Acute generalised chorea can be attributed to multiple causes, including non‐ketotic hyperglycaemia. This cause has been associated with characteristic image signs of striatal hyperdensity on CT scan and hyperintensity on T1 weighted (T1W) MRI.
We report a patient presenting with this syndrome in which a postmortem study was conducted. The findings are discussed together with the neuropathological data available in the literature, contributing towards an explanation of the nature of the imaging signs that has remained elusive.
Case report
A 73‐year‐old woman was admitted to our neurological department for acute generalised chorea of 8 days' duration. There was no relevant personal background or family history.
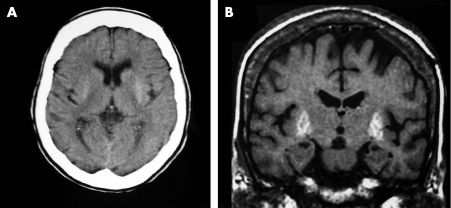
On admission, the patient presented with orofacial dyskinesias and choreic movements in the neck, trunk, upper and lower limbs. The aetiological diagnostic work‐up for acute chorea revealed severe hyperglycaemia on admission (>27.8 mmol/l), bicytopenia with anaemia (erythrocyte count 2.8×106/mm3, haemoglobin 8.1 g/dl) and thrombocytopenia (104 000/μl), and an isolated antiphospholipid antibody positive titre. The remaining investigation for acute chorea was normal. The imaging studies revealed a spontaneous bilateral hyperdensity in the putamen and caudate nuclei on the admission brain CT scan. The brain MRI (1.5T; Signa Horizon, General Electrics Medical Systems, Milwaukee, Wisconsin, USA), conducted 2 weeks after admission, showed a bilateral putaminal hyperintensity in T1W images exclusively (fig 1).
Figure 1 Image findings. (A) CT brain scan on admission—bilateral hyperdense putamen and caudate nuclei. (b) MRI brain scan conducted 2 weeks after admission—bilateral hyperintense putamen on T1W images.
Chorea persisted beyond glycaemia normalisation. The patient eventually died 32 days after admission as a result of unresolved sepsis, having begun with fever 4 days after admission. A postmortem examination was conducted.
In the neuropathological study, paraffin embedded representative sections of the left hemisphere, brainstem and cerebellum were stained with haematoxylin–eosin, Bodian–Luxol, Perls and Van Gieson stains. The basal ganglia region was studied using anterior and posterior coronal sections. Microscopic examination revealed generalised wall fibrosis of the small perforating arteries associated with dilatation of the perivascular spaces of the deep white matter. Multiple lacunes in the basal ganglia and thalamus were found in association with macrophage proliferation. Astrocytic gliosis and extravascular hemosiderin deposits together with ferruginateous deposits on perforating vessels were observed in the posterior zone of the putamen. No vascular amyloid or calcium deposits were observed.
Discussion
In our case, the triad of acute chorea, non‐ketotic hyperglycaemia and a hyperdense and hyperintense putamen on CT and T1W MRI was documented. The bicytopenia and an antiphospholipid antibody positive titre could support an autoimmune aetiology in the form of a secondary antiphospholipid syndrome caused by infection. However, no prothrombotic state was documented in the clinical and laboratory data and the neuropathological data provided no evidence of disseminated intravascular coagulation. In addition, patient age did not favour a primary autoimmune aetiology.
Regarding the signal changes observed on imaging studies, a critical appraisal of the published case reports with neuropathological results3,4,5 emphasises the heterogeneity of the available data in terms of the time delay in neuropathological specimen collection, neuropathological findings, timing of imaging studies, characteristics of basal ganglia findings and the presence of concurrent relevant lesions. In two reported cases,4,5 an association was postulated between the presence of reactive astrocytes and MRI changes. However, in the former case no significant hyperglycaemia was documented and an additional area of temporoparietal infarction was identified which could be associated with chorea onset. In the latter case, the findings were not observed in other regions with an identical signal alternation, namely the pallidum. In a third report,3 calcium deposits and focal micro‐haemorrhages were found in the lesioned putamen and caudate where a confluent area of infarction was also observed. Although calcium was observed only on non‐recent infarction areas, an association with the signal changes on CT and MRI was postulated. In addition, signal changes on MRI were localised to the anterior putamen only and did not involve the putamen more extensively, as usually observed in this syndrome.
Studies focusing on CT and MRI findings have also been inconclusive. Chu et al1 conducted a gradient echo and diffusion weighted MRI study and suggested that signal changes corresponded to cytotoxic oedema. Conversely, another study2 involving similar imaging procedures drew the same conclusions as our report. Nevertheless, both studies did not have access to neuropathology data which would confirm the accuracy of the imaging interpretation.
In our case, neuropathological findings were consistent with small previous haemorrhages in the striatum. This favours the hypothesis of petechial haemorrhages as the cause of this syndrome, suggested to be secondary to erythrocyte diapedesis due to hyperglycaemia induced blood–brain barrier dysfunction.6 The observed vessel wall changes were consistent with a diabetes vasculopathy, which also provides an explanation for brain barrier dysfunction. Thus the initial CT changes correspond to blood and the later MRI findings to the presence of hemosiderin. Because of the transitory nature of this syndrome, a careful analysis of the reported cases, namely the timing for image and neuropathological data collection, is essential to fully understand its aetiology. Using other MRI sequences such as gradient echo or diffusion weighted imaging will further help in characterising image–neuropathology correlations.
References
- 1.Chu K, Kang D W, Kim D E.et al Diffusion‐weighted and gradient echo magnetic resonance findings of hemichorea–hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol 200259448–452. [DOI] [PubMed] [Google Scholar]
- 2.Lai S L, Tseng Y L, Hsu M C.et al Magnetic resonance imaging and single‐photon emission computed tomography changes in hypoglycemia‐induced chorea. Mov Disord 200419475–478. [DOI] [PubMed] [Google Scholar]
- 3.Nath J, Jambhekar K, Rao C.et al Radiological and pathological changes in hemiballism–hemichorea with striatal hyperintensity. J Magn Reson Imaging 200623564–568. [DOI] [PubMed] [Google Scholar]
- 4.Ohara S, Nakagawa S, Tabata K.et al Hemiballism with hyperglycemia and striatal T1‐MRI hyperintensity: an autopsy report. Mov Disord 200116521–525. [DOI] [PubMed] [Google Scholar]
- 5.Shan D E, Ho D M, Chang C.et al Hemichorea–hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol 199819863–870. [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata A, Koike F, Arasaki K.et al Blood brain barrier destruction in hyperglycemic chorea in a patient with poorly controlled diatetes. J Neurol Sci 199916390–93. [DOI] [PubMed] [Google Scholar]