = 240×180 mm; matrix size = 320×240; 24 coronal slices with a thickness of 4 mm and a gap of 1.2 mm between slices); and (c) single‐shot spin EPI with the same acquisition parameters as for the cord, except that there were 18 contiguous axial slices with a slice thickness of 4 mm. These slices were positioned with the same orientation as the dual‐echo scan, with the central slice positioned to match the central slice of the dual‐echo set. For each section, diffusion‐weighting gradient pulses were applied in 12 non‐collinear orientations, with the same orientation scheme and b factors as for the sequence used for the cord. Two acquisitions for each set of diffusion data were performed and averaged after magnitude reconstruction to improve the signal‐to‐noise ratio.
MRI images were transferred to a workstation (Sun Microsystem, Mountain View, California, USA) for postprocessing, which was performed by two experienced observers by consensus, unaware of the participants' identity. Cervical cord and brain DT MRI data were first corrected for eddy current‐induced distortions introduced by diffusion‐weighting gradient pulses, by using an algorithm that maximises mutual information between the diffusion‐unweighted and diffusion‐weighted images.30 Then, the diffusion tensor was calculated for each voxel of spinal cord using linear regression,31 and the eigenvalues and the eigenvectors of the tensor matrix were derived. The eigenvalues were averaged to give the mean diffusivity and used to calculate the fractional anisotropy.32 From cervical cord mean diffusivity and fractional anisotropy maps, the corresponding histograms were produced as described previously.25 Figure 1 shows two illustrative examples of mean diffusivity and fractional anisotropy maps derived from a normal control and a patient with ALS. To correct for the between‐patient difference in cord volume, each histogram was normalised by dividing the height of each histogram bin by the total number of pixels included. Signal intensity artefacts at the edges of the images were carefully excluded and the borders of the segmented tissue were drawn to exclude pixels at the edge of the cord to minimise partial volume averaging. For each histogram, only average mean diffusivity and fractional anisotropy were derived. This was because of the strong correlation existing among all histogram‐derived metrics33; as a consequence, this approach was chosen to minimise the number of comparisons and, therefore, reduce the risk of type I error. Conventional MRI scans of the cervical cord were analysed to assess the presence of areas with increased signal intensity.
Figure 1 Illustrative examples of mean diffusivity (images on the left of each pair) and fractional anisotropy (images on the right of each pair) maps obtained from a healthy volunteer (A) and a patient with amyotrophic lateral sclerosis (B).
The original magnetisation‐prepared rapid acquisition gradient echo data were reformatted, and a set of five contiguous, 3‐mm thick axial slices was reconstructed using the centre of the C2–C3 disc as the caudal landmark. Then, a semiautomated technique was used to segment the cord tissue and to measure the cross‐sectional cord area at the level of each slice.34 Values from the five slices were averaged to obtain a single value for each participant.
Conventional MRIs of the brain were analysed to assess the presence and location (subcortical precentral gyrus, centrum semiovale, posterior limb of the internal capsule, cerebral peduncles, pons and pyramids) of areas with increased signal intensity. On the brain fractional anisotropy and mean diffusivity maps, regions of interest were manually applied along the left and right corticospinal tracts on all axial slices, extending from the pyramids to the top of the internal capsule, as detailed elsewhere.19 All regions of interest were of the same size (17 mm2), and the corticospinal tracts were localised on the basis of a priori anatomical knowledge and reference to relevant literature.35,36 The overall average values of mean diffusivity and fractional anisotropy for the brain corticospinal tracts were entered into the statistical analysis; these values were calculated by averaging the values obtained for the right and left tracts at the four anatomical locations studied (posterior limb of the internal capsule, cerebral peduncles, pons and pyramids).
Student's t test for paired data was used to compare average mean diffusivity and fractional anisotropy values of the cervical cord and the brain corticospinal tracts between controls and ALS. To exclude a potential effect of cord atrophy on cervical cord DT MRI metrics, an analysis of variance model was also run after correction for the mean cord cross‐sectional area. Univariate correlations were assessed using Spearman's rank correlation coefficient.
Results
No abnormalities were seen on the conventional brain and cervical cord MRI scans obtained from controls. On the conventional brain MRI scans, hyperintesities of the corticospinal tract were detected bilaterally from 15 of the 28 (54%) patients with ALS studied. In particular, hyperintense signals were seen in the subcortical precentral gyrus (n = 4), centrum semiovale (n = 11), posterior limb of the internal capsule (n = 13), cerebral peduncles (n = 14), pons (n = 3) and pyramids (n = 3). No macroscopic cervical cord abnormalities were seen on the conventional MRI scans from patients with ALS.
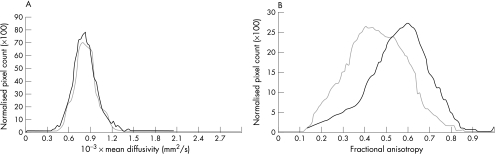
The cross‐sectional cervical cord area was 79.5 (standard deviation (SD) 6.2) mm2 in healthy people and 70.2 (SD 7.9) mm2 in patients with ALS (p<0.001). Table 1 reports the cervical cord DT MRI metrics from ALS and controls. Compared with controls, patients with ALS had significantly lower cervical cord average fractional anisotropy (p = 0.002; after correction for cord cross‐sectional area, this difference remained significant at p<0.05). Figure 2 shows mean diffusivity and fractional anisotropy of the cervical cord from controls and patients with ALS. Average mean diffusivity (0.75 (0.03) v 0.78 (0.04) mm2/s × 103; p = 0.01) and average fractional anisotropy (0.57 (0.03) v 0.49 (0.04); p<0.001) of the brain portion of the corticospinal tracts were different between controls and patients with ALS.
Table 1 Cervical cord mean diffusivity and fractional anisotropy in patients with amyotrophic lateral sclerosis and controls.
| Patients with ALS | Controls | |
|---|---|---|
| MD (mm2/s ×10−3) | 0.88 (0.06) | 0.85 (0.11) |
| FA | 0.48 (0.04) | 0.52 (0.02) |
ALS, amyotrophic lateral sclerosis; FA, fractional anisotropy; MD, mean diffusivity.
Values are mean (SD).
For statistical analysis, see text.
Figure 2 Mean diffusivity (A) and fractional anisotropy (B) of the cervical cord from controls (black line) and patients with amyotrophic lateral sclerosis (grey line).
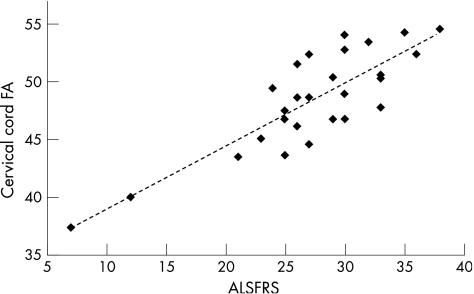
In patients, cord and brain average fractional anisotropy values correlated moderately (r = 0.37, p = 0.05). On the other hand, no significant correlation was found between cord and brain average mean diffusivity values. Cord average fractional anisotropy correlated strongly with the ALSFRS (r = 0.74, p<0.001; fig 3), whereas cord average mean diffusivity (r = –0.17, p = 0.37) and cross‐sectional area (r = –0.007; p = 0.97) did not. A moderate correlation between average fractional anisotropy of the brain corticospinal tracts and ALSFRS was also observed (r = 0.39, p = 0.01).
Figure 3 Scatterplot of the correlation between patients' cervical cord fractional anisotropy (FA) and ALS Functional Rating Scale (ALSFRS).
Discussion
Recent studies have shown that DT MRI can grade cervical cord damage in demyelinating conditions.25,26 In this study, we used DT MRI to: (1) quantify the extent of tissue damage to the cervical cord of patients with ALS; and (2) gain additional insights into the nature of such damage by investigating the relationship with diffusivity measures in the brain portion of the corticospinal tracts. We also assessed the correlation between cord diffusivity changes and the degree of patients' disability as a preliminary step to define new magnetic resonance markers of disease severity. To this end, we selected a relatively large group of patients with probable or definite ALS, who represented the general patient population both in terms of their clinical and conventional MRI characteristics.37,38
The first result of this study was to show that, in comparison with healthy people, patients with ALS have a significantly lower average cord fractional anisotropy. As a fractional anisotropy decrease reflects a loss of fibre bundle directionality,32 this finding indicates the presence of distortion of cord tissue geometry and agrees with previous pathological data showing a pronounced corticospinal tract degeneration in the cord from patients with ALS39 and a reduction in the number of lower motor neurones in the anterior horns of the grey matter.40 Interestingly, we did not find a corresponding increase in average mean diffusivity in our patient sample. This mismatch between mean diffusivity and fractional anisotropy results might be due to glial proliferation, a common finding in ALS,39,40 which in turn is likely to be the result of axonal degeneration and loss of lower motor neurones,40 as supported by the reduction in cervical cord cross‐sectional area that we found in the patients with ALS when compared with controls. Reactive gliosis secondary to tissue loss would lead to a “pseudonormalisation” of mean diffusivity values, which would reduce fractional anisotropy, as glial cells do not have the same anisotropic morphology as the tissue they replace. The presence of cell debris resulting from partially degenerated or disintegrated nerve fibres along the corticospinal tracts41,42 is also likely to contribute to a pseudonormalisation of mean diffusivity and to a reduction in fractional anisotropy. Albeit the presence of cord atrophy (as was the case in this study) increases the likelihood of partial volume effect from the cerebrospinal fluid (CSF), we do not believe that the observed cord fractional anisotropy decrease is influenced a great deal by CSF contamination of pixels at the edge of the cord. This is because of at least three reasons. Firstly, partial volume effect should have influenced mean diffusivity and fractional anisotropy values in concert. Secondly, fractional anisotropy values were also tested after correction for cervical cord cross‐sectional areas and statistical significance was maintained. Finally, contamination from the CSF was further minimised by considering, in the analysis, only pixels away from the edge of the cord.
The second important finding of the study was to show that the loss of fibre coherence in the cord was only moderately related to similar changes occurring in the brain portion of the corticospinal tracts. This suggests a heterogeneous distribution of the various components of the pathological process between the brain and the cord. Previous studies have shown that ALS can cause corticospinal tract damage through a combined action of anterograde degeneration of axons and their myelin sheaths after the loss of pyramidal cortical motor neurones43,44 and disorders of axonal transport,45 both of which result in an axonopathy with a caudocranial evolution. Postmortem46,47,48 and DT MRI19 studies also reported an uneven involvement of the corticospinal tracts with variable patterns of degeneration. Clearly, the hypothesis of a heterogeneous distribution of the process across the CNS cannot be investigated by histopathological studies (most of which are typically performed on end‐stage or near end‐stage disease49) and calls for a longitudinal assessment of the brain and cord diffusivity changes in ALS.
The third and perhaps most intriguing finding of this study was to show a strong correlation between the extent of distortion of cord microstructural geometry and the severity of disability of patients with ALS. Such a correlation might be further improved in the future by the use of high‐field MRI scanners with increased image resolution, which would allow selective segmentation of those parts of the cord (ie, the corticospinal tracts and the anterior portion of the grey matter) that are the targets of the ALS pathological process. Although such an approach was not feasible with the present MRI data set, the correlation we found is of interest considering that optimal MRI quantities reflecting the severity of the clinical manifestations of ALS are still lacking.
The limitations of this study are: firstly, the magnetic resonance scanner used was not equipped with coils allowing parallel imaging to be performed. As this may result in image distortions, we tried to minimise them by using the minimum TE allowed by scanner constraints and by using a rectangular FOV of 37.5%, which enabled us to limit the number of phase‐encoding steps of the k‐space in the phase‐encoding direction. This, together with the high slew rate of the scanner gradients, resulted in the acquisition of DT MR images of good quality (fig 1), with an acquisition time of about 80 s (a short acquisition time is very helpful for patients with disability, such as those with ALS, who might not tolerate long MRI sessions). Secondly, the interslice gap used was high, thus resulting in a relatively coarse image resolution.
Although cord DT MRI longitudinal studies are needed to establish the magnitude of the correlation between anisotropy changes over time and accumulation of irreversible disability, this study suggests that the present DT MRI sequence designed for cervical cord imaging of patients with disability may be a useful adjunctive tool to monitor ALS evolution, either natural or modified by treatment.
Abbreviations
ALS - amyotrophic lateral sclerosis
ALSFRS - ALS Functional Rating scale
CNS - central nervous system
CSF - cerebrospinal fluid
DT MRI - diffusion tensor magnetic resonance imaging
ETL - echo train length
FOV - field of view
TSE - turbo spin echo
Footnotes
Competing interests: None.
References
- 1.Talbot K. Motor neuron disease. Postgrad Med J 200278513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha J A, Reis C, Simoes F.et al Diagnostic investigation and multidisciplinary management in motor neuron disease. J Neurol 20052521435–1447. [DOI] [PubMed] [Google Scholar]
- 3.Goodin D S, Rowley H A, Olney R K. Magnetic resonance imaging in amyotrophic lateral sclerosis. Ann Neurol 198823418–420. [DOI] [PubMed] [Google Scholar]
- 4.Mirowitz S, Sartor K, Gado M.et al Focal signal‐intensity variations in the posterior internal capsule: normal MR findings and distinction from pathologic findings. Radiology . 1989;172535–539. [DOI] [PubMed]
- 5.Oba H, Araki T, Ohtoma K.et al Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology . 1993;189843–846. [DOI] [PubMed]
- 6.Cheung G, Gawel M J, Cooper P W.et al Amyotrophic lateral sclerosis: correlation of clinical and MR imaging findings. Radiology . 1995;194263–270. [DOI] [PubMed]
- 7.Thorpe J W, Moseley I F, Hawkes C H.et al Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry 199661314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato Y, Matsumura K, Kinosada Y.et al Detection of pyramidal tract lesions in amyotrophic lateral sclerosis with magnetization‐transfer measurements. Am J Neuroradiol 1997181541. [PMC free article] [PubMed] [Google Scholar]
- 9.Waragai M. MRI and clinical features in amyotrophic lateral sclerosis. Neuroradiology 199739847–851. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann E, Ochs G, Pelzl A.et al The corticospinal tract in amyotrophic lateral sclerosis: an MRI study. Neuroradiology 19984071–75. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe J L, Vermathen M, Miller R.et al Reduced MTR in the corticospinal tract and normal T2 in amyotrophic lateral sclerosis. Magn Reson Imaging 1998161163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis C M, Simmons A, Jones D K.et al Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999531051–1058. [DOI] [PubMed] [Google Scholar]
- 13.Bowen B C, Pattany P M, Bradley W G.et al MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. Am J Neuroradiol 200021647–658. [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht M J, Fellner F, Fellner C.et al MRI‐FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2‐, T1‐ and proton‐density‐weighted images. J Neurol Sci 200118637–44. [DOI] [PubMed] [Google Scholar]
- 15.Hecht M J, Fellner F, Fellner C.et al Hyperintense and hypointense MRI signals of the precentral gyrus and corticospinal tract in ALS: a follow‐up examination including FLAIR images. J Neurol Sci 200219959–65. [DOI] [PubMed] [Google Scholar]
- 16.Ellis C M, Simmons A, Andrews C.et al A proton magnetic resonance spectroscopic study in ALS: correlation with clinical findings. Neurology 1998511104–1109. [DOI] [PubMed] [Google Scholar]
- 17.Ellis C M, Suckling J, Amaro E., Jret al Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 2001571571–1578. [DOI] [PubMed] [Google Scholar]
- 18.Pierpaoli C, Jezzard P, Basser P.et al Diffusion tensor MR imaging of the human brain. Radiology 1996201637–648. [DOI] [PubMed] [Google Scholar]
- 19.Toosy A T, Werring D J, Orrell R W.et al Diffusion tensor imaging detects cortico‐spinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003741250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe O, Yamada H, Masutani Y.et al Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel‐based analysis. NMR Biomed 200417411–416. [DOI] [PubMed] [Google Scholar]
- 21.Graham J M, Papadakis N, Evans J.et al Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004632111–2119. [DOI] [PubMed] [Google Scholar]
- 22.Sach M, Winkler G, Glauche V.et al Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004127340–350. [DOI] [PubMed] [Google Scholar]
- 23.Cosottini M, Giannelli M, Siciliano G.et al Diffusion‐tensor MR imaging of cortico‐spinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology 2005237258–266. [DOI] [PubMed] [Google Scholar]
- 24.Cercignani M, Horsfield M A, Agosta F.et al Sensitivity‐encoded diffusion tensor MR imaging of the cervical cord. Am J Neuroradiol 2003241254–1256. [PMC free article] [PubMed] [Google Scholar]
- 25.Valsasina P, Rocca M A, Agosta F.et al Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. NeuroImage 200526822–828. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti B, Valsasina P, Judica E.et al Grading cervical cord damage in neuromyelitis optica and MS by diffusion tensor MRI. Neurology 200667161–163. [DOI] [PubMed] [Google Scholar]
- 27.Miller R G, Munsat T L, Swash M.et al Consensus guidelines for the design and implementation of clinical trials in ALS. World Federation of Neurology Committee in Research. J Neurol Sci 19991692–12. [DOI] [PubMed] [Google Scholar]
- 28.The ALS CNTF Treatment Study (ACTS) Phase I‐II Study Group The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. Arch Neurol 199653141–147. [PubMed] [Google Scholar]
- 29.Jones D K, Horsfield M A, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 199942515–525. [PubMed] [Google Scholar]
- 30.Studholme C, Hill D L, Hawkes D J. Automated three‐dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimisation of voxel similarity measures. Med Phys 19972425–35. [DOI] [PubMed] [Google Scholar]
- 31.Basser P J, Mattiello J, Le Bihan D. Estimation of the effective self‐diffusion tensor from the NMR spin‐echo. J Magn Reson 1994103247–254. [DOI] [PubMed] [Google Scholar]
- 32.Pierpaoli C, Basser J. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 199636893–906. [DOI] [PubMed] [Google Scholar]
- 33.Rovaris M, Bozzali M, Santuccio G.et al In vivo assessment of the brain and cervical cord pathology of patients with primary progressive multiple sclerosis. Brain 20011242540–2549. [DOI] [PubMed] [Google Scholar]
- 34.Losseff N A, Webb S L, O'Riordan J I.et al Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996119701–708. [DOI] [PubMed] [Google Scholar]
- 35.Hirayama K, Tsubaki T, Toyokura Y.et al The representation of the pyramidal tract in the internal capsule and basis pedunculi. Neurology 196212337–342. [DOI] [PubMed] [Google Scholar]
- 36. Ross ED. Localization of the pyramidal tract in the internal capsule by whole brain dissection. Neurology 19803059–64. [DOI] [PubMed] [Google Scholar]
- 37.Andersen P M, Borasio G D, Dengler R.et al EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. Eur J Neurol 200512921–938. [DOI] [PubMed] [Google Scholar]
- 38.Kalra S, Arnold D. Neuroimaging in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 20034243–248. [DOI] [PubMed] [Google Scholar]
- 39.Waragai M, Shinotoh H, Hayashi M.et al High signal intensity on T1 weighted MRI of the anterolateral column of the spinal cord in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 19976288–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawyer T, Netsky M G. Amyotrophic lateral sclerosis. A clinicoanatomic study of 53 cases. Arch Neurol Psychiatry 195369171–192. [DOI] [PubMed] [Google Scholar]
- 41.Chou S M. Pathognomy of intraneuronal inclusions in ALS. In: Tsubaki T, Toyokura Y, eds. Amyotrophic lateral sclerosis. Tokyo: University of Tokyo Press, 1979135–176.
- 42.Hirano A, Iwata M. Pathology of motor neurons with special reference to amyotrophic lateral sclerosis and related diseases. In: Tsubaki T, Toyokura Y, eds. Amyotrophic lateral sclerosis. Tokyo: University of Tokyo Press, 1979107–133.
- 43.Lowe J, Lennox G, Leigh P N. Disorders of movement and system degeneration. In: Graham DJ, Lantos PL, eds. Greenfield's neuropathology. vol 2. London: Arnold, 1997281–366.
- 44.Chou S M. Pathology of motor system disorder. In: Leigh PN, Swash M, eds. Motor neuron disease: biology and management. London: Springer‐Verlag, 199553–92.
- 45.Cavanagh J B. The ‘dying back' process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med 1979103659–664. [PubMed] [Google Scholar]
- 46.Bertrand I, Van Bogaert L. La sclerose laterale amyotrophique (anatomie pathologique). Rev Neurol 192525779–806. [Google Scholar]
- 47.Davison C. Amyotrophic lateral sclerosis: origin and extent of upper motor neuron lesion. Neurol Psychiatr 1941461039–1056. [Google Scholar]
- 48.Brownell B, Oppenheimer D R, Hughes J T. The central nervous system in motor neuron disease. J Neurol Neurosurg Psychiatry 197033357–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin J E, Swash M. The pathology of motor neuron disease. In: Leigh PN, Swash M, eds. Motor neuron disease: biology and management. London: Springer‐Verlag, 199593–118.