Abstract
Background
The mismatch between perfusion and diffusion lesions on magnetic resonance perfusion‐weighted imaging (PWI)/diffusion‐weighted imaging (DWI) may help identify patients for thrombolysis. Evidence underlying this hypothesis was assessed.
Methods
All papers describing magnetic resonance PWI/DWI findings in patients with acute ischaemic stroke, and their functional and/or radiological outcome at 1 month, with or without thrombolysis were systematically reviewed.
Results
11 papers fulfilled the inclusion criteria. Among these, there were 5 different mismatch definitions and at least 7 different PWI methods. Only 3 papers including 61 patients with and 18 without mismatch provided data on mismatch, outcome and influence of thrombolysis. Mismatch (v no mismatch) without thrombolysis was associated with a non‐significant twofold increase in the odds of infarct expansion (odds ratio (OR) 2.2, 95% confidence interval (CI) 0.34 to 14.1), which did not change with thrombolysis (OR 2.0, 95% CI 0.37 to 10.9). Half of the patients without mismatch also had infarct growth (with or without thrombolysis). No data were available on functional outcome.
Conclusions
Standardised definitions of mismatch and perfusion are needed. Infarct growth may occur even in the absence of mismatch. Currently, data available on mismatch are too limited to guide thrombolysis in routine practice. More data are needed from studies including patients with and without mismatch, and randomised treatment allocation, to determine the role of mismatch.
Ischaemic stroke is a global problem, for which few acute treatments are available. Thrombolysis has to be given rapidly and, when guided by plain computed tomography scan of the brain, carries a risk of intracranial haemorrhage. Imaging the mismatch between diffusion‐weighted imaging (DWI) and perfusion‐weighted imaging (PWI) on magnetic resonance imaging (MRI) (or presumed reversible ischaemia on computed tomography perfusion1) might help identify patients with tissue at risk of infarction (even beyond the current 3 h time window), thereby avoiding thrombolysis in those with little chance of benefit.2,3 These techniques are used increasingly where technology is available, and in acute‐stroke trials (http://www.strokecenter.org/trials).4
The increasing use of this approach in trials and routine practice suggests that there are clear definitions of what constitutes mismatch and substantial evidence to justify its use. However, it is now known that the DWI lesion is not irreversible (initial DWI lesions may disappear spontaneously or after thrombolysis5), and that the appearance of PWI lesion depends on which of the many methods were used to calculate it. Different perfusion parameters (eg, mean transit time (MTT), regional cerebral blood flow6 and arterial input function7) give different perfusion lesion volumes in the same patient. Thus, it is unclear whether the presence (v absence) of mismatch affects prognosis. If mismatch is to be used to select patients for treatment, then the key point is to determine whether thrombolysis has a greater effect in the presence than in the absence of mismatch. This requires a randomised controlled trial in which patients with and without mismatch are randomly selected to receive thrombolysis or control treatment, an expensive and difficult undertaking given the large sample size needed.8
As there is already a considerable body of literature available on the magnetic resonance mismatch concept, we undertook this systematic review to assess all current evidence on the effect of magnetic resonance PWI/DWI mismatch in patients with acute ischaemic stroke on outcome (clinical and radiological) and whether this is modified by thrombolysis. We set rigorous prespecified inclusion and exclusion criteria based on scientific principles for observational studies and randomised trials to minimise bias.
Methods
Design
We sought papers describing PWI/DWI mismatch and outcome in the presence or absence of thrombolysis. We included papers published in full to obtain detailed methodological data and results.
Search strategy
We developed a search strategy with the Cochrane Stroke Group for articles published between January 1996 and May 2005. We searched Medline and EMBASE (using the terms “diffusion weighted”, “perfusion weighted”, “thrombolysis” and “magnetic resonance imaging”, exploded to maximise findings) and reference lists in the identified articles for further relevant papers.
Inclusion criteria
We included prospective studies of human acute stroke, of at least 20 patients, imaged at presentation with acute stroke using magnetic resonance, DWI and PWI, with clinical assessment at baseline and follow‐up at least 1 month after stroke using a recognised assessment scale, and where possible radiological follow‐up, in which patients did or did not receive thrombolysis.
Exclusion criteria
We excluded articles published before 1996 (before that neither magnetic resonance PWI/DWI nor thrombolysis was widely used in acute stroke), retrospective studies (because of the potential for bias), with <20 patients (very small sample sizes are prone to bias and provide little robust data to inform clinical practice), and with functional and/or radiological outcome assessed at less than a month after stroke (before that would be too early to assess functional outcome; radiologically, ischaemic lesions may still be evolving,9 “fogging” may cause underestimation,10 and oedema may cause overestimation of the final lesion volume11).
Data extraction
Data were collected on a standardised assessment form by one reviewer. Queries were independently checked by another reviewer. We collected the sample size, patients' clinical characteristics, clinical scores (eg, National Institutes of Health Score (NIHSS)), time from onset of symptom to imaging, details of the magnetic resonance sequences performed and post‐processing techniques, definition of PWI/DWI mismatch, evidence of infarct expansion (increase in the lesion volume from the acute baseline DWI to final T2), whether interpretation of the magnetic resonance images was blinded to clinical details or imaging, details of patients excluded from analysis, whether administration of thrombolysis was randomised or not, and any information on functional or radiological outcome in those who received or did not receive thrombolysis with or without PWI/DWI mismatch. We compared imaging at presentation with final follow‐up performed at 1 month or more. We did not examine scan data from intermediary time points (if available) because they are unreliable for estimation of functional outcome or final infarct extent (see above). We were careful to avoid including duplicate publications of the same patients. We defined poor functional outcome as modified Rankin Score (mRS) ⩾1 or Barthel Index<90.
Analysis
We summarised study population demographics, proportions with or without mismatch, number treated with thrombolysis, with poor functional outcome or infarct expansion. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to determine associations between mismatch, infarct expansion, functional outcome and any influence of thrombolysis. We aimed at comparing functional and radiological outcomes in patients with mismatch with those without mismatch, and to determine whether thrombolysis changed the relationship between mismatch and functional or radiological outcome.
Results
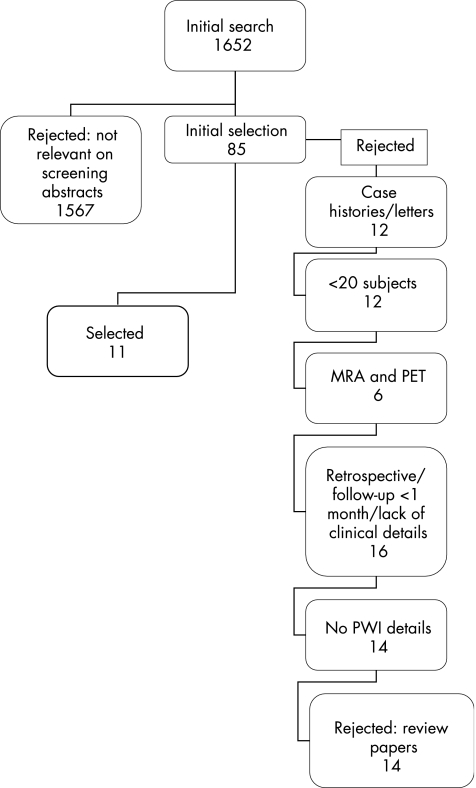
The search identified 1652 papers on any aspect of DWI and PWI, of which 85 were potentially relevant to DWI/PWI mismatch and outcome with or without thrombolysis. Eleven papers (641 patients) fulfilled all prespecified inclusion criteria (for reasons for rejection, often more than one reason, see fig 1).
Figure 1 Selection of papers for systematic review. MRA, magnetic resonance angiography; PET, positron emission tomography; PWI, perfusion‐weighted imaging.
Ten included reports11,12,13,14,15,16,17,18,19,20 were prospective observational studies, and one4 was a placebo‐controlled, double‐blind, randomised, dose‐finding phase II trial of desmoteplase. These papers derive from six research groups. Median time to baseline magnetic resonance imaging ranged from 1.5 to 6 h after stroke. Seven papers had radiological follow‐up at 1 month or more11,12,13,14,15,16 in most patients (table 1).
Table 1 Methodological details of included studies.
| Author | Publication date | Sample size | Incomplete imaging*/died | Clinical score | Other outcome scores at ⩾ 1 month | Time to acute MRI | Time to final MRI (days) |
|---|---|---|---|---|---|---|---|
| Beaulieu C11 | 1999 | 21 | 6 | NIHSS | None | Mean (SD) 5.2 (1.2) h | Mean (SD) 42 (22) |
| Barber P12 | 2004 | 49 | 4 | NIHSS | BI and mRS | Median 4 h (IQR 3.3–5) | Median 84 (IQR 70–89) |
| Rohl L14 | 2001 | 22 | 1 | SSS | BI | Mean 5 h | Range 22–42 (1@102) |
| Parsons M15 | 2002 | 40 | 4 | NIHSS | mRS | Treatment group mean (SD) 3.8 (1.2) h Controls 3.7 (1.2) | Treatment group mean (SD) 77.9 (17.1) Controls 81.4 (12) |
| Barber P13 | 1999 | 26 | 5 | CNS | BI and mRS | Mean (SD) 12.1 (7.6) h | Mean (SD) 90.1 (30.3) |
| Hacke W4† | 2005 | 104 | 18 | NIHSS | BI and mRS | Median 325 min | Due at 30 |
| Derex L16 | 2004 | 49 | 5 | NIHSS | mRS | Mean (SD) 3 h 37 (52) mins | Due at 60 |
| Schellinger P17 | 2001 | 51 | 1 | NIHSS and SSS | BI and mRS | Mean (SD) 3.33 (1.29) h | Due at 5 |
| Chalela J19 | 2004 | 42 | 5 | NIHSS | mRS | Median to: DWI 84 min, PWI 92 min | Median to: DWI 253 mins, PWI 269 mins |
| Rother J18 | 2002 | 139 | 10 | NIHSS | mRS | Median 180 min (75–360) | Due at 7 |
| Ribo M20 | 2005 | 122 | Not specified | NIHSS | mRS | Median group A: 136 min (60–180); group B: 223 min (185–360) | CT 24–48 h |
BI, Barthel Index; CNS, central nervous system; mRS, modified Rankin Score; NIHSS, National Institutes of Health Score; SSS, Scandinavian Stroke Scale.
The most common baseline clinical score was the NIHSS, used in all but two papers,19,20 by an individual explicitly stated to be trained in its use. In all but one paper,11 other scores (eg, Barthel Index and mRS) were recorded at follow‐up. Eight papers gave incomplete details of blinding of clinical and radiological assessors. Three papers (27%)17,18,20 did not mention blinding at all.
Measurement of perfusion lesion
All groups used gadolinium‐based dynamic susceptibility contrast imaging to assess perfusion, the dose varying from 0.1 to 0.2 mmol/kg. The method for perfusion lesion assessment varied: four papers calculated time to peak11,16,18,20; the others used some form of MTT measurement, quantitative in three cases (table 2).12,14,15
Table 2 Definition and frequency of PWI/DWI mismatch in studies meeting methodological inclusion criteria.
| Author | Definition of PWI/DWI mismatch | Workstation/ visual measurement | Number with mismatch | Dose of gadolinium (mmol/kg)* | Perfusion measure | Number treated with thrombolysis |
|---|---|---|---|---|---|---|
| Beaulieu C11 | Differences of at least ±10% | Workstation | 11 (52%) PWI>DWI 7 (33%) PWI⩽DWI | 0.2 | TTP* | 11 (52%) |
| Barber P12 | PWI >acute DWI | Workstation | 77% | 0.1 | MTT† | 12 (24%) |
| Rohl L14 | >10% difference between acute DWI lesion and MTT map lesion | Workstation | 18 (82%) | 0.1 | MTT† | None |
| Parsons M15 | Acute MTT (delay >4 s) lesion volume 20%>DWI lesion | Workstation | 16 (84%) treatment group 16 (76%) control group | 0.2 | MTT† | 19 (48%) |
| Barber P13 | PWI >acute DWI | Workstation | 14 (56%) | 0.1 | rMTT* | None |
| Hacke W4 | ⩾20% PWI/DWI mismatch | Visual initially then workstation | 104 (100%) | 0.1 | MTT* | 75 (72%) |
| Derex L16 | PWI/DWI volume ratio of ⩾1.2 | Workstation | 42 (85%) | 0.1 | TTP* | All (100%) |
| Schellinger P17 | PWI/DWI volume ratio of >1.2 | Workstation | 40/51 (78%) | 25 ml | MTT* | 24 (47%) |
| Chalela J19 | MTT lesion minus DWI lesion at each time point. | Workstation | Not specified | 0.1 | MTT* | All (100%) |
| Rother J18 | PWI/DWI volume ratio of >1.2 | Workstation | 120/139 (86.3%) | 25 ml | TTP* | 76 (55%) |
| Ribo M20 | Group B: DWI/PWI mismatch >50% | Visual | Group B:43/122 (35%) | Bolus | TTP* | All (100%) |
DWI, diffusion‐weighted imaging; MTT, mean transit time; PWI, perfusion‐weighted imaging; TTP, time to peak.
*Semi‐quantitative measure.
Assessment of perfusion/diffusion mismatch
There was no consistent definition of mismatch. Among the 11 papers from 6 research groups, there were 5 different definitions of mismatch. Mismatch was determined by “visual inspection” in two studies,4,20 and by measuring lesion volumes on a workstation in the rest, using various lesion boundary definitions (eg, MTT >4 s compared with the contralateral side) (table 2).
Overall assessment of PWI/DWI mismatch on outcome and effect of thrombolysis
Among the 11 studies initially included in the review, we were able to extract data regarding mismatch and outcome (without or with thrombolysis) only from three.11,14,15 Although all of these studies recruited at least 20 patients, not all of the original sample contributed to the data, mainly due to incomplete imaging (table 1). In the rest, it was not possible to separate the results for thrombolysis for patients with and those without thrombolysis from those of patients without mismatch,12,16,18 although some did have outcome data. Some papers only reported recanalisation and outcome, not mismatch.13,17 All three papers with usable data included patients with and without mismatch, and two11,15 examined the effects of thrombolysis. In total, there were 61 patients with, and 18 without mismatch. Final follow‐up scans were not available (patient died or scan was not performed) for 7 of 61 patients, some with mismatch at baseline and some without. Only two papers reported functional outcome.14,15
PWI/DWI mismatch and outcome: no thrombolysis
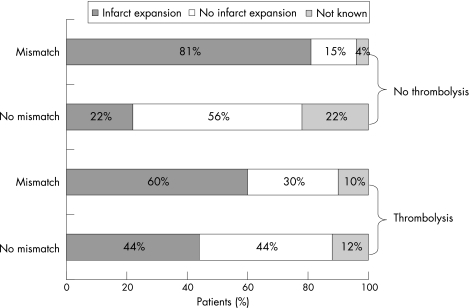
Two papers14,15 provided data on mismatch and functional outcome, and three11,14,15 on infarct expansion at 1 month or more (total n = 50 patients) in patients who did not receive thrombolysis: 41 of 50 patients had mismatch (by any definition), of whom 33 (80%) had any infarct expansion and 6 (20%) did not (follow‐up scans were missing for two patients); 9 of 50 patients had no mismatch, of whom 5 (56%) developed infarct expansion. Therefore, mismatch was associated with a non‐significant twofold increase in the odds of infarct expansion (OR 2.2, 95% CI 0.34 to 14.1). Note that the wide CIs include the possibility of both a reduction and an increase in the risk. Data on functional outcome were not presented in a way that allowed calculation of ORs. However, the mean Barthel Index or mRS at final follow‐up was non‐significantly worse in those with mismatch at baseline than in those without mismatch (table 3, fig 2).
Table 3 Details of patients with and without mismatch not treated with thrombolysis.
| Author | Number with mismatch | Number without mismatch | Baseline NIHSS: mismatch Mean (range) | Baseline NIHSS: no mismatch Mean (range) | Outcome score: mismatch Mean (range) | Outcome score: no mismatch Mean (range) | Mismatch and no infarct expansion | Mismatch and infarct expansion | No mismatch and no infarct expansion | No mismatch and infarct expansion | No final follow‐up scan |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beaulieu C11 | 7 | 1 | 14 (6–24) | 7 | No data | No data | 2 | 3 | 1 | 0 | 2 (both mismatch) |
| Rohl L14 | 18 | 3 | SSS 38 (11–56) | SSS 41(31–56) | BI 86 (25–100) | BI 98 (94–100) | 3 | 15 | 1 | 2 | 0 |
| Parsons M15 | 16 | 5 | 15 (7–20) | 16(10–20) | mRS 3 (0–6) | mRS 2 (1–4) | 1 | 15 | 3 | 0 | 2 (both no mismatch) |
| Total | 41 | 9 | 6 (15%) | 33 (81%) | 5 (56%) | 2 (22%) | 4 | ||||
| Best case* scenario | 8 (20%) | 33 (81%) | 7 (78%) | 2 (22%) | 0 | ||||||
| Worst case scenario† | 6 (15%) | 35 (85%) | 5 (56%) | 4 (44%) | 0 |
BI, Barthel Index; mRS, modified Rankin Score; NIHSS, National Institutes of Health Score; SSS, Scandinavian Stroke Scale.
*Assumes no infarct growth occurred in patients with missing scans.
†Assumes infarct growth occurred in patients with missing scans.
Figure 2 Fate of the acute magnetic resonance diffusion‐weighted imaging lesion in patients with or without mismatch and/or thrombolysis.
PWI/DWI mismatch and outcome: with thrombolysis
Two studies provided data on mismatch, radiological outcome (but only one on functional outcome15) and thrombolysis (total n = 29).11,15 Note that in one,11 thrombolysis was actually given immediately before the first MRI, meaning that the baseline scans may have already been affected by thrombolysis, thus making interpretations about the effects of recombinant tissue‐type plasminogen activator (rt‐PA) in these patients difficult. However, we included these data on the basis that the effects of rt‐PA are not instantaneous. With this in mind, we can conclude that 12 of 20 (60%) patients with mismatch had infarct growth (data missing for two patients) after thrombolysis, whereas 8 of 20 (40%) did not; 4 of 9 (44%) patients without mismatch had infarct expansion (data missing for one patient) with thrombolysis, 5 of 9 (50%) did not. Thus, there was a similar OR for the risk of infarct expansion in the presence of mismatch with thrombolysis (OR 2, 95% CI 0.37 to 10.9) as that without thrombolysis. The wide CIs include the possibility of either reduction or increase in the risk. The one paper with clinical outcome data15 compared patients with mismatch who received thrombolysis with 16 historical controls with mismatch who did not receive thrombolysis (table 2), but such historical comparisons are prone to bias and are unreliable. Hence, there are no meaningful data on clinical outcomes in patients with or without mismatch in the presence of thrombolysis (table 4, fig 2).
Table 4 Details of patients with and without mismatch treated with thrombolysis.
| Author | Number with mismatch | Number without mismatch | Baseline NIHSS: mismatch Mean (range) | Baseline NIHSS: no mismatch Mean (range) | Outcome score: mismatch | Outcome score: no mismatch | Mismatch and no infarct expansion | Mismatch and infarct expansion | No mismatch and no infarct expansion | No mismatch and infarct expansion | No final follow‐up scan |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beaulieu C11 | 4 | 6 | 14 (8–24) | 7 (2–14) | No data | No data | 0 | 3 | 3 | 3 | 1 (mismatch patient) |
| Parsons M15 | 16 | 3 | 15 (9–220 | 15 (12–19) | mRS 2 (1–6) | mRS 4 (2–6) | 6 | 9 | 1 | 1 | 2 (1 mismatch, 1 without) |
| Total | 20 | 9 | 6 (30%) | 12 (60%) | 4 (44%) | 4 (44%) | 3 | ||||
| Best case scenario* | 8 (40%) | 12 (60%) | 5 (56%) | 4 (44%) | 0 | ||||||
| Worst case scenario† | 6 (30%) | 14 (70%) | 4 (44%) | 5 (56%) | 0 |
mRS, modified Rankin Score; NIHSS, National Institutes of Health Score.
*Assumes no infarct growth occurred in patients with missing scans.
†Assumes infarct growth occurred in patients with missing scans.
To account for missing follow‐up scans, we calculated “best” (assuming that all the patients with missing data did not have infarct expansion) and “worst” (assuming that all the patients with missing data did have infarct expansion) case scenarios (tables 3 and 4). In the best case scenario, mismatch without thrombolysis was associated with a 14‐fold increase in the risk of infarct expansion (OR 14.4, 95% CI 2.5 to 83.2), and mismatch with thrombolysis was associated with a twofold increase in the risk of infarct expansion (OR 2.5, 95% CI 0.48 to 12.9). In the worst case scenario, mismatch without thrombolysis was associated with a sevenfold increase in the risk of infarct expansion (OR 7.3, 95% CI 1.5 to 35.2) and mismatch with thrombolysis was associated with a twofold increase in the risk of infarct expansion (OR 1.86, 95% CI 0.37 to 9.49). However, note that the marked change in OR when outcome changed for only a few patients, combined with the wide CIs, indicates that these data, although promising, are unreliable and highly unstable, and require confirmation in large, methodologically sound studies.
Discussion
This review highlights the urgent need for more data to confirm and refine or refute the mismatch concept, and for standardisation of methods to assess mismatch and PWI lesions. Despite more than 1500 papers reporting some aspect of PWI/DWI mismatch, many advocating its use to identify “tissue at risk”, there is very limited evidence even to say whether patients with mismatch have a different outcome from those without mismatch, or crucially, what the effect of rt‐PA might be. These data were very fragmented and difficult to manage systematically, and there was little that was ultimately usable. Indeed, some might argue that we should have excluded even more, such as the study that performed magnetic resonance DWI/PWI just after rt‐PA. However, we reasoned that others may have adopted the same approach without explicitly mentioning it, and that this was a relatively minor flaw among many more fundamental ones. We did not seek additional unpublished information from authors because personal communications are not peer‐reviewed and may be misleading, and the published literature is the accessible knowledge base. Therefore, current opinion should not be based on information that is absent from the published literature, because unpublished information is not accessible to all to evaluate and form their own opinion.
The inclusion criteria for the review included studies with >20 patients because of the well‐known problem of bias in smaller studies. Other criteria such as type and site of arterial occlusion were outside the scope of this systematic review and were therefore not included.
The lack of standardisation of the DWI and PWI imaging is a major problem. Perfusion imaging used different doses of contrast and different processing techniques, and measured different parameters in different ways. It is unclear whether one should use complex and time‐consuming methods of analysis of PWI data incorporating the arterial input function (and if so which7) or, as suggested recently, use simpler semiquantitative methods such as Tmax, which may be just as good.21 There was no consistency in the definition or measurement of mismatch either. The 5 definitions from the 6 research groups ranged from a PWI lesion >acute DWI lesion12,13,19 to a PWI lesion 50% >acute DWI lesion.20 Two papers specified visual inspection, but the others measured lesion volume on a workstation. Few studies12,13,16 commented on the observer reliability of any of these PWI/DWI assessments.
Nonetheless, the pattern suggested by fig 2 does indeed, rather tantalisingly, suggest that patients with mismatch are more likely than patients without mismatch to have infarct growth, and that the proportion of patients with mismatch who had infarct growth may be reduced by thrombolysis. However, there are no data on functional outcome (more relevant than radiological outcomes), and these data are not from randomised comparisons, but from rather small observational studies of different patients with widely differing definitions, sometimes with historical controls. Clearly, some patients without mismatch definitely get infarct growth, so the absence of mismatch does not mean that there is no “tissue at risk” of infarct growth. This suggests little justification for excluding patients without mismatch either from routine acute stroke treatments or possibly from trials. If the mismatch theory is correct, then there is an urgent need to gather more robust evidence to support its use.
To move forward, common standards and definitions for mismatch are needed. A large randomised trial of thrombolysis against control in patients with and without mismatch is needed to reliably determine whether the degree of mismatch really does influence response to thrombolytic treatment. The EPITHET (http://www.strokecenter.org/trials) study has this design, although patients are recruited on the basis of their computed tomography results and not on the basis of magnetic resonance mismatch, which may lead to an imbalance of patients with and without mismatch in the treatment and control groups. Certainly, until there is better evidence, patients without mismatch should probably not be denied any routine acute treatments, because about 50% will get infarct expansion that might be prevented by acute treatments such as thrombolysis. The lack of data underpinning the mismatch theory should also be acknowledged in the design of any future trials of novel therapeutic agents (eg, new thrombolytic or neuroprotective agents) planning to use mismatch as an inclusion criterion. These problems also apply to computed tomography perfusion. It certainly should not be assumed that presumed reversible ischaemia on computed tomography perfusion identifies “tissue at risk” without adequate data, and standards should be agreed urgently.
Acknowledgements
We thank Brenda Thomas of the Cochrane Stroke Review Group for her help with the search strategy and the Health Foundation who funded Dr Ingrid Kane.
Abbreviations
DWI - diffusion‐weighted imaging
MRI - magnetic resonance imaging
mRS - modified Rankin Score
MTT - mean transit time
NIHSS - National Institutes of Health Score
PWI - perfusion‐weighted imaging
rt‐PA - recombinant tissue‐type plasminogen activator
Footnotes
Competing interests: None.
References
- 1.Parsons M W, Pepper E M, Chan V.et al Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 200558672–679. [DOI] [PubMed] [Google Scholar]
- 2.Schlaug G, Benfield A, Baird A E.et al The ischemic penumbra. Operationally defined by diffusion and perfusion MRI. Neurology 1999531528–1537. [DOI] [PubMed] [Google Scholar]
- 3.Shih L C, Saver J L, Alger J R.et al Perfusion‐weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003341425–1430. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Albers G, Al Rawi Y.et al The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI‐based 9‐h window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 20053666–73. [DOI] [PubMed] [Google Scholar]
- 5.Fiehler J, Foth M, Kucinski T.et al Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke 20023379–86. [DOI] [PubMed] [Google Scholar]
- 6.Butcher K, Parsons M, Baird T.et al Perfusion thresholds in acute stroke thrombolysis. Stroke 2003342159–2164. [DOI] [PubMed] [Google Scholar]
- 7.Thijs V N, Somford D M, Bammer R.et al Influence of arterial input function on hypoperfusion volumes measured with perfusion‐weighted imaging. Stroke 20043594–98. [DOI] [PubMed] [Google Scholar]
- 8.Schulz K, Grimes D A. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 20053651657–1661. [DOI] [PubMed] [Google Scholar]
- 9.Warach S, Gaa J, Siewert B.et al Acute human stroke studied by whole brain echo planar diffusion‐weighted magnetic resonance imaging. Ann Neurol 199537231–241. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien P, Sellar R J, Wardlaw J M. Fogging on T2‐weighted MR after acute ischaemic stroke: how might this occur and what are the implications? Neuroradiology 200446635–641. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu C, de Crespigny A, Tong D C.et al Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 199946568–578. [DOI] [PubMed] [Google Scholar]
- 12.Barber P A, Parsons M W, Desmond P M.et al The use of PWI and DWI measures in “proof‐of‐concept” stroke trials. J Neuroimaging 200414123–132. [PubMed] [Google Scholar]
- 13.Barber P A, Davis S M, Darby D G.et al Absent middle cerebral artery flow predicts the presence and evolution of the ischemic penumbra. Neurology 1999521125–1132. [DOI] [PubMed] [Google Scholar]
- 14.Rohl L, Geday J, Ostergaard L.et al Correlation between diffusion‐ and perfusion‐weighted MRI and neurological deficit measured by the Scandinavian Stroke Scale and Barthel Index in hyperacute subcortical stroke (< or = 6 hours). Cerebrovasc Dis 200112203–213. [DOI] [PubMed] [Google Scholar]
- 15.Parsons M W, Barber P A, Chalk J.et al Diffusion‐ and perfusion‐weighted MRI response to thrombolysis in stroke. Ann Neurol 20025128–37. [DOI] [PubMed] [Google Scholar]
- 16.Derex L, Nighoghossian N, Hermier M.et al Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci 20042253–9. [DOI] [PubMed] [Google Scholar]
- 17.Schellinger P D, Fiebach J B, Jansen O.et al Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol 200149468–469. [PubMed] [Google Scholar]
- 18.Rother J, Schellinger P D, Gass A.et al Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke 2002332438–2445. [DOI] [PubMed] [Google Scholar]
- 19.Chalela J A, Kang D W, Luby M.et al Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol 200455105–112. [DOI] [PubMed] [Google Scholar]
- 20.Ribo M, Molina C A, Rovira A.et al Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3‐ to 6‐hour window using multimodal transcranial Doppler/MRI selection protocol. Stroke 200536602–606. [DOI] [PubMed] [Google Scholar]
- 21.Butcher K, Parsons M, MacGregor L.et al Refining the perfusion‐diffusion mismatch hypothesis. Stroke 2005361153–1159. [DOI] [PubMed] [Google Scholar]