Abstract
Objective
To identify the proportion of patients in a large idiopathic normal pressure hydrocephalus (INPH) cohort with large head circumference (HC) who presumably have congenital hydrocephalus that has not become clinically apparent until late in life.
Methods
HC was measured in 158 patients diagnosed with communicating INPH and assigned to HC centile range adjusted for height and sex. The proportion of patients with INPH and HC above the 97th, 90th or 50th centiles was compared with the proportion expected in a normal population.
Results
The proportion of patients with HC >90th centile was significantly larger than would be expected in a normal distribution (19.6% vs 10%, p = 0.0001), as was the proportion of patients with HC >97th centile (8.9% vs 3%, p = 0.0001). The relative association between INPH and HC >97th centile was nearly tripled (relative association 2.95; CI 1.36 to 6.41), but the relative association between INPH and HC >50th centile was not significantly higher than predicted (relative association 1.13; CI 0.95 to 1.34).
Conclusion
A significantly larger proportion of elderly adults with INPH have a HC greater than the 90th or 97th centile than predicted by population norms, supporting the concept that compensated congenital hydrocephalus that does not become symptomatic until late in life is one aetiology of INPH, but is not responsible for all INPH. The mechanism that leads to the development of INPH in most patients remains elusive.
Idiopathic normal pressure hydrocephalus (INPH) in older people can lead to a disabling syndrome affecting gait and balance, cognition and urinary continence.1 Although INPH is usually considered an acquired form of hydrocephalus because the onset of symptoms is not until late in life, there is evidence that a subset of patients with INPH may actually have congenital hydrocephalus or a history of benign external hydrocephalus (BEH) of childhood leading to moderate ventriculomegaly. In 1989, Graff‐Radford observed that several patients with INPH had a large head circumference (HC), suggesting they had asymptomatic congenital communicating hydrocephalus that became symptomatic with ageing.2 A larger study of patients with clinically diagnosed INPH also demonstrated disproportionate numbers with a large HC.3 Based on the finding that patients with clinically suspected INPH have an average intracranial volume greater than that of controls, it has been proposed that BEH is the precursor to INPH for all patients.4,5
We previously found that 15% of patients with the syndrome of hydrocephalus in young and middle‐aged adults (SHYMA) had congenital hydrocephalus, defined as HC >97th centile; however, 65% of patients with SHYMA had obstructive hydrocephalus, which INPH is typically considered not to include, and thus the SHYMA data cannot be extrapolated to the INPH population.6 We have diagnosed INPH for over 10 years using a protocol of spinal catheter insertion for controlled cerebrospinal fluid (CSF) drainage, CSF pressure monitoring, or both, which is considered the most accurate prognostic test by the INPH Consensus Guidelines,7,8 and we routinely measure HC in our patients. The purpose of this study is to identify the proportion of patients with a large HC in a INPH cohort who presumably have congenital hydrocephalus that has not become clinically apparent until late in life.
Methods
We retrospectively reviewed the records of all patients who (1) presented at age ⩾66 years for initial evaluation of INPH at the Johns Hopkins Adult Hydrocephalus Program; (2) were seen in our clinic between November 1999 and October 2005; and (3) were diagnosed with INPH according to a standard protocol that includes assessing clinical response to CSF removal via lumbar puncture or continuous CSF drainage via spinal catheter.1 The study was approved by the institutional review board.
We excluded patients with obstructive hydrocephalus, secondary hydrocephalus (eg, history of meningitis, encephalitis, subarachnoid or intracerebral haemorrhage, or traumatic brain injury), and congenital skull malformations or disorders associated with enlarged HC (eg, neurofibromatosis).
HC was measured to the nearest 0.5 cm with a tape measure placed around the head over the glabella and the occipital protuberance. All HC data were collected by the same physician (MAW) using the same tape measure. Maximum adult height, to account for loss of height secondary to osteoporosis, was reported by the patient or family to the nearest ½ inch, which we then converted to centimetres.
HC centile range was determined using an adult HC chart that incorporates sex, height and HC.9 We used a HC chart specifically created for adults because HC continues to increase until age 20–25 years and paediatric HC charts end at age 18 years.10
Statistical analysis
Data were analysed using STATA 9. We compared the expected and actual proportion of patients with HC greater than the 50th, 90th and 97th centiles by using the one‐sample test of proportions. We also calculated the relative risk that a patient with INPH also has a HC above the 50th, 90th or 97th centile. For this paper, we use the term relative association rather than relative risk because it is not necessarily a pathological condition to have HC above average.
Results
Table 1 shows the demographic data for the 158 patients who fitted the inclusion criteria. Because the published norms for adult HC were based on a Caucasian population sample,9 we performed two analyses of the cohort: (1) the entire cohort, including 150 Caucasians, 6 African‐Americans, 1 native American and 1 Asian, and (2) the subset of 150 Caucasian patients only.
Table 1 Demographic characteristics for the entire idiopathic normal pressure hydrocephalus cohort and Caucasian subset of patients.
| Cohort | No of patients | Men | Women | Age range (years) | Average age at presentation (years) | Average age of symptom onset (years) |
|---|---|---|---|---|---|---|
| Entire | 158 | 93 (59%) | 65 (41%) | 66–91 | 76 (5.2) | 72 (5.1) |
| Caucasian subset | 150 | 89 (59%) | 61 (41%) | 66–91 | 76 (5.1) | 73 (5.1) |
Numbers in parentheses are SD.
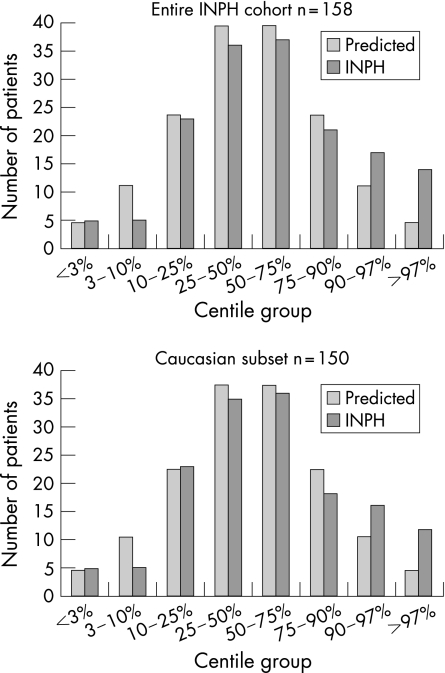
Histograms of the entire cohort and the Caucasian subset (fig 1), and quantile–normal plots (not shown) demonstrate correspondence between the HC data and a normal distribution, except for a disproportionate number of patients in the largest two centile ranges (>90% and >97%). The proportion of patients with HC >90th centile is significantly larger than would be expected in a normal distribution for both the entire cohort (19.6% vs 10%, p = 0.0001) and the Caucasian subset (18.6% vs 10%, p = 0.0004) (table 2). Similarly, the proportion of patients with HC >97th centile is significantly larger than would be expected in a normal distribution for both the entire cohort (8.9% vs 3%, p = 0.0001), and the Caucasian subset (8% vs 3%, p = 0.0003).
Figure 1 Histograms demonstrating the distribution of head circumference in eight centile groups for patients with idiopathic normal pressure hydrocephalus (INPH) compared with the predicted normal distribution.
Table 2 Proportion and relative association of head circumference above the 50th, 90th and 97th centiles.
| HC | Entire cohort | Caucasian subset | Entire cohort | Caucasian subset | ||||
|---|---|---|---|---|---|---|---|---|
| Proportion | p value | Proportion | p value | Relative association of large HC | CI | Relative association of large HC | CI | |
| >50th centile | 56% | 0.1315 | 55% | 0.2207 | 1.13 | 0.95 to 1.34 | 1.09 | 0.91 to 1.31 |
| >90th centile | 19.6% | 0.0001 | 18.6% | 0.0004 | 1.96 | 1.26 to 3.06 | 1.87 | 1.18 to 2.95 |
| >97th centile | 8.9% | 0.0001 | 8% | 0.0003 | 2.95 | 1.36 to 6.41 | 2.67 | 1.19 to 5.95 |
HC, head circumference.
The relative association between INPH and HC >97th centile was nearly tripled for both the entire cohort (relative association 2.95; CI 1.36 to 6.41) and the Caucasian subset (relative association 2.67; CI 1.19 to 5.95), but the relative association between INPH and HC >50th centile was not significantly higher than predicted (relative association 1.13; CI 0.95 to 1.34) for the entire cohort (table 2).
Discussion
Our results show that there is a greater proportion of patients with INPH with HC above the 90th and 97th centiles than expected; however, the remainder of the HC distribution curve for the patients with INPH matches the expected normal distribution of HC in adults, and is not significantly shifted towards larger‐than‐average HC. Our findings are consistent with the concept that there is a subset of patients with INPH who most likely have asymptomatic congenital hydrocephalus and do not become symptomatic until late in life. This is the largest study of HC in patients with INPH, and the first to evaluate a cohort of patients diagnosed using methods consistent with the INPH Consensus Guidelines.
HC measurement became a routine monitoring tool for infants and children in the 1940s, and had become a standard part of the well‐child examination by 1969.11 It is well accepted that head enlargement due to hydrocephalus occurs only during infancy until the cranial sutures close. The tracking of HC gained importance during the 1950s after shunt surgery became an effective treatment for hydrocephalus, as head enlargement was an important clinical manifestation of developing hydrocephalus that was then confirmed by transillumination of the skull, skull x rays, pneumo‐encephalography, or percutaneous aspiration of subdural or ventricular fluid (intracranial tapping).11,12 With the introduction of CT of the head in the 1970s, and MRI in the 1980s, it became easier in the US and other parts of the developed world to confirm the diagnosis of hydrocephalus and assess the need for shunt surgery for children whose HC was either above the 97th centile or found to be enlarging.
Because the patients in our study were born between 1908 and 1937, before HC measurement for children was routine, it is unlikely that they would have been found to have a large HC as children. Even if their physicians had noted a large HC on physical examination, such children would most likely not have been treated if they were asymptomatic.
The relationship between large HC (or macrocephaly), congenital hydrocephalus and INPH has been the subject of speculation. BEH, the most common cause of childhood macrocephaly and mild ventriculomegaly, has been proposed as the precursor of INPH, as an MRI study found that the intracranial volumes of 22 men and 29 women with suspected normal pressure hydrocephalus were significantly larger than those of 110 consecutive age‐ and sex‐matched controls.4 Bradley et al5 hypothesised that INPH is a “two‐hit” disease, starting with BEH. In their hypothesis, early increased resistance to CSF resorption leads to dependence on CSF flow through the extracellular space of the brain parenchyma to achieve normal CSF resorptive capacity. Decades later, changes to the brain parenchyma associated with deep white matter ischaemia, which occurs more frequently in INPH than in the normal population, compromises the extracellular CSF pathways, leading to insufficient CSF resorptive capacity, further ventricular enlargement and finally symptomatic INPH.13,14
Although Bradley and colleagues5 propose that congenital hydrocephalus (ie, BEH) is the “first hit” of the two‐hit mechanism responsible for all INPH, our results suggest that congenital hydrocephalus could serve as the first hit mechanism in, at most, 19.6% of our INPH cohort with HC >90th centile. The remainder of the cohort with normal HC presumably has acquired hydrocephalus rather than congenital hydrocephalus. Therefore, the mechanism by which INPH develops in most patients remains elusive. Possibilities include (1) altered CSF production, circulation or resorption either following long‐forgotten events associated with subarachnoid haemorrhage or inflammation, such as concussion or viral meningitis8 or (2) susceptibility to comorbid pathology such as hypertension and white matter disease.13,14
The proportion of older adults with symptoms of INPH and large HC might be greater than the results in our study, as our selection criteria specifically excluded obstructive hydrocephalus. Indeed, we have encountered such patients who would represent either late‐onset idiopathic aqueductal stenosis (LIAS) or longstanding overt ventriculomegaly in adults. Partial or compensated obstructive hydrocephalus may also serve as the first hit in a two‐hit model of symptomatic hydrocephalus developing in adulthood. Patients with LIAS have no overt symptoms until they develop headaches (usually in early adulthood) or INPH symptoms (usually in late adulthood.)15 Longstanding overt ventriculomegaly in adults, a form of LIAS with macrocephaly and radiological or clinical evidence of long‐lasting increased intracranial pressure, may also present as a shunt‐responsive adult syndrome of headaches or as the normal pressure hydrocephalus triad.16,17
The long delay between early HC enlargement due to congenital communication or obstructive hydrocephalus and mid‐life headaches or late‐life INPH symptoms implies that the ability of the brain and the CSF circulatory system to compensate for hydrocephalus may be adequate for decades.6,15,16 The mechanisms underlying such longstanding compensation and subsequent decompensation at the neuronal and glial levels are poorly understood. In experimental models with young animals, the evolution of symptomatic hydrocephalus is associated with destruction of periventricular white matter, and treatment with a shunt correlates with improved cortical connectivity via periventricular white matter tracts.18,19 Whether clinically normal patients with compensated hydrocephalus have normal neuronal and glial function and cortical connectivity is uncertain, as it is possible that they have subtle, unrecognised impairment.
There are other potential causes of a large HC in infancy that would persist through adult life. Benign hereditary macrocephaly is not associated with hydrocephalus and, because it is a normal variant, would not be expected to be so prevalent as to alter the shape of the normal HC distribution curve. We excluded one patient with neurofibromatosis, which is associated with macrocephaly.20 On the other hand, not all children with congenital hydrocephalus develop HC >90th centile.21,22 Therefore, it is conceivable that a portion of patients with INPH and normal HC in our cohort also had unrecognised congenital hydrocephalus; however, in the absence of neuroimaging from early childhood, it would be virtually impossible to detect these patients on the basis of clinical evaluation and neuroimaging in adulthood.23 Interestingly, Alzheimer's disease has a well‐established association with smaller than average HC.14,24,25
In summary, we find that a significantly larger than expected proportion of elderly adults with INPH has a HC greater than the 90th and 97th centiles in comparison to population norms, supporting the concept that compensated congenital hydrocephalus is one aetiology of INPH that does not become symptomatic until late in life. The fact that the remainder of the HC distribution curve in INPH is normal suggests that the underlying aetiologies for most INPH are not acquired in infancy, but rather after closure of the cranial sutures—that is, in childhood, adolescence or adulthood. Given the standard use of HC measurement in children and the widespread use of CT and MRI, the proportion of patients with congenital hydrocephalus who are not identified until they develop INPH symptoms will diminish over time, because their hydrocephalus is likely to be identified early in life even if asymptomatic. It remains to be determined how best to monitor and decide when to treat children and adults with incidentally discovered hydrocephalus to prevent irreversible neurological impairment while simultaneously avoiding premature treatment.
Abbreviations
BEH - benign external hydrocephalus
CSF - cerebrospinal fluid
HC - head circumference
INPH - idiopathic normal pressure hydrocephalus
LIAS - late‐onset idiopathic aqueductal stenosis
SHYMA - syndrome of hydrocephalus in young and middle‐aged adults
Footnotes
Funding: None.
Competing interests: RKW has no personal or institutional financial interest in drugs, materials or devices described in this report. In the 12 months prior to the submission of this manuscript, MAW has received honoraria from Medtronic for speaking on the topic of adult hydrocephalus. The Johns Hopkins Adult Hydrocephalus Program has received support from Medtronic for research related to adult hydrocephalus, but not related to the topic of this manuscript.
References
- 1.McGirt M J, Woodworth G, Coon A L.et al Diagnosis, treatment, and analysis of long‐term outcomes in idiopathic normal‐pressure hydrocephalus. Neurosurgery 200557699–705. [DOI] [PubMed] [Google Scholar]
- 2.Graff‐Radford N R, Godersky J C. Symptomatic congenital hydrocephalus in the elderly simulating normal pressure hydrocephalus. Neurology 1989391596–1600. [DOI] [PubMed] [Google Scholar]
- 3.Krefft T A, Graff‐Radford N R, Lucas J A.et al Normal pressure hydrocephalus and large head size. Alzheimer Dis Assoc Disord 20041835–37. [DOI] [PubMed] [Google Scholar]
- 4.Bradley W G, Safar F G, Furtado C.et al Increased intracranial volume: a clue to the etiology of idiopathic normal‐pressure hydrocephalus? Am J Neuroradiol 2004251479–1484. [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley W G, Jr, Bahl G, Alksne J F. Idiopathic normal pressure hydrocephalus may be a “two hit” disease: benign external hydrocephalus in infancy followed by deep white matter ischemia in late adulthood. J Magn Reson Imaging 200624747–755. [DOI] [PubMed] [Google Scholar]
- 6.Cowan J A, McGirt M J, Woodworth G.et al The syndrome of hydrocephalus in young and middle‐aged adults (SHYMA). Neurol Res 200527540–547. [DOI] [PubMed] [Google Scholar]
- 7.Marmarou A, Bergsneider M, Klinge P.et al The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal‐pressure hydrocephalus. Neurosurgery 200557S17–28 discussion iiv. [DOI] [PubMed] [Google Scholar]
- 8.Relkin N, Marmarou A, Klinge P.et al Diagnosing idiopathic normal‐pressure hydrocephalus. Neurosurgery 200557S4–16. [DOI] [PubMed] [Google Scholar]
- 9.Bushby K M, Cole T, Matthews J N.et al Centiles for adult head circumference. Arch Dis Child 1992671286–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichorn D H, Bayley N. Growth in head circumference from birth through young adulthood. Child Dev 196233257–271. [DOI] [PubMed] [Google Scholar]
- 11.McLaurin R L. Diagnostic study of suspected hydrocephalus. JAMA 19692081359–1364. [PubMed] [Google Scholar]
- 12.Drake J M, Kestle J R, Tuli S. CSF shunts 50 years on—past, present and future. Childs Nerv Syst 200016800–804. [DOI] [PubMed] [Google Scholar]
- 13.Krauss J K, Regel J P, Vach W.et al Vascular risk factors and arteriosclerotic disease in idiopathic normal‐pressure hydrocephalus of the elderly. Stroke 19962724–29. [DOI] [PubMed] [Google Scholar]
- 14.Bech‐Azeddine R, Hogh P, Juhler M.et al Idiopathic normal pressure hydrocephalus: clinical comorbidity correlated to cerebral biopsy findings and outcome of CSF shunting. J Neurol Neurosurg Psychiatry 200778157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuhara T, Luciano M G. Clinical features of late‐onset idiopathic aqueductal stenosis. Surg Neurol 200155132–6 discussion 67. [DOI] [PubMed] [Google Scholar]
- 16.Kiefer M, Eymann R, Strowitzki M.et al Gravitational shunts in longstanding overt ventriculomegaly in adults. Neurosurgery 200557109–19 discussion 19. [DOI] [PubMed] [Google Scholar]
- 17.Oi S, Shimoda M, Shibata M.et al Pathophysiology of long‐standing overt ventriculomegaly in adults. J Neurosurg 200092933–940. [DOI] [PubMed] [Google Scholar]
- 18.Eskandari R, McAllister JP I I, Miller J M.et al Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J Neurosurg 2004101196–210. [DOI] [PubMed] [Google Scholar]
- 19.Del Bigio M R, Wilson M J, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 200353337–346. [DOI] [PubMed] [Google Scholar]
- 20.Bale S J, Amos C I, Parry D M.et al Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet 199140206–210. [DOI] [PubMed] [Google Scholar]
- 21.Medina L S, Frawley K, Zurakowski D.et al Children with macrocrania: clinical and imaging predictors of disorders requiring surgery. Am J Neuroradiol 200122564–570. [PMC free article] [PubMed] [Google Scholar]
- 22.Maytal J, Alvarez L A, Elkin C M.et al External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. Am J Roentgenol 19871481223–1230. [DOI] [PubMed] [Google Scholar]
- 23.Prassopoulos P, Cavouras D, Golfinopoulos S.et al The size of the intra‐ and extraventricular cerebrospinal fluid compartments in children with idiopathic benign widening of the frontal subarachnoid space. Neuroradiology 199537418–421. [DOI] [PubMed] [Google Scholar]
- 24.Golomb J, Wisoff J, Miller D C.et al Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry 200068778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortimer J A, Snowdon D A, Markesbery W R. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol 200325671–679. [DOI] [PubMed] [Google Scholar]