Abstract
Background
Familial hemiplegic migraine (FHM) is an unusual migraine syndrome characterised by recurrent transient attacks of unilateral weakness or paralysis as part of the migraine aura. Genetically and clinically heterogeneous, FHM1 is caused by mutations in CACNA1A and FHM2 by mutations in ATP1A2.
Aim
Three children with prolonged hemiplegia were tested for mutations in CACNA1A or ATP1A2.
Methods
Mutations in CACNA1A and ATP1A2 were screened for by denaturing high performance liquid chromatography and confirmed by sequencing. Expression studies were performed to characterise the functional consequences of these mutations.
Results
No mutation was found in the FHM1 gene while three mutations were identified in the FHM2 gene. All three mutations were missense: two were novel and one was de novo; none was found in controls. Functional studies in HeLa cells showed complete loss of mutant pump function without interfering with the wild‐type pump, consistent with haploinsufficiency.
Conclusion
We identified novel disease causing mutations in the FHM2 gene. Genetic screening for FHM should be considered in a child with prolonged hemiplegia even if there is no prior history or family history of migraine or hemiplegic episodes.
Familial hemiplegic migraine (FHM) is a rare form of migraine with aura, typically inherited in an autosomal dominant fashion although sporadic cases have also been observed. A typical attack of FHM begins with unilateral paresthesia and weakness lasting from 30 to 60 min, followed by a severe pulsatile headache (often contralateral) lasting several hours. Other neurological symptoms may accompany the hemiparesis, including hemianoptic field defects, aphasia, confusion and even coma. Some patients with FHM have brainstem and cerebellar symptoms, and there appears to be an overlap between the clinical features of FHM and basilar migraine.1
Genetic heterogeneity has clearly been documented for FHM.2 Mutations in three genes have been identified in families with FHM: CACNA1A (FHM1 OMIM 141500), encoding a neuronal calcium channel subunit3; ATP1A2 (FHM2; MIM 602481), encoding a catalytic subunit of a sodium potassium ATPase4; and SCN1A (FHM3; MIM 609634), encoding a neuronal sodium channel subunit,5 previously shown to be involved in rare epilepsy syndromes.6,7 These genes code for transmembrane ion channels and ion pumps heavily expressed in the brain. Mutations in CACNA1A also cause episodic ataxia type 2 (EA2; MIM 108500)3 and most families with hemiplegic migraine caused by mutation in CACNA1A also have ataxia and interictal nystagmus.8 Families with mutations in ATP1A2 and SCN1A often have seizures in addition to hemiplegic migraine.4,5
In this report, we describe three children with mutations in ATP1A2 who presented with prolonged hemiplegic episodes lasting more than a week. In two of the children, this was their first episode of hemiplegia. The third child had prior, more typical episodes of hemiplegic migraine (lasting about 30 min), but had no family history of FHM. In this case, we were able to document that the mutation was de novo as neither parent harboured the mutation.
Patients and methods
Patients
Each child was hospitalised with a prolonged severe attack of hemiplegia. Clinical details are summarised in table 1.
Table 1 Clinical and genetic features of probands with mutations in ATP1A2.
| Patient No | Mutation | Duration of severe hemiplegia (days) | Associated symptoms | Trigger | Ictal MRI | Ictal EEG | Baseline exam | Family member affected |
|---|---|---|---|---|---|---|---|---|
| 1 | A606T | 5 | Headache | unknown | Normal | Unilateral slowing | Normal | Mother |
| 2 | N717K | 7 | Headache, confusion | Viral gastroenteritis | ⇑ DWI/ ⇓ ADC | Unilateral slowing | Normal | None |
| 3 | R1002Q | 10 | Headache, aphasia | Mild head trauma | Normal | Unilateral slowing | Normal | Father |
ADC, apparent diffusion coefficient; DWI, diffusion weighted imaging.
Patient No 1
A 9‐year‐old girl developed left‐sided numbness and paralysis after 2 days of a severe right‐sided headache. The hemiparesis was dense for 5 days and then gradually resolved over the next few days. During hospitalisation, a lumbar puncture, brain MRI and magnetic resonance angiography were normal. An EEG showed slow background activity in the right hemisphere. The child had no previous episodes of hemiparesis but her mother reported episodes of hemiparesis lasting from 30 min to an hour, accompanied by headache and vomiting, since the age of 32 years.
Patient No 2
A 7‐year‐old boy developed headache, somnolence, confusion and left hemiplegia shortly after contracting a viral gastroenteritis, manifested by nausea, vomiting and diarrhoea. Lumbar puncture and an initial brain MRI were normal but a follow‐up MRI scan 2 days later showed increased signal intensity on diffusion weighted imaging and decreased signal intensity on apparent diffusion coefficient mapping in the right parietal region, sparing the cortex. He remained densely hemiplegic for 7 days but the hemiplegia gradually resolved over the next few weeks. Six days after the onset of hemiplegia, he had a generalised tonic–clonic seizure that was treated with phenytoin. The child had a prior history of episodes of more typical hemiplegic migraine, lasting about 30 min followed by unilateral headache, often induced by minor head trauma. There was no family history of hemiplegic migraine.
Patient 3
A 10‐year‐old girl struck her head while skating without losing consciousness. She developed headache a few hours later and, 2–3 hours after that, she developed right‐sided numbness and hemiplegia, along with a global aphasia. Her symptoms persisted for 10 days. The weakness resolved completely in 3 weeks but it took 7 weeks for language to return to normal. Brain MRI on days 2 and 10 were normal. An EEG showed background slowing on the left that gradually normalised over several weeks. Her father had typical episodes of hemiplegic migraine beginning at the age of 7 years and also had episodes of visual aura without headache. He had at least one seizure, for which he was treated with phenobarbital for five years. Subsequently, he has remained seizure‐free without medication.
Mutation screening and identification
Informed consent was obtained from the probands (all Caucasians) and their parents in accordance with Institutional Review Board procedures. DNA was extracted from peripheral blood by standard techniques. All exons and flanking introns of CACNA1A and ATP1A2 were amplified from each proband. We used denaturing high performance liquid chromatography to screen for polymorphisms.9 Direct sequencing of amplicons with unusual elution profiles was performed to identify specific nucleotide changes. All mutations and determination of cosegregation in relatives were confirmed by restriction fragment length polymorphisms from separate PCR reactions. DNA samples from 100 Caucasian adults of both sexes, who did not suffer from migraine, were screened via restriction fragment length polymorphisms to check for the presence of each mutation.
All mutations were described according to the HGVS mutation nomenclature (http://www.hgvs.org/mutnomen/) for cDNA ATP1A2 GenBank NM000702.2.
Mutagenesis
Full length wild‐type ATP1A2 cDNA clones with Q116R and N127D to confer ouabain resistance and wild‐type ATP1A2 and ATP1B2 cDNA clones in mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, California, USA) were generous gifts from G Casari (Human Molecular Genetics Unit, Dibit‐Dan Raffaele Scientific Institute, Milan, Italy). Each mutation was introduced into the ouabain resistant wild‐type ATP1A2 cDNA clone using QuikChange (Stratagene, La Jolla, California, USA) and confirmed by bidirectional sequencing.
Functional studies
ATP1A2 and ATP1B2 constructs required for functional ion pump formation were cotransfected by Lipofectamine 2000 (Invitrogen) in human HeLa cells.4 One day post‐transfection, cells were treated with 1 μM ouabain (Sigma, St Louis, Missouri, USA) to block endogenous ion pump activity.4,10 Cell viability was quantified using the 3‐(4,5‐dimethylthiazol‐2yl)‐2,5‐diphenyltetrazolium bromide reduction assay at 24, 48 and 72 h post‐ouabain challenge.10
Results
Three distinct mutations in ATP1A2 were detected: A606T in patient No 1, N717K in patient No 2 and R1002Q in patient No 3 (table 1, fig 1A). All three were missense mutations affecting amino acids highly conserved in ion pumps in human and other species (fig 1A). In patient Nos 1 and 3, the affected parent also had the mutation and the unaffected parent did not have the mutation. In patient No 2 with confirmed paternity, we found that neither parent had the mutation, demonstrating that the mutation arose de novo. Screens of CACNA1A were normal in all three probands.
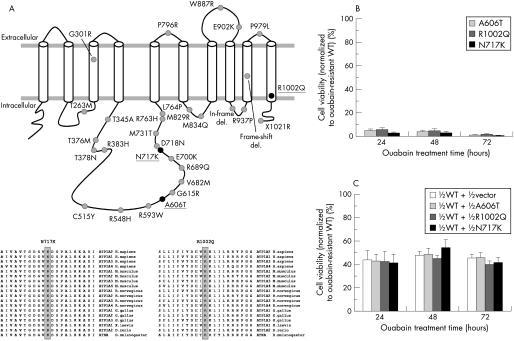
Figure 1 (A) Mutations causing prolonged hemiplegia in three children, indicated by black circles on the ATP1A2 encoded Na+/K+‐ATPase membrane topology. The predicted structure consists of 10 transmembrane segments with a large cytoplasmic loop connecting the 4th and 5th transmembrane segments. The grey circles represent previously reported FHM2 mutations. Shown on the bottom are amino acid alignments spanning the two novel mutations from related proteins in human and other organisms. (B) Viability of cells transfected with the mutant ATP1A2 cDNAs, normalised to the viability of cells transfected with the ouabain resistant wild‐type ATP1A2 cDNA. Untransfected cells, cells transfected with the vector and cells transfected with the wild‐type ouabain sensitive ATP1A2 cDNA were tested as controls, all with barely detectable cell viability (data not shown). Three independent experiments were performed, each with triplicate measurements. (C) Viability of cells transfected with equimolar mutant and the ouabain resistant wild‐type ATP1A2 cDNAs to simulate the heterozygous state in patients. Results were normalised to cells transfected with twice the amount of ouabain resistant wild‐type ATP1A2 cDNA used in the experiments. Three independent experiments were performed; each with triplicate measurements.
HeLa cells transfected with ouabain resistant wild‐type ATP1A2 thrived in ouabain, yet cells transfected with the mutant constructs did not survive treatment with ouabain (fig 1B), supporting the hypothesis that all three mutations resulted in loss of function. Simulated heterozygous states obtained by co‐transfecting equal amounts of wild‐type and mutant constructs exhibited intermediate behaviour, suggesting haploinsufficiency (fig 1C).
Discussion
We identified three distinct ATP1A2 mutations in three children hospitalised for a prolonged attack of hemiplegia. All three mutations altered highly conserved amino acid residues, and all resulted in loss of function of the sodium–potassium pump when expressed in cell culture. The mutation A606T has been previously reported in a Swiss German family with FHM2,11 and the other two, N717K and R1002Q, were novel. In one child, we were able to document that the mutation N717K arose spontaneously.
Although episodes of hemiplegic migraine typically last from 30 to 60 min, patients with more prolonged episodes have been reported with both FHM18 and FHM2. The diagnosis of FHM typically rests on identifying a prior history of typical attacks or a positive family history of FHM. In two of our patients, the initial hemiplegic episode was prolonged, suggesting a possible stroke, and in the third patient there was no family history of hemiplegic migraine. As noted earlier, FHM1 is typically associated with cerebellar findings on examination while FHM2 is often associated with seizures. All of our patients had normal neurological examinations between attacks, and only one experienced seizures occurring during the prolonged attack of hemiplegia. Hemiplegic migraine, particularly FHM2, should be considered in any child presenting with a prolonged bout of hemiplegia. Genetic diagnosis and stratification of such patients are important in defining the phenotypic spectrum and characterising the natural history.
To date, more than 20 mutations in ATP1A2 have been reported to cause FHM2.4,11,12,13,14,15,16,17,18,19 The mutations are scattered throughout the gene, although the majority are concentrated in the region that codes for the intracellular linker between the 4th and 5th transmembrane segments, an important regulatory domain for ion transport. Only two prior mutations have been found in the transmembrane segments of the protein—the R1002Q mutation is the first reported in the 10th transmembrane segment. Consistent with prior functional studies on several different mutations,4,18,19 our functional data suggest that the disease causing mechanism for FHM2 is haploinsufficiency of the sodium–potassium ATPase alpha 2 isoform.
Acknowledgements
Supported by the National Institutes of Health (NIH) grant P50 DC05224.
Abbreviations
FHM - familial hemiplegic migraine
Footnotes
Competing interests: None.
References
- 1.Haan J, Terwindt G M, Ophoff R A.et al Is familial hemiplegic migraine a hereditary form of basilar migraine? Cephalalgia 199515477–481. [DOI] [PubMed] [Google Scholar]
- 2.Carrera P, Piatti M, Stenirri S.et al Genetic heterogeneity in Italian families with familial hemiplegic migraine. Neurology 19995326–33. [DOI] [PubMed] [Google Scholar]
- 3.Ophoff R A, Terwindt G M, Vergouwe M N.et al Familial hemiplegic migraine and episodic ataxia type‐2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 199687543–552. [DOI] [PubMed] [Google Scholar]
- 4.De Fusco M, Marconi R, Silvestri L.et al Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 200333192–196. [DOI] [PubMed] [Google Scholar]
- 5.Dichgans M, Freilinger T, Eckstein G.et al Mutation in the neuronal voltage‐gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005366371–377. [DOI] [PubMed] [Google Scholar]
- 6.Escayg A, MacDonald B T, Meisler M H.et al Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 200024343–345. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Del‐Favero J, Ceulemans B.et al De novo mutations in the sodium‐channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 2001681327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducros A, Denier C, Joutel A.et al The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med 200134517–24. [DOI] [PubMed] [Google Scholar]
- 9.Jen J, Wan J, Graves M.et al Loss‐of‐function EA2 mutations are associated with impaired neuromuscular transmission. Neurology 2001571843–1848. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Peterson D A, Kimura H.et al Mechanism of cellular 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) reduction. J Neurochem 199769581–593. [DOI] [PubMed] [Google Scholar]
- 11.Riant F, De Fusco M, Aridon P.et al ATP1A2 mutations in 11 families with familial hemiplegic migraine. Hum Mutat 200526281. [DOI] [PubMed] [Google Scholar]
- 12.Vanmolkot K R, Kors E E, Hottenga J J.et al Novel mutations in the Na+, K+‐ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann Neurol 200354360–366. [DOI] [PubMed] [Google Scholar]
- 13.Kaunisto M A, Harno H, Vanmolkot K R.et al A novel missense ATP1A2 mutation in a Finnish family with familial hemiplegic migraine type 2. Neurogenetics 20045141–146. [DOI] [PubMed] [Google Scholar]
- 14.Spadaro M, Ursu S, Lehmann‐Horn F.et al A G301R Na+/K+ ‐ATPase mutation causes familial hemiplegic migraine type 2 with cerebellar signs. Neurogenetics 20045177–185. [DOI] [PubMed] [Google Scholar]
- 15.Swoboda K J, Kanavakis E, Xaidara A.et al Alternating hemiplegia of childhood or familial hemiplegic migraine? A novel ATP1A2 mutation. Ann Neurol 200455884–887. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosini A, D'Onofrio M, Grieco G S.et al Familial basilar migraine associated with a new mutation in the ATP1A2 gene. Neurology 2005651826–1828. [DOI] [PubMed] [Google Scholar]
- 17.Pierelli F, Grieco G S, Pauri F.et al A novel ATP1A2 mutation in a family with FHM type II. Cephalalgia 200626324–328. [DOI] [PubMed] [Google Scholar]
- 18.Vanmolkot K R, Stroink H, Koenderink J B.et al Severe episodic neurological deficits and permanent mental retardation in a child with a novel FHM2 ATP1A2 mutation. Ann Neurol 200659310–314. [DOI] [PubMed] [Google Scholar]
- 19.Vanmolkot K R, Kors E E, Turk U.et al Two de novo mutations in the Na,K‐ATPase gene ATP1A2 associated with pure familial hemiplegic migraine. Eur J Hum Genet 200614555–560. [DOI] [PubMed] [Google Scholar]