Abstract
Background
Low levels of cerebrospinal fluid (CSF) β‐amyloid 1–42 (Aβ42) and high total tau (T‐tau) are diagnostic for manifest Alzheimer's disease. It is not known, however, whether these biomarkers may be risk indicators for cognitive decline in otherwise healthy older people.
Methods
The longitudinal relationship between CSF markers, Aβ42 and T‐tau, measured in 1992, and change in Mini‐Mental State Examination (ΔMMSE) score between 1992 and 2002 were investigated in 55 women (aged 70–84 years, mean (SD) MMSE score = 28.3 (1.5)), who were participants in the Prospective Population Study of Women in Gothenburg, Sweden. These women did not have dementia when they experienced lumbar puncture in 1992–3.
Results
Over the 8‐year follow‐up period, ΔMMSE (range = +3 to −21 points) was correlated with Aβ42 (Spearman's r = 0.40, p = 0.002), such that lower levels of Aβ42 were related to greater decline. This was also observed after excluding 4 women who developed dementia between 1992 and 2002 (Spearman's r = 0.34, p = 0.019). A multivariate logistic regression model predicting a decline of ⩾5 points on the MMSE (observed in six women), or a risk of developing dementia over the 8‐year follow‐up period (observed in four women), including age, education, Aβ42 and T‐tau as covariates, showed that Aβ42 was the sole predictor of significant cognitive decline or dementia (OR per 100 pg/ml Aβ42 = 2.24, 95% CI 1.19 to 4.22, p = 0.013).
Conclusions
Low levels of CSF Aβ42 may predict cognitive decline among older women without dementia.
Alzheimer's disease (AD) is rapidly increasing with advancing age, with reported prevalence estimates of 30% at age 85 years, and as high as 50% at age 95 years.1 AD is expected to reach epidemic proportions between 2010 and 2050, when the number of people with the disease is projected to be more than double. Juxtaposed against this harrowing background is the fact that risk indicators for AD in healthy older people are lacking.
β‐amyloid 1–42 (Aβ42) and total tau (T‐tau) are two known biomarkers of manifest AD that are detectable in cerebrospinal fluid (CSF). Low levels of CSF Aβ42 and high levels of CSF T‐tau in cases of AD have been described in both clinical and population‐based samples.2,3,4,5,6 Aβ42 is the 42‐amino acid fragment of amyloid precursor protein. It accumulates in the brain and is the principal component of senile plaques. Aβ42 in CSF has been suggested to reflect the deposition of β‐amyloid in senile plaques, with lower levels excreted to the CSF. T‐tau is a microtubule‐associated protein that, on hyperphosphorylation, is the primary component of neurofibrillary tangles in AD.7 T‐tau in CSF has been suggested to reflect neuronal and axonal degeneration in AD and/or the formation of neurofibrillary tangles.7 Thus, both these markers potentially reflect central pathogenic processes in a brain with AD. In addition, clinical evidence is accumulating that low levels of CSF Aβ42 and high levels of T‐tau may also be useful for predicting progression of mild cognitive impairment (MCI) to AD.8
Despite known associations between CSF Aβ42 and T‐tau, and manifest AD, little is known about the utility of these biomarkers as risk indicators of cognitive decline in healthy older people who are representative of the population. Thus, we investigated whether lower CSF Aβ42 and higher CSF T‐tau predict cognitive decline, as measured using change in Mini‐Mental State Examination (ΔMMSE) score over 8 years, in a population‐based sample of older women aged 70–84 years.
Methods
This analysis originates from the Prospective Population Study of Women in Gothenburg, Sweden. The Prospective Population Study of Women began in 1968–9 with a baseline examination of a representative sample of 1462 women (90.1% participation rate),9 obtained from the Revenue Office Register. The study was approved by the ethics committee for medical research of Gothenburg University, and informed consent was obtained from all participants and/or their relatives.
In 1992–3, 837 women born in 1908, 1914, 1918 and 1922 (aged 70–84 years) and living in Gothenburg were invited to take part in the third study follow‐up.10 Of those, 590 agreed to take part in a psychiatric examination, and 86 consented to lumbar puncture (LP) in 1992–3 (3 born in 1908, 7 in 1914, 33 in 1918 and 43 in 1922). These women were recontacted as part of a fourth follow‐up in 2000–2, and 55 women without dementia in 1992–3 repeated the MMSE (4 born in 1914, 23 in 1918 and 24 in 1922). Reasons for loss to follow‐up include death (n = 15), refusal (n = 9), incomplete MMSE data in 2000–2 due to physical handicaps such as blindness and paresis (n = 6), and missing MMSE (n = 1).
In 1992–3 and 2000–2, psychiatric examinations were performed by psychiatrists (1992–3) or psychiatric research nurses (2000–2).11,12,13 The nurses who conducted the examinations in 2000–2 were trained and supervised by psychiatrists who were involved in the examinations in 1992–3. In 2000–2, nurses and psychiatrists rated symptoms and signs of persons with and those without dementia. Inter‐rater reliability among and between psychiatrists and psychiatric nurses for the MMSE was studied in 82 participants. Overall, a κ of 0.93 was observed on the basis of the evaluation of MMSE quartiles. Inter‐physician ratings (n = 37) resulted in a κ of 0.96, and inter‐nurse ratings (n = 32) resulted in a κ of 0.91. Comparing MMSE quartile rankings for physicians versus nurses (n = 13) resulted in a κ of 0.79.
The psychiatric examination was semi‐structured and included tests of cognitive functioning, including the MMSE,14 ratings of psychiatric symptoms and signs during the preceding month in accordance with the Comprehensive Psychopathological Rating Scale,15 and ratings of dementia and depression symptoms.16 Furthermore, in a semi‐structured telephone interview, close informants were asked about cognitive and psychiatric symptoms in the participants. Dementia, depression and other mental disorders were diagnosed by psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, Revised17 as described previously.13,16 A diagnosis of dementia was based on combined information from the psychiatric interview, close informant interview, case records and hospital registries.
In 1992–3 and 2000–2, participants underwent a physical examination performed by a physician, a battery of blood tests and other assessments. Women were also surveyed for a variety of background factors, including use of psychotropic drugs.18 Categories of psychotropic drugs that were considered in these analyses were not mutually exclusive and included the general class of anxiolytic sedatives (ATC codes N05B and N05C) and subclass, benzodiazepines (ATC codes N05BA and N05CD), as well as antidepressants (ATC codes N06A).
LPs were generally carried out through the L3/L4 interspace. The initial 0.5–1 ml of CSF was discarded if macroscopically stained. The following 12 ml was collected in one tube and gently mixed to avoid gradient effects.19 To eliminate cells and other insoluble material, the samples were centrifuged at 2000 g for 10 min, and then stored at −80°C in 1 ml aliquots in polypropylene vials until analyses. CSF Aβ42 was determined using a sandwich ELISA (Innotest β‐amyloid1–42; Innogenetics, Zwijndrecht, Belgium) constructed to specifically measure Aβ1–42.20,21 To measure levels of CFS T‐tau, sandwich ELISA (Innotest hTAU‐Ag; Innogenetics, Ghent, Belgium) constructed to measure T‐tau (both normal and phosphorylated tau) was used.22
Statistical analyses
Means and SDs were calculated for continuous variables. Spearman's correlation analyses were conducted to measure relationships between continuous variables due to non‐normally distributed CSF markers and MMSE data. ΔMMSE was calculated as MMSE measured in 2000–2 − MMSE measured in 1992–3. Analysis of variance was used to measure differences in mean CSF markers or ΔMMSE by birth cohort, degree of ΔMMSE (losing at least 1 or 5 points), level of education (basic versus more than basic) and dementia. Tests for linear trend in mean levels of CSF markers by age were conducted. χ2 analysis was used to assess relationships between dichotomous categorical variables.
Multivariate logistic regression analyses predicting loss of at least 5 points on the MMSE or a risk of developing dementia over the 8‐year follow‐up period were performed with regard to Aβ42, T‐tau, education and age. Results were considered statistically significant at p<0.05. SPSS V.12.0.1 was used to analyse study data.
Results
Table 1 shows the characteristics of 55 women without dementia who donated CSF in 1992–3 and who completed an MMSE in both 1992–3 and 2000–2, with regard to demographic variables, CSF biomarkers, MMSE scores and dementia status between 1992 and 2002. Although having depression was not an exclusion criteria, MMSE score and ΔMMSE were evaluated in relation to the occurrence of major depressive disorder and total depression in 1992–3 and 2000–2, and no association was found (data not shown).
Table 1 Characteristics of 55 particpants in the Prospective Population Study of Women who underwent Mini‐Mental State Examination at baseline and follow‐up.
| CSF sample* | Range | |
|---|---|---|
| Age (years) | 72.5 (2.6) | 70–78 |
| Basic education | 63% (34/54) | |
| Aβ42 (pg/ml) in 1992–3 | 832.1 (222.3) | 282–1314 |
| T‐tau (pg/ml) in 1992–3 | 324.4 (208.4) | 81–1200 |
| MMSE in 1992–3 | 28.3 (1.5) | 23–30 |
| MMSE in 2000–2 | 26.5 (4.3) | 4–30 |
| ΔMMSE | −1.8 (3.9) | −21–+3 |
| Percent with dementia between 1992 and 2002 | 7.2% (4/55) |
Aβ42, β‐amyloid 1–42; CSF, cerebrospinal fluid; MMSE, Mini‐Mental State Examination; T‐tau, total tau.
*Data are presented as mean (SD) or as % (N/total N).
There were no differences between women who participated in the LP and those who participated in the rest of the psychiatric examination in 1992–3 when compared on the basis of a large number of factors, including age; age range; smoking status; alcohol intake; physical activity level (work and leisure time); body mass index; blood levels of cholesterol, high‐density lipoprotein and triglycerides; systolic and diastolic blood pressures; age at menopause; history of angina pectoris, myocardial infarction and diabetes; and use of a variety of drugs including lipid‐lowering agents, antihypertensive agents and hormone replacement therapy. In addition, there were no differences in mean CSF Aβ42 (p = 0.207) and T‐tau (p = 0.374) levels between those who participated in 1992–3 only and those who repeated the MMSE.
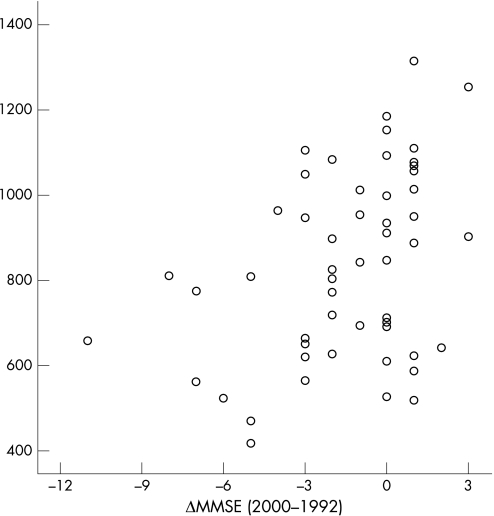
Spearman's rank correlations showed that although CSF T‐tau in 1992–3 was cross‐sectionally correlated with MMSE in 1992–3 (r = −0.30, p = 0.026), CSF Aβ42 in 1992–3 was correlated with MMSE score in 2000–2 (r = 0.33, p = 0.014) and ΔMMSE between 1992 and 2002 (r = 0.40, p = 0.002). Figure 1 shows the relationship between ΔMMSE in 1992–3 and 2000–2, and CSF Aβ42 in 1992–3. This relationship was observed whether or not four individuals who developed dementia over the 8‐year follow‐up period (ΔMMSE = −21 to −2) were included. Lower Aβ42 was also related to degree of ΔMMSE, whether defined as any decline or as a decline of at least 5 (table 2).
Figure 1 Open circles represent change in individual Mini‐Mental State Examination (ΔMMSE) scores over an 8‐year period in relation to baseline levels of cerebrospinal fluid β‐amyloid 1–42.
Table 2 Cerebrospinal fluid biomarker levels by decline in Mini‐Mental State Examination in women without dementia.
| Aβ42 (pg/ml) | T‐tau (pg/ml) | |
|---|---|---|
| Decline in MMSE of ⩾ 5 vs <5 from 1992–2002 | ||
| Decline in MMSE <5, n = 45 | 880.1 (208.6) | 300.4 (154.9) |
| Decline in MMSE ⩾5, n = 6 | 633.5 (183) | 269.8 (81.4) |
| p = 0.029* | p = 0.600 | |
| Any decline in MMSE from 1992–2002 | ||
| No change or gain in MMSE, n = 27 | 904.9 (231.4) | 339.4 (164.1) |
| Any decline in MMSE, n = 24 | 790.5 (191.6) | 248.8 (112.1) |
| p = 0.036* | p = 0.056 |
Aβ42, β‐amyloid 1–42; MMSE, Mini‐Mental State Examination; T‐tau, total tau.
*p Values are age‐adjusted, and are related to comparisons of those experiencing decline versus a lesser amount of decline.
A multivariate logistic regression model predicting those experiencing decline of ⩾5 points on the MMSE or a risk of developing dementia over the 8‐year follow‐up period (n = 10), and including age, education, Aβ42 and T‐tau as covariates, showed that Aβ42 was the sole predictor of significant cognitive decline or dementia (odds ratio per 100 pg/mlAβ42 = 2.24, 95% CI 1.19 to 4.22, p = 0.013). In addition, CSF levels of Aβ42 were not correlated with T‐tau (Spearman's r = 0.027, p = 0.845), thus negating the potential effects of collinearity between biomarkers.
Baseline MMSE score was also evaluated in relation to ΔMMSE, and no apparent relationship was observed. In this sample of 55 women, 51 (93%) scored 27 or better on the 30‐point MMSE in 1992–3. Of those scoring <27, 1 scored 23 (with ΔMMSE = 0), 2 scored 25 (ΔMMSE = −21 and ΔMMSE = −3) and 1 scored 26 (ΔMMSE = −2).
Age was related to mean CSF levels of T‐tau, but not to mean levels of Aβ42 (p = 0.291), MMSE score in 1992–3 (p = 0.293), MMSE score in 2000–2 (p = 0.632) or ΔMMSE (p = 0.897). There was a linear trend for higher levels of T‐tau (p = 0.042) with increasing age (275.8 (135.6) pg/ml in those aged 70 years; 345.1 (245.1) pg/ml in those aged 74 years; 478 (280.5) pg/ml in those aged 78 years). This trend disappeared when those with dementia were excluded (p = 0.222). Use of psychotropic drugs in 1992–3 was not related to mean Aβ42 or T‐tau levels in 1992–3, or to mean change in MMSE score.
Discussion
CSF Aβ42 may potentially be a useful risk indicator of cognitive decline in cognitively healthy older people. This is the first population‐based study to show an independent, longitudinal relationship between CSF Aβ42 and subsequent decline in MMSE score over an extensive follow‐up period among a population‐based group of women without dementia. There is only one other population‐based study of CSF markers showing that Aβ42 was predictive of dementia over a 3‐year follow‐up period, conducted in 85‐year‐old women and men.2 Both our study and the latter study included participants who exhibited relatively high mean baseline MMSE scores (28.3 in both samples), underscoring the predictive nature of the results.
Low levels of CSF Aβ42 and high CSF T‐tau are increasingly used as diagnostic markers to evaluate manifest AD, and may reflect the disease process.20,23 Lower levels of CSF Aβ42 have been related to higher numbers of neuritic plaques in the neocortex and hippocampus in autopsy samples, and pathologically confirmed AD.24 In addition, CSF biomarkers have also been used to predict conversion of MCI to AD in clinical samples5,8,25,26,27; however no data have been presented on their utility related to early stages of cognitive decline, particularly in a population‐based sample.
These data are important when considering the timing of AD neuropathology in relation to clinical manifestations of cognitive decline and dementia. Clinical studies show low CSF Aβ42 and high T‐tau in both early and late AD,4,5,20,26,28 and across categories of AD severity.29 In addition, lower Aβ42 and higher T‐tau have been cross‐sectionally correlated with lower MMSE scores in a clinical sample of patients with AD and controls.30 However, this finding has not always been replicated,29,31 despite patients with AD in these latter studies having higher levels of T‐tau compared with controls. Since most reports on the relationship between CSF markers, cognition and dementia are cross‐sectional, have short lengths of follow‐up and are based on clinical samples, knowledge regarding the predictive value of CSF markers for cognitive decline in populations is lacking. AD pathology starts at least 20–30 years before the clinical onset of the disease, and perhaps even earlier.32 In our study, women who experienced the most cognitive decline over an 8‐year follow‐up period had lower levels of Aβ42 at baseline. These data also support the role of CSF Aβ42 as an earlier pathological marker than T‐tau, which appears to have utility as a later marker of brain pathology, given its strong relationship with dementia and cross‐sectional MMSE score.
Although the findings of this study are intriguing and point to the predictive potential of CSF biomarkers for cognitive decline, and a better understanding of dementia aetiology, there are some limitations that should be mentioned. (1) The study sample is relatively small, thus our findings merit replication. However, the ability to evaluate CSF in a population‐based sample of older people who are healthy and initially without dementia is a rare opportunity. (2) Our sample is comprised only of Caucasian women; hence, our findings have a somewhat limited sex and racial generalisability. (3) A diagnosis of MCI, amnestic or otherwise, has not been made in this sample, and hence we cannot comment on this clinical designation in itself. (4) Our measures of cognition using the MMSE were conducted 8 years apart, with no intervening measures. Thus, we have limited information as to the trajectory of MMSE decline during this period and the temporal relationship between Aβ42 and decline. (5) Although widely used in population‐based studies, the MMSE is a crude measure of global cognition. While this limits to some extent its sensitivity and specificity, the simplicity of this test minimises potential inter‐rater and intra‐rater errors.
In conclusion, in our sample of cognitively healthy older women representative of the Swedish population, a potential early risk indicator of cognitive decline, notably CSF Aβ42, has been observed. Although other studies have shown Aβ42 to be related to AD and the conversion of MCI to AD, this is the first study to show the relationship between Aβ42 and overall age‐related cognitive decline. Future studies of CSF Aβ42 and T‐tau will assist in the characterisation of risk indicators by which to measure risk of cognitive decline and dementia for the initiation of earlier intervention and possibly prevention strategies.
Abbreviations
Aβ42 - β‐amyloid 1–42
AD - Alzheimer's disease
CSF - cerebrospinal fluid
LP - lumbar puncture
MCI - mild cognitive impairment
MMSE - Mini‐Mental State Examination
T‐tau - total tau
Footnotes
Funding: This study was supported by grants from the Swedish Research Council (grant nos 11337, 11267), the Swedish Council for Working Life and Social Research (nos 2835, 2646), the Alzheimer's Association Stephanie B Overstreet Scholars (IIRG‐00‐2159) and the Alzheimer's Association Zenith Award (ZEN‐01‐3151), National Institutes of Health/National Institutes on Aging 1R03AG026098 ‐ 01A1, Stiftelsen Söderström‐Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Stiftelsen Professor Bror Gadelius' Minnesfond, the Swedish Society of Medicine, the Göteborg Medical Society, Alzheimerfonden, Alma och Anna Yhlen's Foundation, the Göteborg Medical Services and Social Services Administrations, and the Fredrik and Rosa von Malmborgs Foundation for Brain Research. The sponsors had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Competing interests: None declared.
References
- 1.Borjesson‐Hanson A, Edin E, Gislason T.et al The prevalence of dementia in 95‐year olds. Neurology 2004632436–2438. [DOI] [PubMed] [Google Scholar]
- 2.Skoog I, Davidsson P, Aevarsson O.et al Cerebrospinal fluid beta‐amyloid 42 is reduced before the onset of sporadic dementia: a population‐based study in 85‐year‐olds. Dement Geriatr Cogn Disord 200315169–176. [DOI] [PubMed] [Google Scholar]
- 3.Hulstaert F, Blennow K, Ivanoiu A.et al Improved discrimination of AD patients using beta‐amyloid(1–42) and tau levels in CSF. Neurology 1999521555–1562. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen N, Minthon L, Davidsson P.et al Evaluation of CSF‐tau and CSF‐Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 200158373–379. [DOI] [PubMed] [Google Scholar]
- 5.Andreasen N, Minthon L, Vanmechelen E.et al Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett 19992735–8. [DOI] [PubMed] [Google Scholar]
- 6.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol 20032605–613. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M, Sisodia S S, Price D L. Neurofibrillary tangles and beta‐amyloid deposits in Alzheimer's disease. Curr Opin Neurobiol 19911441–447. [DOI] [PubMed] [Google Scholar]
- 8.Hansson O, Zetterberg H, Buchhave P.et al Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow‐up study. Lancet Neurol 20065228–234. [DOI] [PubMed] [Google Scholar]
- 9.Bengtsson C, Blohme G, Hallberg L.et al The study of women in Gothenburg 1968–79. A population study. General design, purpose and sampling results. Acta Med Scand 1973193311–318. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson C, Gredmark T, Hallberg L.et al The population study of women in Göteborg 1980–81: the third phase of a longitudinal study. Scand J Soc Med 198917141–145. [DOI] [PubMed] [Google Scholar]
- 11.Bengtsson C, Ahlquist M, Andersson K.et al The Prospective Population Study of Women in Gothenburg, Sweden, 1968–69 to 1992–93. A 24‐year follow‐up study with special reference to participation, representativeness, and mortality. Scand J Primary Health Care 199715214–219. [DOI] [PubMed] [Google Scholar]
- 12.Palsson S, Larsson L, Tengelin E.et al The prevalence of depression in relation to cerebral atrophy and cognitive performance in 70‐ and 74‐year‐old women in Gothenburg. The Women's Health Study. Psychol Med 20013139–49. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Waern M, Sjögren K.et al Midlife respiratory function related to white matter lesions and lacunar infarcts in late life: the Prospective Population Study of Women in Gothenburg, Sweden. Stroke 2006371658–1662. [DOI] [PubMed] [Google Scholar]
- 14.Folstein M F, Folstein S E, McHugh P R. “Mini‐Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 15.Åsberg M, Perris C, Schalling D.et al The CPRS—development and applications of a psychiatric rating scale. Acta Psychiatr Scand 1978271(Suppl)5–27. [DOI] [PubMed] [Google Scholar]
- 16.Skoog I, Nilsson L, Palmertz B.et al A population‐based study of dementia in 85‐year‐olds. N Engl J Med 1993328153–158. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd, revised edn. Washington, DC: American Psychiatric Association, 1987
- 18.Bengtsson C L, Hallberg H, Noppa H.et al Anthropometric data in middle‐aged women. The population study of women in Göteborg 1968–1969. Acta Morphol Neurol Scand 197917133. [PubMed] [Google Scholar]
- 19.Blennow K, Fredman P, Wallin A.et al Protein analyses in cerebrospinal fluid. I. Influence of concentration gradients for proteins on cerebrospinal fluid/serum albumin ratio. Eur Neurol 199333126–128. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen N, Hesse C, Davidsson P.et al Cerebrospinal fluid beta‐amyloid(1–42) in Alzheimer disease: differences between early‐ and late‐onset Alzheimer disease and stability during the course of disease. Arch Neurol 199956673–680. [DOI] [PubMed] [Google Scholar]
- 21.Vanmechelen E, Vanderstichele H. Towards an earlier diagnosis of Alzheimer's disease. J Biotechnol 199866229–231. [DOI] [PubMed] [Google Scholar]
- 22.Blennow K, Wallin A, Agren H.et al Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 199526231–245. [DOI] [PubMed] [Google Scholar]
- 23.Galasko D, Chang L, Motter R.et al High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol 199855937–945. [DOI] [PubMed] [Google Scholar]
- 24.Strozyk D, Blennow K, White L R.et al CSF Abeta 42 levels correlate with amyloid‐neuropathology in a population‐based autopsy study. Neurology 200360652–656. [DOI] [PubMed] [Google Scholar]
- 25.Hampel H, Teipel S J, Fuchsberger T.et al Value of CSF beta‐amyloid1‐42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry 20049705–710. [DOI] [PubMed] [Google Scholar]
- 26.Buerger K, Teipel S J, Zinkowski R.et al CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 200259627–629. [DOI] [PubMed] [Google Scholar]
- 27.Bouwman F H, Schoonenboom S N, van der Flier W M.et al CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging 2006. Epub ahead of print [DOI] [PubMed]
- 28.Arai H. Clinical heterogeneity in mild cognitive impairment—beyond clinical diagnosis: towards imaging amyloid. Rinsho Shinkeigaku 200444924–928. [PubMed] [Google Scholar]
- 29.Riemenschneider M, Buch K, Schmolke M.et al Cerebrospinal protein tau is elevated in early Alzheimer's disease. Neurosci Lett 1996212209–211. [DOI] [PubMed] [Google Scholar]
- 30.Ibach B, Binder H, Dragon M.et al Cerebrospinal fluid tau and beta‐amyloid in Alzheimer patients, disease controls and an age‐matched random sample. Neurobiol Aging 2006271202–1211. [DOI] [PubMed] [Google Scholar]
- 31.Galasko D, Clark C, Chang L.et al Assessment of CSF levels of tau protein in mildly demented patients with Alzheimer's disease. Neurology 199748632–635. [DOI] [PubMed] [Google Scholar]
- 32.Launer L J. The epidemiologic study of dementia: a life‐long quest? Neurobiol Aging 200526335–340. [DOI] [PubMed] [Google Scholar]