Abstract
Background
The issue of when to start treatment in Parkinson's disease (PD) remains controversial. Some favour treatment at diagnosis while others opt for a “wait and watch” policy. The effect of the latter policy on the self reported health status of people with PD is unknown.
Aims
To record self reported health status through longitudinal use of a validated PD specific questionnaire (PDQ‐39) in untreated PD patients in multiple centres in the UK. To compare patients who were left untreated with those who were offered treatment during follow‐up.
Methods
A multicentre, prospective, “real life” observational audit based study addressing patient reported outcomes in relation to self reported health status and other sociodemographic details.
Results
198 untreated PD were assessed over a mean period of 18 months. During two follow‐up assessments, the self reported health status scores in all eight domains of the PDQ‐39 and the overall PDQ‐39 summary index worsened significantly (p<0.01) in patients left untreated. In a comparative group in whom treatment was initiated at or soon after diagnosis, there was a trend towards improvement in self reported health status scores after treatment was started.
Conclusions
This study addresses for the first time self reported health status, an indicator of health related quality of life, in untreated PD. The findings may strengthen the call for re‐evaluation of the policy to delay treatment in newly diagnosed patients with PD.
Drug treatment for Parkinson's disease (PD), particularly in its early stages, remains controversial, as highlighted in a recent review.1 Most controlled trials of early drug therapy in PD focus on dyskinesias and motor symptoms as end points, while self reported health status and non‐motor symptoms such as depression, hallucinations, falls, sleep problems, restless legs and dementia are not adequately considered.2 Furthermore, the external validity of such trials is questionable because of the exclusion, for instance, of elderly patients and those with active comorbid medical problems.2,3 Patients diagnosed with early PD may not be treated initially on the assumption that the condition is too mild to warrant treatment. A “wait and watch” policy is thus undertaken and drug therapy is initiated only when the disability becomes apparent and “functional” impairment occurs.4,5 This policy is dictated in part by the absence of disease modifying therapy.1
The argument for starting PD therapy at diagnosis would be strengthened if an adverse effect on self reported health status of delaying treatment was documented. This can be achieved by using validated self reported health status measures, such as the PD specific questionnaire, PDQ‐39, which incorporates aspects of motor and non‐motor function in PD.2,6 Because of controversy regarding whether such scores truly represent quality of life or, more correctly, a patient centred overview of physical, social and psychological health, we refer to the PDQ‐39 results as a health status rather than quality of life (which some consider a personal issue which is unquantifiable).7
PDLIFE is a prospective multicentre UK based national audit study examining the serial changes in self reported health status of people with PD in the early untreated stage or on monotherapy, using the PDQ‐39. As part of this study, serial self reported health status data and demographic details were collected in PD patients who were not started on specific therapy at the initial consultation and who were subsequently followed for a mean of 18 months.
To our knowledge, this is the first report of a longitudinal follow‐up designed to address primarily self reported health status measures in a cohort of unselected drug naïve PD patients who were clinically judged not to require pharmacological treatment.
Patients and methods
Methods
PDLIFE is an ongoing multicentre prospective audit based study, established by a collaborative clinical group and supported by the UK Parkinson's Disease Society, to address the serial changes in self reported health status measures in response to therapeutic intervention in PD patients over a 5 year period. The initial phase (phase 1) of PDLIFE is complete and includes 10 UK centres. Phase 1 was designed to replicate a “real life” situation as clinical trials of anti‐PD drugs usually concentrate on young patients with minimal comorbid illness presenting to tertiary centres. For this reason, to avoid bias, the centres included in the PDLIFE study ranged from tertiary regional movement disorder clinics to specialised PD clinics for the elderly and clinics in a district general hospital setting.
Patients
Patients fulfilling the UK Brain Bank criteria for PD were potentially eligible.8 As the aim of the study was to address the changes in self reported health status in untreated PD and the effect of any anti‐PD drug related intervention on self reported health status, only untreated patients (drug naïve, DNPD) or those receiving monotherapy with any anti‐PD agent (MTPD) were included. The serial follow‐up design also allowed assessment of patients who were initially untreated at baseline and thereafter were started on treatment. The combination of levodopa and entacapone was included in the MTPD group. Patients receiving anti‐PD drug treatment for more than 5 years, patients in complex or palliative stages of PD and those with an uncertain diagnosis were excluded.
All patients completed a standard audit form at the first visit which noted age, sex, age at diagnosis, family history, occupational history, social support, exposure to toxins, ethnicity, history of head injury, olfactory disturbance, alcohol consumption, comorbidities and a detailed drug history. In addition, Hoehn and Yahr (HY) staging and subtype of Parkinson's disease (akinesia dominant, tremor dominant or mixed) were recorded, and patients completed the PDQ‐39 questionnaire.
PDQ‐39
The PDQ‐39 is the most used and validated disease specific instrument for self reported health status in PD.6,7 The PDQ‐39 has 39 questions and eight domains/dimensions, covering mobility, activities of daily living, emotional well being, stigma, social support, cognition, communication and bodily discomfort. Lower scores indicate better perceived health status (0 = perfect health, 100 = worst health). PDQ‐39 is validated in PD and its clinimetrics have been extensively evaluated. Reliability (evaluated by Cronbach's α for internal consistency) is very satisfactory except for social support (0.66) while construct validity (examined by comparing PDQ‐39 scores with relevant SF‐36 scores, Unified Parkinson's Disease Rating Scale (UPDRS), HY scale and Columbia Rating Scale) shows significant correlation between the scales.6,9 There is sensitivity to change, suggesting that PDQ‐39 can be used to evaluate treatment.10 Higher order factor analysis was used to derive the overall single index score, the PDQ‐39 summary index (PDQ‐39SI), from the eight dimension scores.9 The PDQ‐39SI shows high levels of internal validity and construct validity.
All patients completed the PDQ‐39 themselves. Completion of the UPDRS11 and other scales and instruments was optional. At follow‐up after 6–12 months, a separate form was used noting any interventions in the intervening period (drug treatment started for DNPD or added for MTPD), a clinical global impression scale for patients and physician, HY stage, changes in social or other support services or circumstances, any change in diagnosis (emergence of atypical parkinsonian features at follow‐up), any drug related side effects and the PDQ‐39.
The data in this paper relate specifically to the DNPD patients recruited to PDLIFE and their serial follow‐up over a mean period of 18 months. Other aspects of this study relating to the overall database will not be discussed. Ten centres across the UK contributed during phase 1. The study was registered as a prospective audit of existing clinical practices, using established scales in routine use, and specific ethics approval was not required.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS version 13, Chicago, Illinois, USA). For statistical analysis of the overall mean single index score (PDQ‐39SI) a two way mixed ANOVA (treatment type as the between factor and time period as the within factor) was used. Further comparisons were made where appropriate. Mean separate PDQ‐39 domains were analysed using a three way mixed ANOVA (treatment type as the between factor, time and the eight domains of the PDQ‐39 as the within factors).
Results
The current report is based on a sample of 198 patients that were DNPD at the baseline assessment and were followed‐up for mean of 18 months (fig 1). Mean age of the DNPD patients was 63.4 (9.2) years (range 45–86), 48% were male, mean HY stage was 1.6 (0.67) (range 1–3) and mean duration of disease was 4 years (range 0.3–6).
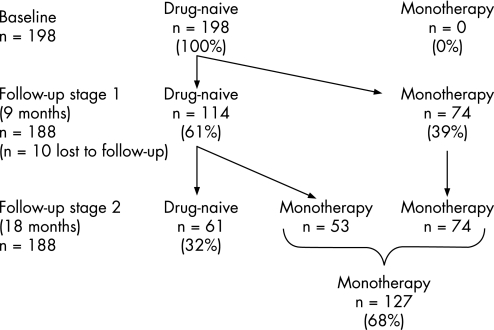
Figure 1 Disposition of the 198 initially drug naïve patients with Parkinson's disease at follow‐up. Of those attending for follow‐up, 61% remained drug naïve at the first follow‐up (at a mean of 9 months) and 32% remained drug naïve at the second follow‐up (at a mean of 18 months).
In all, 95% (n = 188) of the 198 DNPD cases returned for their first follow‐up after a mean period of 9 months (range 6–15) (fig 1). Of these, 60.6% (n = 114) remained untreated (DNPD group). The remaining 39.4% (n = 74) were started on anti‐PD treatment (monotherapy) after the first consultation and the initiation treatment varied between levodopa preparations (51%), dopamine agonists (43%) and others (6%, selegiline, trihexyphenidyl and amantadine).
Of the 114 subjects that remained untreated at the first follow‐up, 61 (53.5% of 114) remained untreated at the second follow‐up, a mean period of 18 months (range 15–20) following the baseline consultation. Demographic details of the DNPD and MTPD patients at the second follow‐up are shown in table 1. Age, HY score, comorbidity and sociodemographic profile were similar between the DNPD patients who were not started on treatment compared with those who were, except for the rate of depression (as noted by a question in the audit form) which was significantly higher in the treated group (0% vs 10%; p<0.001). At follow‐up, HY scores did not change significantly in either group although there was a trend towards increasing HY scores in both groups.
Table 1 Baseline age, Hoehn and Yahr (HY) score, comorbidity and sociodemographic profiles, according to whether patients remained drug naïve at the second follow‐up (n = 61) or were started on monotherapy between the first and second follow‐up (n = 53).
| Drug naïve | Monotherapy | |
|---|---|---|
| Mean age (y) | 66 | 65 |
| Mean HY score | 1.7 | 1.7 |
| % Male | 52 | 40 |
| % Caucasian | 97 | 93 |
| % Comorbidity | ||
| Hypertension | 16 | 16 |
| Diabetes | 8 | 10 |
| Heart disease | 12 | 6 |
| Stroke | 4 | 0 |
| Depression | 0 | 10 |
HY score, Hoehn and Yahr score.
Depression was significantly higher in the monotherapy group compared with the drug naïve group (p<0.001, χ2) but there were no other significant differences.
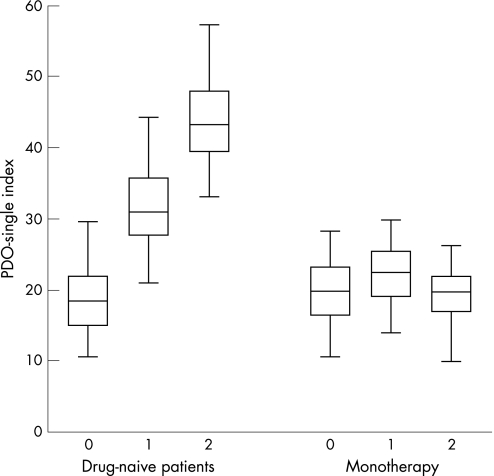
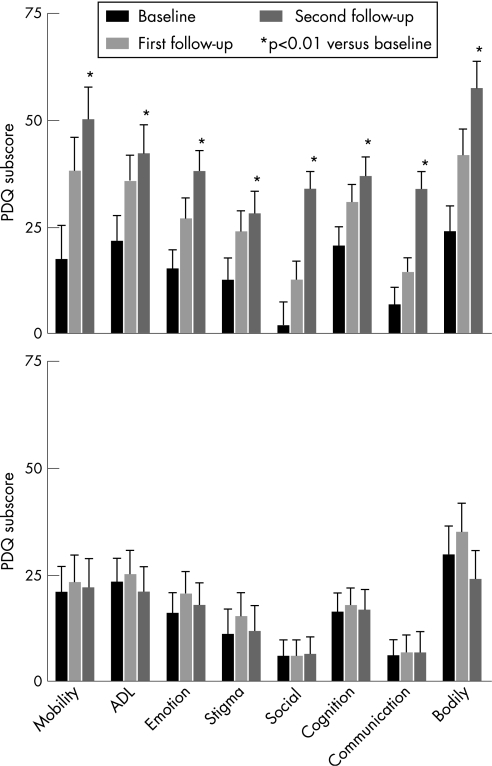
In patients who returned for review and remained drug naïve (DNPD), there was a progressive deterioration in PDQ‐39SI scores at the first follow‐up (p<0.05) with further significant deterioration at the second follow‐up (p<0.05) in those who were left untreated at the first review (n = 61) (fig 2). Deterioration was also significant in all eight domains of the PDQ‐39 (fig 3), with PDQ‐39 scores worsening significantly at the second follow‐up (p<0.01). The effect size for each dimension varied between 0.5 and 1.9 at the first follow‐up (for PDQ‐39 an effect size of 0.2 is considered small, 0.5 is moderate and 0.8 is large) and between 0.2 and 0.8 at the second follow‐up.12 The largest effect size was noted for social stigma (−1.98) in those left untreated at the first follow‐up. In contrast, patients initially drug naïve at baseline and started on treatment between the first and second follow‐up visits (fig 3) showed a trend to deterioration in six out of eight domains of the PDQ‐39 between the baseline and the first follow‐up which appeared to be arrested following treatment between the first and second follow‐up points in which there was a trend towards improvement (n = 53, p>0.05). Calculation of degree of change in the individual domains of the PDQ‐39 by measurement of effect size showed small changes towards improvement in the following domains: activities of daily living (0.2), social support (0.2), cognition (0.13), communication (0.1) and bodily discomfort (0.2).
Figure 2 Mean (95% CI) Parkinson's Disease Questionnaire (PDQ)‐39 Summary Index scores in patients initially drug naïve at baseline but who received anti‐PD drug treatment between the first and second follow‐up consultation (n = 53). There was a trend towards worsening between baseline and the first follow‐up (mixed ANOVA, p>0.05) and thereafter a trend to improvement. For patients who remained drug naïve throughout the follow‐up period (n = 61), the PDQ‐39 Summary Index deteriorated significantly at each visit (overall difference between groups p<0.01, mixed ANOVA). The horizontal line within the box represents the median value, with the edges of the box representing the lower and upper quartiles; the whiskers display the range.
Figure 3 Mean (95% CI) individual dimensions of the Parkinson's Disease Questionnaire (PDQ)‐39 for patients that remained drug naïve throughout the follow‐up consultations (n = 61) (A). *p<0.01 (mixed ANOVA) between baseline and the second follow‐up in all dimensions. In patients initially drug naïve at baseline but who received anti‐PD drug treatment between the first and second follow‐up consultation (n = 53) (B), there was a trend to worsening between baseline and the first follow‐up in six of the eight dimensions (mixed ANOVA, p>0.05) and thereafter a trend to improvement in six of the eight dimensions (B). ADL, activities of daily living.
Discussion
We believe that this is the first report of an observational study of the early stages of PD in patients left untreated at diagnosis, which focuses on overall self reported health status measures rather than on motor disability. Self reported health status measures in PD have also been referred to as measurement of quality of life although this remains controversial. Although the mean age of our patients was similar to that in clinical trials, a greater proportion of older patients (aged up to 86 years) was studied, as reflected by the greater standard deviation. The study was based on the PDQ‐39 questionnaire, whose domains include aspects which cover motor (mobility, activities of daily living) and non‐motor (cognition, emotional well being and bodily discomfort) features of PD. The key finding is that there was a significant deterioration in all eight domains of the PDQ‐39 scale in those patients who were left untreated at the first presentation or at the first review. This pattern worsened if patients did not start treatment at the first review with further deterioration of quality of life scores at the second review. This contrasts with untreated PD patients who were given specific anti‐PD treatment at the first review. In these patients, the PDQ‐39 scores remained stable and showed a trend towards improved quality of life scores in six out of the eight domains of the PDQ‐39 at the second follow‐up, suggesting a beneficial effect of dopaminergic treatment on aspects of the PDQ‐39 scale domains, ranging from mobility to bodily discomfort (fig 3). The observation was supported by the measurement of effect sizes for each domain which showed a moderate to large effect size for each domain of the PDQ‐39 at the first follow‐up in those left untreated at baseline.
As this was an observational, prospective, audit based study, we did not influence decisions on when to begin treatment or drug choice. However, the baseline assessment data allowed us to compare if there were any major variables that might have influenced initiation of treatment by the physician in the MTPD group and not in the DNPD group. As indicated in table 1, a significantly higher (p<0.001) rate of self declared depression in MTPD (0% DNPD vs 10% MTPD) was noted before treatment was started. We assume, therefore, that in these cases the decision to treat could have been based on the clinician's discretion and patient choice and perhaps not functional disability. A divergent trend between self reported health status and motor scores/functional disability has also been noted by the US Parkinson Study Group who made similar observations relating to self reported health status in their CALM‐PD trial which was a randomised comparison of initial levodopa versus initial pramipexole.13 In the CALM‐PD study, although the motor scores improved more with levodopa, the self reported health status scores, as measured by the PDQUALIF14 scale, were similar between levodopa and pramipexole.
There are obvious drawbacks of audit or clinical practice‐led observational studies such as this which merit discussion. Firstly, the longitudinal follow‐up of the 198 DNPD patients was not complete, with 188 returning for follow‐up visits to date in this ongoing work, but does reflect a high retention rate at follow‐up. Secondly, the numbers of patients remaining drug naïve at the second follow‐up were low. In spite of this, there was a robust deterioration in the self reported health status scores, both overall and within individual domains of the PDQ‐39, suggesting the severity of this problem if patients are left untreated. Data on patients treated at baseline and followed up thereafter (n = 74) will be reported later as part of the overall study. Thirdly, this was an observational and not a randomised study of the effect of treatment of anti‐PD drugs, such that only limited conclusions can be reached about the relative merits of levodopa versus dopamine agonist treatment. These issues are more appropriately addressed by randomised studies such as the large national pragmatic trial in the UK comparing the use of various anti‐PD agents used to initiate treatment of PD and their effect on self reported health status using the PDQ‐39 as the primary end point (the PDMED study). Another criticism is the absence of UPDRS scoring, the “gold standard” for assessment of PD motor impairment.11 However, a core aim of the study was to move away from recording of impairment which may not reflect other determinants of self reported health status such as non‐motor symptoms, and furthermore the UPDRS is in the process of amendment.15,16,17
We consider the strengths of the study as the inclusion of “real life” patients with no restriction on age groups or comorbidities, and the longitudinal analysis of 188 DNPD cases, the largest reported collection of untreated PD cases over time focusing on self reported health status measures. While a PD specific non‐motor questionnaire (NMSQuest) and scale have been validated recently, at the time of the study the PDQ‐39 provided an indirect indicator of non‐motor and motor symptoms of PD.18,19 It is also recognised that the UPDRS may not predict health related self reported health status, as exemplified by the comparison of pramipexole and levodopa as initial treatment for drug naïve PD.13 Similar results have also been reported from the pilot validation study of the Unified Non‐motor Symptoms Scale for PD which appears to predict self reported health status in PD in a more robust fashion than the UPDRS in a large population of PD patients across all stages.19 A take home message from this study may be the re‐evaluation of the policy whereby some physicians may elect to delay treatment in newly diagnosed PD, a topic that had recently prompted conflicting views.20,21 However, in these reviews, the argument for starting treatment at diagnosis or delaying treatment in PD was largely based on motor disabilities such as dyskinesias, issues related to neuroprotection and cost of medication, and not what happens to self reported health status or quality of life if such patients are left untreated. Studies by the Global PD Steering Committee and others have indicated that factors other than motor scores dictate overall quality of life in PD.17,22 Our work would suggest that self reported health status of PD needs to be an essential part of this debate.
In conclusion, based on the results of this “real life” observational study of the journey of untreated PD patients, it appears that in patients presenting to doctors who are then left untreated, there is a clinically important and possibly reversible deterioration in all eight domains of the PDQ‐39 scale. Deterioration is observed not only in the motor domains but also in the non‐motor domains, such as cognition, bodily discomfort, emotional well being and communication. This contrasts with PD patients in whom treatment is started where the PDQ‐39 scores remain stable and show no deterioration, regardless of whether they are prescribed levodopa or other anti‐PD treatments. We believe that PD specific measures of self reported health status should be integral to the clinical assessment of patients at review and also while evaluating response to treatment.
Acknowledgements
We are grateful to the Parkinson's Disease Society of the UK for supporting this project and funding LT. We acknowledge the help and support of Professor R Gregory, Dr R Abbott, Professor D J Brooks, Ms L Graham, Mr R Meadowcroft, Ms A Blockley, Ms L Graham and the European Parkinson's Disease Association.
Authors' contribution
Data collection: K Ray Chaudhuri, L Taurah, D Grosset, L Taurah, D J Burn, D MacMahon, L Findley, O Foster, K Patel, A Forbes, K Turner, A Bowron, C Clough, B Castleton, S Smith, G Carey, T Murphy, J Hill, U Brechany, P McGee, S Reading, G Brand.
Manuscript writing: D Grosset, L Taurah, D J Burn, D MacMahon, K Ray Chaudhuri.
Design of study—working committee: K Ray Chaudhuri, L Kelly, M Baker, S Ford, K Breen, L Taurah, D MacMahon, L Findley, O Foster, K Patel, A Forbes, K Turner, C Clough, S Smith, U Brechany, P McGee, M Baker, N Qizilbash, A Williams, J Hearne.
Statistical analysis: Dr Peter KH Walton, Andy Smallman from Dendrite Clinical Systems Ltd, D Grosset, L Taurah.
All authors contributed to the structure, revision and writing of the article.
Abbreviations
DNPD - drug naïve patient with Parkinson's disease
HY stage - Hoehn and Yahr stage
MTPD - patient receiving monotherapy for Parkinson's disease
PD - Parkinson's disease
PDQ - Parkinson's Disease Questionnaire
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Funding: The study was supported by the UK Parkinson's Disease Society and we also acknowledge an initial educational grant from GSK Pharmaceuticals in 1999 made to the UK Parkinson's Disease Society to set up the software used for data analysis for this study. Dendrite Corporation helped with the statistical software at the conception of the study.
Competing interests: None.
References
- 1.Montgomery E, Pan T, Le W.et al Slowing Parkinson's disease progression: recent dopamine agonist trials. Neurology 200462343–345. [PubMed] [Google Scholar]
- 2.Chaudhuri K R, Healy D, Schapira A H V. The non motor symptoms of Parkinson's disease. Diagnosis and management. Lancet Neurology 20065235–245. [DOI] [PubMed] [Google Scholar]
- 3.Clarke C E, Guttman M. Dopamine agonist monotherapy in Parkinson's disease. Lancet 20023601767–1769. [DOI] [PubMed] [Google Scholar]
- 4.Marras C, Lang A E. Measuring motor complications in clinical trials for early Parkinson's disease. J Neurol Neurosurg Psychiatry 200374143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olanow C W, Watts R L, Koller W C. An algorithm (decision tree) for the management of Parkinson's disease (2001): Treatment guidelines. Neurology 200156(Suppl 5)S1–S886. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson C, Peto V, Fitzpatrick R.et al Self‐reported functioning and well‐being in patients with Parkinson's disease: Comparison of the Short‐form Health Survey (SF‐36) and the Parkinson's Disease Questionnaire (PDQ‐39). Age Ageing 199524505–509. [DOI] [PubMed] [Google Scholar]
- 7.Morrish P K. Quality of life in Parkinson's disease. Parkinson Dis 200241–3. [Google Scholar]
- 8.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkinson C, Peto V, Fitzpatrick R.et al The Parkinson's disease questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age Ageing 199726353–357. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga H, Kasai T, Yoshidome H. Clinical findings, status of care, comprehensive quality of life, daily life therapy and treatment at home in patients with Parkinson's disease. Eur Neurol 199738(Suppl 2)64–69. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton R L, and members of the UPDRS Development Committee Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, eds. Recent developments in Parkinson's disease. Florham Park, NJ: Macmillan Healthcare Information, 1987153–163.
- 12.Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ‐39 Parkinson's disease questionnaire. Age Ageing 200130299–302. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson Study Group Pramipexole versus levodopa as initial treatment for Parkinson's disease. A 4 year randomised controlled trial. Arch Neurol 2004611044–1053. [DOI] [PubMed] [Google Scholar]
- 14.Welsh M, McDermott M P, Holloway R G.et al Development and testing of the Parkinson's disease quality of life scale. Mov Disord 200318637–645. [DOI] [PubMed] [Google Scholar]
- 15.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 200318738–750. [DOI] [PubMed] [Google Scholar]
- 16.Dodel R C, Dubois B, Fahn S.et al Addressing non‐motor impairments in Parkinson's disease: the new version of the UPDRS. Mov Disord. 2005;20: S83(P 227), (Suppl 10)
- 17.Global Parkinson's Disease Steering Committee Factors impacting on quality of life in Parkinson's disease; results from an international survey. Mov Disord 20021760–67. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri K R, Martinez‐Martin P, Schapira A H V.et al An international multicentre pilot study of the first comprehensive self‐completed non motor symptoms questionnaire for Parkinson's disease: The NMSQuest study. Mov Disord 200621916–923. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhuri K R, Martinez‐Martin P, Schapira A H V.et al International validation of the first unified non motor symptoms scale for Parkinson's disease. Results from the first pilot NMSS study. Neurology 200666(Suppl 2)S13.001 [Google Scholar]
- 20.Schapira A H, Obeso J. Timing of treatment initiationin Parkinson's disease: A need for reappraisal? Ann Neurol 200659559–562. [DOI] [PubMed] [Google Scholar]
- 21.Aminoff M J. Treatment should not be initiated too soon in Parkinson's disease. Ann Neurol 200659562–564. [DOI] [PubMed] [Google Scholar]
- 22.Schrag A, Selia C, Jahanshahi M.et al The EQ‐5D—a generic quality of life measure—is a useful instrument to measure quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 20006967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]