Abstract
Biosynthetic regulation of catA, the gene encoding catechol 1,2-dioxygenase (EC 1.13.1.1), was studied in an Acinetobacter calcoaceticus mutant strain unable to metabolize benzoate. Benzoate and muconate independently induced the enzyme. In glucose-grown cells, benzoate yielded higher enzyme levels than did muconate, whereas muconate was the more effective inducer in succinate-grown cells.
Full text
PDF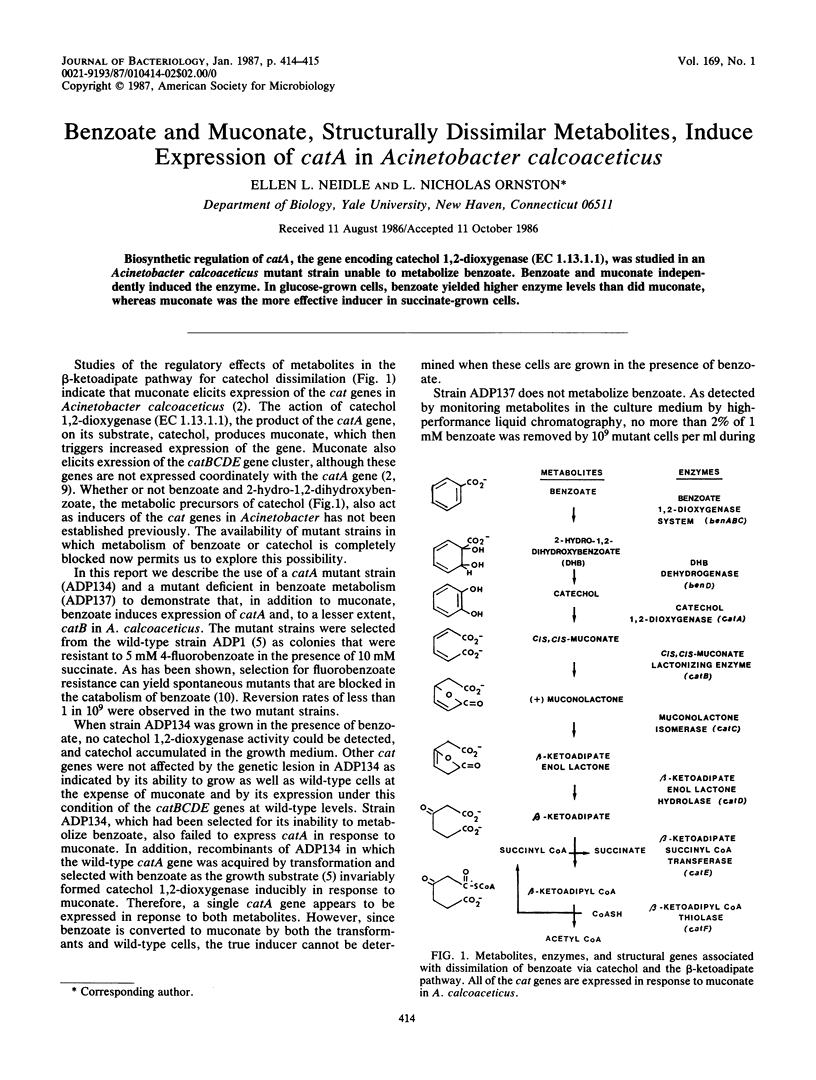

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., McKenzie D. I. Catechol oxygenases of Pseudomonas putida mutant strains. J Bacteriol. 1976 Sep;127(3):1098–1107. doi: 10.1128/jb.127.3.1098-1107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., KATAGIRI M., ROTHBERG S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957 Dec;229(2):905–920. [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catechol 1,2-dioxygenase structural gene catA in Escherichia coli. J Bacteriol. 1986 Nov;168(2):815–820. doi: 10.1128/jb.168.2.815-820.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Shanley M. S., Neidle E. L., Parales R. E., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catBCDE genes in Pseudomonas putida and Escherichia coli. J Bacteriol. 1986 Feb;165(2):557–563. doi: 10.1128/jb.165.2.557-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore G. J., Ribbons D. W. p-Cymene pathway in Pseudomonas putida: selective enrichment of defective mutants by using halogenated substrate analogs. J Bacteriol. 1980 Aug;143(2):816–824. doi: 10.1128/jb.143.2.816-824.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



