Abstract
Background
Therapeutic management of gait disorders in patients with advanced Parkinson's disease (PD) can sometimes be disappointing, since dopaminergic drug treatments and subthalamic nucleus (STN) stimulation are more effective for limb‐related parkinsonian signs than for gait disorders. Gait disorders could also be partly related to norepinephrine system impairment, and the pharmacological modulation of both dopamine and norepinephrine pathways could potentially improve the symptomatology.
Aim
To assess the clinical value of chronic, high doses of methylphenidate (MPD) in patients with PD having gait disorders, despite their use of optimal dopaminergic doses and STN stimulation parameters.
Methods
Efficacy was blindly assessed on video for 17 patients in the absence of l‐dopa and again after acute administration of the drug, both before and after a 3‐month course of MPD, using a Stand–Walk–Sit (SWS) Test, the Tinetti Scale, the Unified Parkinson's Disease Rating Scale (UPDRS) part III score and the Dyskinesia Rating Scale.
Results
An improvement was observed in the number of steps and time in the SWS Test, the number of freezing episodes, the Tinetti Scale score and the UPDRS part III score in the absence of l‐dopa after 3 months of taking MPD. The l‐dopa‐induced improvement in these various scores was also stronger after the 3‐month course of MPD than before. The Epworth Sleepiness Scale score fell dramatically in all patients. No significant induction of adverse effects was found.
Interpretation
Chronic, high doses of MPD improved gait and motor symptoms in the absence of l‐dopa and increased the intensity of response of these symptoms to l‐dopa in a population with advanced PD.
The significant, long‐term benefits of dopaminergic treatment1 and bilateral stimulation of the subthalamic nucleus (STN)2 have been well documented for limb‐related syndromes in patients with advanced Parkinson's disease (PD). However, after several years of disease progression (and regardless of the ongoing treatment), axial signs in general and gait disorders in particular (including reduced step length, freezing and postural instability) become more prominent and lead to falls and even institutionalisation. Therapeutic management of the condition is disappointing, since dopaminergic treatments and STN stimulation are more effective for other limb‐related parkinsonian signs than for gait disorders as such.2,3 However, an interesting therapeutic approach could involve the combined modulation of l‐dopa bioavailability (to potentiate the partial dopa‐sensitivity of gait disorders) and the non‐dopaminergic system, particularly the norepinephrine system, which has been previously suspected to be involved in gait disorders.4,5 This “norepinephrine hypothesis” could explain the positive results on freezing of gait observed in some open‐label studies on small populations of patients with advanced PD using the synthetic norepinephrine precursor l‐threo‐dihydroxyphenylserine6,7 or tinazidine, an α‐2 adrenergic agonist.4 However, these results have never been confirmed—probably because l‐threo‐dihydroxyphenylserine is a weak precursor of norepinephrine and only slightly influences striatal, extracellular dopamine levels.8
Methylphenidate (MPD, Ritalin) is an amphetamine‐like psychomotor stimulant, which influences both the dopaminergic and norepinephrine systems. Indeed, MPD inhibits the dopamine transporter (DAT), particularly in the striatum.9 The DAT is one of the most important determinants of extracellular dopamine concentrations, as demonstrated in DAT knock‐out mice.10 Through inhibition of the DAT, MPD blocks presynaptic dopamine re‐uptake.11 To a lesser extent, MPD also influences the norepinephrine system through presynaptic norepinephrine transporter inhibition.11,12,13 Hence, by targeting the DAT and the norepinephrine transporter, MPD might disperse dopamine widely and consign dopamine storage and release to regulation by norepinephrine neurones as well as by dopaminergic neurones.13 Effects of MPD may be mediated by restoration of the dopaminergic/norepinephrine neurotransmitter balance.13,14
A pilot study on five patients with PD with motor fluctuations showed that low doses of MPD (0.2 mg/kg) combined with l‐dopa led to greater peak right‐hand tapping speed.15 The effects of doses of up to 0.4 mg/kg of MPD were also assessed in a double‐blind, placebo‐controlled procedure; MPD seemed to lack an effect when given alone but did potentiate the effects of l‐dopa on walking speeds and dyskinesia.9 Recently, positive effects on gait speed, fall risk and attention were demonstrated in an open‐label study using an acute, low dose (20 mg) of MPD.16 It therefore seemed interesting to determine whether higher doses and longer‐term treatment could improve the MPD‐induced partial response for gait disorders. Indeed, up to 70% of the dopamine nerve terminals (and consequently 70% of DAT activity) are lost in severe PD.17 An oral dose of 0.25 mg/kg MPD may only occupy half of the striatal DATs in humans,12 whereas oral doses of 0.5–0.8 mg/kg allow a higher occupancy and lead to high extracellular dopamine concentrations.13,18,19 Moreover, high doses of MPD could also increase the norepinephrine properties of MPD.
Our research hypothesis was the improvement of gait by MPD. The aim of this study was to assess the clinical value of a high‐dose, 3‐month course of MPD (1 mg/kg) in STN‐stimulated patients with advanced PD (free of motor fluctuations) having gait disorders despite their use of optimal dopaminergic doses and STN stimulation parameters. The primary outcome measure was the completion time in the Stand–Walk–Sit (SWS) Test.20 Efficacy was blindly assessed on video in the absence of l‐dopa and then again after acute administration of the latter drug, to assess the potential norepinephrine and/or dopaminergic effects of MPD on gait speed and step length.
Patients and methods
Patients
Patients with PD21 were studied after having obtained their written, informed consent and after approval by the local ethics committee. The subjects were consecutively selected from our active case file over a 3‐month period. The inclusion criteria were as follows: STN‐stimulated patients with advanced PD having severe gait disorders (including freezing), which were not related to “off periods” in motor fluctuations and which occurred despite satisfactory segmental motor control by stimulation and dopa‐therapy. Age and cognitive status were not included in exclusion criteria. A total of 17 patients were included (12 men and 5 women), with a mean age of 67 (64–71) years (median value (1st–3rd quartile)), a mean disease duration of 17 (15–20) years and a mean stimulation duration of 4 years.3,4,5 They had been offered stimulation of the STN to correct severe motor fluctuations and l‐dopa‐induced dyskinesia. All patients received l‐dopa treatment combined with a dopamine agonist (six taking ropinirole and three taking pergolide) and/or a catechol‐O‐methyl transferase inhibitor (n = 7), with an l‐dopa equivalent dose of 675 (600–787) mg. Before the study, axial and limb motor control during STN stimulation and dopa‐therapy were optimised for each patient. Their motor fluctuations had almost disappeared since the initiation of STN stimulation, and the patients had little or no dyskinesia during “on” periods.
Each patient's cognitive status was measured by their score (out of 144) on the Mattis Dementia Rating Scale. The median Mattis and Mini‐Mental State Examination scores were 130 (121–138) and 28/30 (25.5–29), respectively. Of the 17 patients, 7 had developed dementia, according to the Diagnostic and statistical manual of mental disorders, fourth edition criteria, in the years following initiation of STN stimulation.
Experimental design
A repeated‐measures design was applied with one factor (condition) and four levels (no l‐dopa, taking l‐dopa alone, taking MPD alone and on‐both).
Dosage schedule
Patients received a daily dose of 1 mg/kg of MPD three times daily (at 8:00, 12:00 and 16:00 h) for 3 months, including a 1‐month titration phase (which consisted of increasing the daily dosage by about 50% each week until achievement of the weight‐adjusted target dosage of five to eight 10‐mg tablets per day). Tolerance of MPD was monitored for each patient throughout the treatment period and, if necessary, dose reduction was permitted.
Motor symptoms
Efficacy was blindly assessed on video in the absence of l‐dopa, and then after acute administration of l‐dopa later the same morning, before and after 3 months of MPD treatment. The condition in the absence of l‐dopa and MPD was designated “off dopa/off MPD” and was necessarily the first condition examined in the morning after a night of treatment withdrawal, with the last intake of l‐dopa at 20:00 h. The assessment criterion was the Unified Parkinson's Disease Rating Scale (UPDRS) part III score. The condition after acute administration of l‐dopa and in the absence of MPD was designated “on dopa/off MPD”. The condition after 3 months of MPD and in the absence of l‐dopa was designated “off dopa/on MPD” and the condition after 3 months of MPD and following acute administration of l‐dopa was designated “on dopa/on MPD”. Since the end of the l‐dopa effect cannot be assessed with certainty, evaluations in the absence of l‐dopa were performed at 8:30 h and those involving l‐dopa were performed at 9:30 h. The l‐dopa dose was 100 mg in the on dopa/off MPD and on dopa/on MPD conditions, corresponding to the usual, first morning dose used by patients to relieve their symptoms during STN stimulation. The last dose of 1 mg/kg of MPD was taken at 7:00 h for the off dopa/on MPD and on dopa/on MPD conditions. For each condition, the completion time and the number of steps and freezing episodes (clinically defined by a sudden motor block of >10 s) in the SWS test,20 the Tinetti Scale score,22 the UPDRS part III score23 and the Dyskinesia Rating Scale score24 were recorded by a neurologist (DD), and were blindly rated on video by two independent neurologists (LD and PK). Each rater watched and scored the videos (presented in a random order) alone, after one or two visualisations, as necessary.
Non‐motor symptoms
Efficacy was assessed openly before and after treatment with MPD. It concerned parts I and II of the UPDRS and an assessment of sleepiness on the Epworth Sleepiness Scale25 by a neurologist (DD). The Lille Apathy Rating Scale score26 and performance in attention tasks were scored by a neuropsychologist (KD). Sustained attention was assessed in terms of performance in a simple reaction time task (mean response time and number of omissions). Selective attention was assessed in terms of performance in a choice reaction time task (mean response time and number of errors). A psychiatric examination was also performed by a psychiatrist (OC) to detect potential changes induced by the MPD treatment. After a semistructured interview, the Montgomery Asberg Depression Rating Scale (MADRS)27 and the Brief Psychiatric Rating Scale28 were rated.
Adverse events
Adverse events were reported spontaneously and through a monthly questionnaire citing the effects commonly reported by people taking MPD (dry mouth, anorexia nervosa, blurred vision, insomnia, headaches, palpitations, nausea, abdominal pain, confusion, allergy, purpura, fever, athralgia, alopecia, drowsiness, dyskinesia). The events were rated according to their severity, from slight (1) to severe (3). A number of clinical parameters were assessed: cardiovascular and general health status, weight, lying and standing arterial blood pressure, heart rate and the ECG (blindly assessed by a cardiologist (MK)). A general biological profile was performed.
Data analysis
We anticipated a mean (SD) completion time of 20 (3) s in the SWS test. We hypothesised that there would be a 3 s difference when taking MPD (in the absence or presence of l‐dopa). With an α risk of 0.05 and an a priori power of 90% in our analysis, 13 subjects would be needed. However, we assumed a priori that the data were not normally distributed and thus necessitated the use of a non‐parametric test; this led to a power loss of 25% and therefore to a required sample size of 17 subjects.
Conover's non‐parametric test with one factor (condition) and four levels (no l‐dopa, taking l‐dopa alone, taking MPD alone and taking both) was performed for each parameter from the blind‐assessed analyses.29 Main effects were analysed with respect to Mauchly's sphericity test. The Greenhouse–Geisser epsilon correction of degrees of freedom was applied, if required. To explain significant main effects when comparing the four levels, we performed contrast studies (using the Bonferroni post hoc test to take into account the type 1 error). All the six post hoc tests were calculated between the four levels and therefore had a significance level of 0.01.
In the open‐label study, the efficacy results before and after 3 months of taking MPD were compared using a Wilcoxon test. A significance level of 0.05 was chosen. We used SPSS software (V.11.5) for all statistical analyses.
The absence of a normal distribution for the results required their presentation with medians (quartiles 1 and 3).
Results
All the patients completed the study. Sixteen were maintained at a dose of 1 mg/kg of MPD/day. For one patient (number 10), the dose was decreased to 0.8 mg/kg because of facial flush. Stimulation and dopaminergic treatment parameters were not modified during the study.
Gait and motor assessment
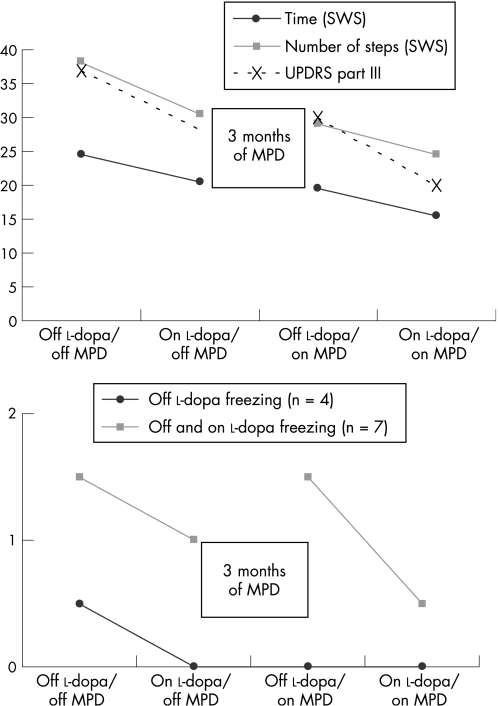
In a blind assessment, we observed a significant main effect of the condition (Conover test with one factor and four levels) on the completion time (F(2,15) = 11.5, p = 0.001) and the number of steps (F(3,14) = 8.6, p = 0.002) in the SWS Test (table 1). Contrast studies revealed an improvement in the off dopa/on MPD condition when compared with off dopa/off MPD and in the on dopa/on MPD condition when compared with on dopa/off MPD. We noted a complementary effect for MPD and l‐dopa, with a significant improvement in the on dopa/on MPD condition when compared with the on dopa/off MPD and off dopa/on MPD conditions (fig 1). The same significant effects were obtained for the Tinetti Scale (F(3,14) = 3.8, p = 0.036) and the UPDRS part III (F(3,14) = 8.8, p = 0.002) scores.
Table 1 Blind assessment (offline video) of motor performance in the absence of l‐dopa and following acute administration of l‐dopa, before and after 3 months of methylphenidate treatment.
| Blind assessment | Without MPD | Under MPD | ||
|---|---|---|---|---|
| Off l‐dopa | On l‐dopa | Off l‐dopa | On l‐dopa | |
| SWS Test: Completion time | 25 (22, 33) | 19.5 (15, 28) | 20 (17, 29)* | 15.5 (13, 20]† |
| SWS steps | 38 (35, 44) | 29 (26, 35) | 30 (28, 37)* | 24 (22, 29)† |
| UPDRS part III | 34 (23.5, 43)* | 30.5 (18.5, 36)† | 29 (22.5, 33.5)* | 21 (16, 27)† |
| Tinetti Scale score | 8 (7, 10)* | 10 (9, 11)† | 8.5 (7.5, 11)* | 11 (10, 12)† |
| Dyskinesia Rating Scale score | 0 (0, 1) | 2 (0, 5.5) | 0 (0, 1) | 3.5 (0, 4) |
| Freezing in off l‐dopa and on l‐dopa conditions | 7 patients | 7 patients | 5 patients | 4 patients |
| Number of episodes | 1.5 (1, 2) | 1 (1, 1.5) | 1.5 (0.25, 2.5) | 0.5 (0, 1) |
| Only off l‐dopa freezing | 4 patients | — | 0 patient | 0 patient |
| Number of episodes | 0.5 (0.5, 0.75) | 0* | 0† | |
| Only on l‐dopa freezing | — | 1 patient | 1 patient | 1 patient |
| Number of episodes | — | 1 | 2 | 1.5 |
–, no assessment; MPD, methylphenidate; SWS, Stand–Walk–Sit Test; UPDRS, Unified Parkinson's Disease Rating Scale.
The median values (1st quartile, 3rd quartile) are specified.
Significant differences in the completion time and the number of steps in the SWS Test and in the UPDRS part III score were observed when comparing the four conditions.
*Significant difference (p<0.05) before and after MPD, in the absence of l‐dopa.
†Significant difference before and after MPD, when taking l‐dopa (p<0.05).
Figure 1 Blind assessment (offline video) of the motor performance in the absence of l‐dopa and following acute administration of l‐dopa, before and after 3 months of methylphenidate (MPD) treatment. The values indicated in the figures are median values. Contrast studies, using a Bonferroni post hoc test value of 2.2, revealed the same significant effects for the completion time and the number of steps of the Stand–Walk–Sit test and the Unified Parkinson Disease Rating Scale (UPDRS) part III. We observed significant improvement in the off dopa/on MPD condition when compared with off dopa/off MPD, and in the on dopa/on MPD condition when compared with on dopa /off MPD. We noted a complementary effect with MPD and l‐dopa, with a significant improvement in the on dopa/on MPD condition when compared with the on dopa/off MPD and off dopa/on MPD conditions. The extent of off dopa freezing in the off dopa/off MPD condition was significantly higher than in the other three conditions. There were no significant differences in the “on dopa” freezing.
Of the 17 patients, 12 displayed between 1 and 3 freezing episodes during the different conditions of the SWS Test (1 patient had as many as 20 episodes in the on l‐dopa condition). Only four patients exhibited freezing during the off dopa/off MPD condition, which disappeared in all patients in the other three conditions of treatment (F(1,3) = 144, p = 0.001). Before MPD treatment, 7 patients exhibited freezing during both the off dopa/off MPD and on dopa/off MPD conditions; this phenomenon tended to decrease in the on dopa/on MPD condition but the improvement was not significant (p = 0.1). Only one patient exhibited freezing during the on dopa/off MPD condition and did not improve after MPD treatment. Festination was not recorded in any of the 17 patients. The Dyskinesia Rating Scale score tended to increase in the on dopa/on MPD condition compared with the on dopa/off MPD condition, but again the change was not significant.
Assessment of non‐motor symptoms
A significant decrease in the UPDRS part I (z = 2.8, p = 0.005) and UPDRS part II (z = 2.4, p = 0.015) scores was clearly observed after 3 months of taking MPD, compared with the period before treatment. Sleepiness (as assessed on the Epworth Sleepiness Scale) was also significantly reduced (z = −3.2, p = 0.002; table 2). We did not observe any sleep attacks before or after the course of MPD.
Table 2 Open‐label assessment of the motor, the cognitive and the psychiatric performances on the Unified Parkinson's Disease Rating Scale part I and II, the Epworth Sleepiness Scale, the Montgomery Asberg Depression Rating Scale score, the Brief Psychiatric Rating Scale and the Lille Apathy Rating Scale.
| Open‐label assessment | Before MPD treatment | After 3 months on MPD |
|---|---|---|
| UPDRS part I | 4 (3, 5) | 3 (2, 4)* |
| UPDRS part II | 21 (6.75, 24) | 19 (19, 22)* |
| ESS | 7 (6, 10) | 6 (3, 6)* |
| MADRS | 8 (7, 13) | 8 (6, 12) |
| BPRS | 33 (30, 38.5) | 32 (28, 36) |
| LARS | −21 (−26, −19) | −21 (−25, −14) |
| Sustained attention | ||
| Mattis score ⩾130 | SRT 643 (630, 774) | SRT 641 (588, 710)* |
| Errors 0(0, 0) | Errors 0(0, 0) | |
| Mattis score <130 | SRT 643 (595, 731) | SRT 747 (635, 1184) |
| Errors 0(0, 0) | Errors 0(0, 0) | |
| Selective attention | ||
| Mattis score ⩾130 | CRT 935 (744, 994) | CRT 900 (836, 1010) |
| Errors 0(0, 0.5) | Errors 0(0, 2) | |
| Mattis score <130 | CRT 900 (898, 939) | CRT 1104 (904, 1364)* |
| Errors 0 (0, 0.75) | Errors 0 (0, 3) |
BPRS, Brief Psychiatric Rating Scale; CRT, choice reaction time; ESS, Epworth Sleepiness Scale; LARS, Lille Apathy Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale; MPD, methylphenidate; SRT, simple reaction time; UPDRS, Unified Parkinson's Disease Rating Scale.
Maintained attention (SRT and number of errors) and selective attention (CRT and number of errors) were analysed according to the global cognitive efficiency on the Mattis score before MPD treatment (for patients with a Mattis score ⩾130 and those with a Mattis score <130). The median values (1st quartile, 3rd quartile) are specified.
*Significant difference (p<0.05).
The patients were neither depressed nor apathetic, as evidenced by their low scores on the MADRS and the Lille Apathy Rating Scale. The MADRS, Brief Psychiatric Rating Scale and Lille Apathy Rating Scale scores were not significantly influenced by MPD treatment.
After 3 months of taking MPD, there were no significant differences in terms of performance in (1) the simple reaction time task (mean response time (z = −0. 3; p = 0.7) and the number of omissions (z = −0.7; p = 0.4)); and (2) the choice reaction time task (mean response time (z = −1.1; p = 0.3) and number of errors (z = −0.7; p = 0.4)). Of the 10 patients without dementia with a Mattis score ⩾130, sustained attention was improved after a 3‐month course of MPD: the mean response time in the simple reaction time task decreased significantly (z = −2.03; p = 0.04), whereas response accuracy was maintained (table 2). Performance in the choice reaction time task did not change (z = −0.3, p = 0.4). Of the seven patients with dementia with a Mattis score <130, sustained attention was not modified after 3 month of taking MPD (z = −1.3, p = 0.1). However, we did see a worsening in selective attention, since the mean reaction time on the choice reaction time task increased significantly (z = −1.9; p = 0.05), despite equivalent levels of accuracy.
Acceptability
Cardiovascular and general health status, lying and standing arterial blood pressure, heart rate, the ECG and the clinical biochemistry results were not significantly modified by MPD treatment. None of our patients displayed increases in arterial blood pressure or heart rate. The adverse events revealed only minor incidents: dry mouth (n = 7), anorexia (n = 6), nervosa (n = 6), weight loss (n = 4), headaches (n = 2), palpitations (n = 2) and dyskinesia (n = 2).
Discussion
Main outcomes
Chronic administration of high doses of MPD improved gait in the absence of l‐dopa, as assessed by the walking speed, the number of steps and the number of freezing episodes. The l‐dopa‐induced improvement in these various parameters was also greater after the 3‐month period of treatment with MPD than before. Interestingly, this improvement was obtained in patients having gait disorders but with good limb motor control by dopaminergic treatment and STN stimulation—a common problem in advanced PD. Moreover, the slight increase in dyskinesia in patients taking MPD and l‐dopa (compared with l‐dopa alone) was not significant. The motor benefit of MPD was also obtained in patients with dementia (despite a slight worsening in performance in the choice reaction time); this is of particular interest, since gait disorders are frequently associated with dementia in advanced PD.30 MPD dramatically reduced excessive daytime sleepiness in all patients, improved the simple reaction times in patients without dementia and did not induce depression and apathy in patients who did not have these conditions before MPD treatment.
Comparison with previous studies
Previous studies using lower doses and/or acute administration of MPD additionally showed an improvement in tapping and walking speeds, but only when MPD was associated with l‐dopa.9,15,16 One of these studies also noted the absence of an increase in the severity of dyskinesia following acute administration of l‐dopa in patients taking MPD, although the duration of dyskinesia was prolonged.9 Using a subjective measure of fatigue on a visual analogue scale and an acute MPD dose of 0.4 mg/kg, the decrease in excessive daytime sleepiness was equally not significant,9 suggesting also that chronic administration of high doses of MPD has a greater effect on sleepiness. An MPD‐induced improvement in choice reaction times (but not simple reaction times) was observed in five patients in off l‐dopa conditions.15 Equally, MPD improved attention in 21 patients taking l‐dopa.16 Even though a number of factors (differences in a patient's cognitive status, the presence of l‐dopa, and the dosage and duration of MPD administration) could all lead to differing effects on attention, MPD generally seems to have a psychoactive effect in patients without dementia.
Hypothetical mechanisms of action
Hence, it seems that the stimulation profile of MPD depends on the dosage and the duration of administration. Acute, low doses of MPD potentiated the effect of l‐dopa,9 whereas chronic, high doses could potentiate exogenous l‐dopa and increase the extracellular concentrations of endogenous dopamine. Interestingly, the decrease in the daily dose of l‐dopa obtained in STN‐stimulated patients (compared with non‐stimulated patients) could possibly favour the appearance of gait disorders (since the latter require higher doses of l‐dopa than those with parkinsonian signs in the limbs) and could possibly help explain the benefit observed after administration of MPD. Since the half life of MPD is short (2–5 h),31 chronic administration of high doses of MPD could result in the steadier inhibition of neurotransmitter transporters (ie, within the plateau region of the drug's pharmacological action), compared with acute and/or low doses of MPD. Chronic administration could also induce more pharmacodynamic and molecular changes in the corticostriatal circuits, which in turn might contribute to the observed clinical improvements. These molecular changes have been particularly studied in terms of (1) behavioural sensitisation for the risk of drug misuse in young MPD‐treated patients with attention deficit hyperactivity disorder (ADHD); and (2) locomotor sensitisation in animal models.31,32 Furthermore, the complementary effects of l‐dopa and MPD suggest the involvement of a mechanism other than central dopamine enhancement (such as norepinephrine transporter inhibition, leading to high concentrations of extracellular norepinephrine).11,12,13 The broad interactions between the norepinephrine and dopaminergic systems could also partly explain the indirect potentiation of exogenous l‐dopa activity by norepinephrine, as demonstrated in both rats and humans by using α2 adrenergic antagonists.33,34 Lastly, another mechanism of action might be involved in the increase in frontal cortex norepinephrine concentration, since the latter was suspected of being related to freezing.4,5 An increase in the norepinephrine concentration may also be evidenced by the dramatic reduction in sleepiness seen here, since an increase in the dopamine concentration would lead to excessive daytime sleepiness.35 Several mechanisms could be proposed to explain the lack of a significant worsening in dyskinesia: the pharmacodynamic phenomenon of sensitisation,9 the decrease in the daily dose of l‐dopa in patients undergoing STN stimulation and (possibly) a norepinephrine effect thought to decrease dyskinesia.36
Acceptability
MPD was well tolerated in our elderly patients with advanced PD. The same, common adverse events observed here were found in adults without PD treated with MPD for ADHD.37 Besides the central dopamine enhancement, the mechanism of action could involve the norepinephrine system, since the same adverse events occur with atomoxetine (the first non‐stimulant norepinephrine transporter inhibitor used in ADHD).37,38
Study limitations
The lack of randomisation of the different conditions could have induced a general learning effect with the repeated tasks and constitutes a study limitation. However, the order in which the conditions were performed was chosen because of the indeterminate long‐term effects of l‐dopa: the latter would have impeded assessment of the subsequent condition and would probably have had a more serious confounding effect on the results. The sample size was low but enabled the identification of significant effects. Given that the effects of MPD have to be assessed in the presence and absence of l‐dopa under standardised conditions, and to limit the placebo effect, all the motor assessments were blindly analysed by two separated neurologists. These interesting results must therefore be confirmed in a larger population (including a placebo group) in a study with greater statistical power.
Conclusion
Blind assessment demonstrated that chronic, high doses of MPD improved gait and motor symptoms in the presence and absence of l‐dopa in an elderly population of patients with advanced PD undergoing STN stimulation. Our results support the potential value of a large‐scale, double‐blind, placebo‐controlled trial on PD for evaluation of the clinical implications featured here, together with long‐term studies to determine the cardiovascular acceptability of MPD treatment in this type of population.
Acknowledgements
We thank Eléni Pélécanos, Francine Niset and the Délégation de la Recherche Clinique du CHU de Lille for collecting the data, the CHU de Lille for promoting the study and Dr David Fraser (Biotech Communication, Damery, France) for proofreading the manuscript.
Abbreviations
ADHD - attention‐deficit hyperactivity disorder
DAT - dopamine transporter
MADRS - Montgomery Asberg Depression Rating Scale
MPD - methylphenidate
PD - Parkinson's disease
STN - subthalamic nucleus
SWS - Stand–Walk–Sit
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Competing interests: None declared.
References
- 1.Nutt J G. Long‐term L‐DOPA therapy: challenges to our understanding and for the care of people with Parkinson's disease. Exp Neurol 20031849–13. [DOI] [PubMed] [Google Scholar]
- 2.Krack P, Batir A, Van Blercom N.et al Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 20033491925–1934. [DOI] [PubMed] [Google Scholar]
- 3.Krystkowiak P, Blatt J L, Bourriez J L.et al Effects of subthalamic nucleus stimulation and levodopa treatment on gait abnormalities in Parkinson disease. Arch Neurol 20036080–84. [DOI] [PubMed] [Google Scholar]
- 4.Maertens de Noordhout A, Pepin J L, Delwaide P J. Open study of tinazidine in the treatment of freezing gait. 11th International Symposium on Parkinson's disease 26–30 March 1994, Rome
- 5.Mizuno Y, Kondo T, Mori H. Various aspects of motor fluctuations and their management in Parkinson's disease. Neurology 199444S29–S34. [PubMed] [Google Scholar]
- 6.Narabayashi H, Yokochi F, Ogawa T.et al Analysis of L‐threo‐3,4‐dihydroxyphenylserine effect on motor and psychological symptoms in Parkinson's disease. No To Shinkei 199143263–268. [PubMed] [Google Scholar]
- 7.Tohgi H, Abe T, Takahashi S. The effects of L‐threo‐3,4‐dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect 1993527–34. [DOI] [PubMed] [Google Scholar]
- 8.Sarre S, Smolders I, Thorre K.et al Biotransformation of locally applied precursors of dopamine, serotonin and noradrenaline in striatum and hippocampus: a microdialysis study. J Neural Transm 19971041215–1228. [DOI] [PubMed] [Google Scholar]
- 9.Nutt J G, Carter J H, Sexton G J. The dopamine transporter: importance in Parkinson's disease. Ann Neurol 200455766–773. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov R R, Jones S R, Fumagalli F.et al Re‐evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev 199826148–153. [DOI] [PubMed] [Google Scholar]
- 11.Keating G M, McClellan K, Jarvis B. Methylphenidate (OROS formulation). CNS Drugs 200115495–500. [DOI] [PubMed] [Google Scholar]
- 12.Volkow N D, Wang G J, Fowler J S.et al Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 19981551325–1331. [DOI] [PubMed] [Google Scholar]
- 13.Madras B K, Miller G M, Fischman A J. The dopamine transporter and attention‐deficit/hyperactivity disorder. Biol Psychiatry 2005571397–1409. [DOI] [PubMed] [Google Scholar]
- 14.Overtoom C C, Verbaten M N, Kemner C.et al Effects of methylphenidate, desipramine, and L‐dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behav Brain Res 20031457–15. [DOI] [PubMed] [Google Scholar]
- 15.Camicioli R, Lea E, Nutt J G.et al Methylphenidate increases the motor effects of L‐dopa in Parkinson's disease: a pilot study. Clin Neuropharmacol 200124208–213. [DOI] [PubMed] [Google Scholar]
- 16.Auriel E, Hausdorff J M, Herman T.et al Effects of methylphenidate on cognitive function and gait in patients with Parkinson's disease: a pilot study. Clin Neuropharmacol 20062915–17. [DOI] [PubMed] [Google Scholar]
- 17.Benamer H T, Patterson J, Wyper D J.et al Correlation of Parkinson's disease severity and duration with 123I‐FP‐CIT SPECT striatal uptake. Mov Disord 200015692–698. [DOI] [PubMed] [Google Scholar]
- 18.Volkow N D, Wang G, Fowler J S.et al Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 200121RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeman P, Madras B. Methylphenidate elevates resting dopamine which lowers the impulse‐triggered release of dopamine: a hypothesis. Behav Brain Res 200213079–83. [DOI] [PubMed] [Google Scholar]
- 20.Gibb W R G, Lees A J. The prevalence of the lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 198851745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langston J W, Widner H, Goetz C G.et al Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 199272–13. [DOI] [PubMed] [Google Scholar]
- 22.Tinetti M E, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 199045P239–P243. [DOI] [PubMed] [Google Scholar]
- 23.Fahn S, Elton R L, members of the UPDRS development committee Unified Idiopathic Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Caln D, Goldstein M, eds. Recent developments in Parkinson's disease. NJ: MacMillan Healthcare Information, 1987153–163.
- 24.Goetz C G, Stebbins G T, Shale H M.et al Utility of an objective Dyskinesia Rating Scale for Parkinson's disease: inter‐ and intrarater reliability assessment. Mov Disord 19949390–394. [DOI] [PubMed] [Google Scholar]
- 25.Johns M W. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 199114540–545. [DOI] [PubMed] [Google Scholar]
- 26.Sockeel P, Dujardin K, Devos D.et al The Lille Apathy Rating Scale, a new instrument for detecting and quantifying apathy: validation in Parkinson's disease. J Neurol Neurosurg Psychiatry 200677579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery S A, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979134382–389. [DOI] [PubMed] [Google Scholar]
- 28.Flemenbaum A, Zimmermann R L. Inter‐ and intra‐rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep 197332783–792. [DOI] [PubMed] [Google Scholar]
- 29.Conover W J, Iman R L. Rank transformation as a bridge between parametric and nonparametric statistics. Am Stat Assoc 198235124–129. [Google Scholar]
- 30.Suchowersky O. Parkinson's disease: medical treatment of moderate to advanced disease. Curr Neurol Neurosci Rep 20022310–316. [DOI] [PubMed] [Google Scholar]
- 31.Fone K C, Nutt D J. Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Curr Opin Pharmacol 2005587–93. [DOI] [PubMed] [Google Scholar]
- 32.Dafny N, Yang P B. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 200668393–405. [DOI] [PubMed] [Google Scholar]
- 33.Chopin P, Colpaert F C, Marien M. Effects of alpha‐2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6‐hydroxydopamine lesions of the nigrostriatal pathway. J Pharmacol Exp Ther 1999288798–804. [PubMed] [Google Scholar]
- 34.Savola J M, Hill M, Engstrom M.et al Fipamezole(JP‐1730) is a potent alpha2 adrenergic receptor antagonist that reduces levodopa‐induced dyskinesia in the MPTP‐lesioned primate model of Parkinson's disease. Mov Disord 200318872–883. [DOI] [PubMed] [Google Scholar]
- 35.Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev 20059185–200. [DOI] [PubMed] [Google Scholar]
- 36.Rascol O, Arnulf I, Peyro‐Saint Paul H.et al Idazoxan, an alpha‐2 antagonist, and L‐dopa‐induced dyskinesias in patients with Parkinson's disease. Mov Disord 200116708–713. [DOI] [PubMed] [Google Scholar]
- 37.Christman A K, Fermo J D, Markowitz J S. Atomoxetine, a novel treatment for attention‐deficit‐hyperactivity disorder. Pharmacotherapy 2004241020–1036. [DOI] [PubMed] [Google Scholar]
- 38.Starr H L, Kemner J. Multicenter, randomized, open‐label study of OROS methylphenidate versus atomoxetine: treatment outcomes in African‐American children with ADHD. J Natl Med Assoc 20059711S–16S. [PMC free article] [PubMed] [Google Scholar]