Abstract
We describe a patient with advanced Parkinson's disease who developed pathological gambling within a month after successful bilateral subthalamic nucleus (STN) stimulation. There was no history of gambling. On neuropsychological testing, slight cognitive decline was evident 1 year after surgery. Stimulation of the most dorsal contact with and without medication induced worse performances on decision making tests compared with the more ventral contact. Pathological gambling disappeared after discontinuation of pergolide and changing the stimulation parameters. Pathological gambling does not seem to be associated with decision making but appears to be related to a combination of bilateral STN stimulation and treatment with dopamine agonists.
Pathological gambling in Parkinson's disease (PD) is a behavioural complication which has been related to the use of dopamine agonists1,2 but also to levodopa therapy.3 Bilateral subthalamic nucleus (STN) stimulation has been shown to improve levodopa sensitive motor symptoms in PD but negative effects on behaviour have been reported, such as mania, depression, apathy, drug dependence and compulsive self‐stimulatory behaviour.3,4,5,6,7 Recently, improvement of pathological gambling after bilateral STN stimulation has been described.8,9 We report a patient with advanced PD who developed pathological gambling within a few weeks after successful bilateral STN stimulation.
Case report
Patient
A 63‐year‐old, right‐handed man with a 10 year history of PD underwent bilateral STN surgery for severe pharmacoresistant response fluctuations. Before surgery, his medication consisted of 600/150 mg levodopa/carbidopa slow release and pergolide 6–8 mg daily dose. He complained about slight forgetfulness but neuropsychological evaluation was normal (table 1).
Table 1 Neuropsychological and neurological data at baseline and at follow‐up.
| Baseline (0 wk) | FU 1 (26 wk) | FU 2 (52 wk) | FU 3 (156 wk) | FU 4 (157 wk) | FU 5 (163 wk) | FU 6 (180 wk) | |
|---|---|---|---|---|---|---|---|
| Stimulation parameters | |||||||
| Contacts, right/left monopolar | 1‐/1‐2‐ | 1‐/1‐2‐ | 1‐/1‐2 | Off | 3‐/2‐3‐ | 3‐/2‐3‐ | |
| Amplitude (V) right/left | 3.2/2.5 | 3.2/2.5 | 3.2/2.5 | 2.6/2.5 | 2.6/2.5 | ||
| Pulse width (μs) | 60 | 60 | 60 | 60 | 60 | ||
| Frequency (Hz) | 185 | 185 | 185 | 130 | 130 | ||
| UPDRS‐III (off/on) | 56/34 | 21/13 | 21/13 | /14 | 22/ | /18 | |
| H&Y (off/on) | 3/3 | 3/2,5 | 3/2,5 | /2,5 | 3/ | /2 | |
| Medication | |||||||
| Pergolide (mg) | 6–8 | 4 | 3 | 2 | 2 | 2 | 0 |
| LEU | 880 | 750 | 510 | 760 | 760 | 760 | 560 |
| Executive function | |||||||
| Category fluency (T) | 47 | 43 | 44 | 37 | 46 | 33 | 35 |
| COWAT letter fluency (T) | 52 | 51 | 50 | 55 | 56 | 60 | 63 |
| Stroop colour–word (T) | 39 | 33 | 44 | 34 | 28 | 32 | 37 |
| Trailmaking B (T) | 49 | 51 | 60 | 42 | 47 | 44 | 56 |
| PASAT number correct | 47 | 33 | 25 | 29 | 39 | 38 | 38 |
| Memory | |||||||
| AVLT immediate recall (T) | 51 | 37 | 38 | 37 | 33 | 37 | 45 |
| AVLT delayed recall (T) | 46 | 30 | 34 | 31 | 35 | 35 | 39 |
| Decision‐making | |||||||
| IGT disadvantageous choices% | 34 | 56 | 78 | 70 | |||
| Go/no go commission errors% | 48 | 43 | 68 | 77 | |||
| Questionnaires | |||||||
| DEX Questionnaire self/proxy | 11/23 | 10/29 | 6/22 | 15/42 | 11/56 | 9/50 | 10/28 |
| PDQL | 87 | 71 | 70 | 66 | 66 | 70 | |
| MADRS | 8 | 9 | 3 | 7 | 9 | 9 |
AVLT, Auditory Verbal Learning Test; COWAT, Controlled Oral Word Association Test; DEX, Dysexecutive Questionnaire; FU, Follow‐up; H&Y, Hoehn and Yahr scale; IGT, Iowa Gambling Task; LEU, levodopa equivalent unit; MADRS, Montgomery and Asberg Depression Rating Scale; PASAT, Paced Auditory Serial Addition Test; PDQL, Parkinson's Disease Quality of Life; T, normally distributed score with a mean of 50 and SD of 10, corrected for age and education; UPDRS, Unified Parkinson's Disease Rating Scale.
Significant changes in cognitive test scores (compared with baseline) are printed in bold typeface.
Surgery and postoperative management
In 2002, the patient underwent a one stage bilateral stereotactic procedure using frame based MRI, visualising the STN on T2 weighted images, verifying the atlas based target (12 mm lateral (x), 2 mm posterior (y) and 6 mm inferior (z) to the mid‐commissural point (MCP)), and macrostimulation to determine the final position for electrode placement. The electrodes (model 3389; Medtronic, Minneapolis, USA) were implanted with the deepest contact 8 mm below the MCP on the right and 6 mm below the MCP on the left. At discharge, monopolar stimulation at contact 1 was used on both sides with an amplitude of 1.9 V on the left and 2.4 V on the right, pulse width of 60 μs and frequency of 185 Hz. There was marked motor improvement with a reduction in anti‐PD medication to 400/100 mg levodopa/carbidopa slow release and pergolide 3 mg daily dose.
Follow‐up
Six months after operation, the patient was satisfied with the results of surgery on motor functioning although he noticed increased emotional lability. His wife reported memory decline but she found this acceptable considering the large positive effect on motor functioning. Neuropsychological testing with stimulation on showed a marked decline in memory and in selective attention, compared with the preoperative status (table 1, FU 1). Twelve months after operation, the patient complained of forgetfulness and word finding difficulties. His wife reported a decline in his cognitive functioning. She mentioned slight behaviour changes, namely increased lability, impulsivity and vivid dreams, but denied the presence of hallucinations, apathy, irritability or euphoria. Neuropsychological functioning with stimulation on was relatively stable at 12 months compared with the 6 month follow‐up except for an improvement on the Stroop Colour–Word Card which measures selective attention (table 1, FU 2).
Three years after operation, during a follow‐up visit at the movement disorders clinic, the patient informed the neurologist that he was suffering from pathological gambling with a preference for slot machines, which had started 1 month after surgery. Despite regular follow‐ups at the outpatient clinic over the preceding 3 years, the patient had never before mentioned pathological gambling. According to his wife and children, the patient was previously “as stingy as a Dutchman”. Because of increasing debts, the house had to be sold and his wife wanted a divorce. The patient had been admitted to a psychiatric institution because of a suicide attempt. Two more suicide attempts followed. Subsequently, the patient was admitted to the neurological ward.
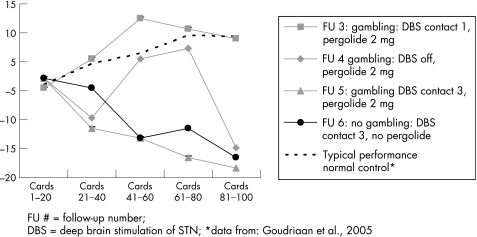
At this time, neuropsychological evaluation was repeated, 156 weeks after STN implantation (table 1, FU 3). The patient now disclosed a history of alcohol abuse in his thirties which he had overcome. His mood was characterised as slightly depressed. Compared with the 12 month follow‐up, there was a decline in category fluency, and selective and divided attention. Two decision making tests were added to the test battery: the Iowa Gambling Task (IGT),10 an ecologically valid decision task involving weighing of immediate rewards against long term losses, and a “go/no go” discrimination task to investigate abnormal reward processing (for a description of both tasks, as applied to pathological gambling, see Goudriaan and colleagues11). Parallel versions were used in each test session. Performance on the IGT was normal, with 34% disadvantageous choices (see fig 1 for performance curve). Performance on the go/no go task was at chance level.
Figure 1 Advantageous minus disadvantageous decks during five consecutive learning stages on the Iowa Gambling Task. DBS, deep brain stimulation; FU, follow‐up. *Data from Goudriaan and colleagues.11
One week after switching off the neurostimulation, the patient claimed to feel less of an urge to gamble. There was no change on the standard neuropsychological tests or on the go/no go task (table 1, FU 4). Performance on the IGT was worse compared with stimulation on, with 56% disadvantageous choices. There was a marked increase in motor impairment, necessitating the neurostimulation to be switched on. Because a chronic stimulation effect, inducing the pathological gambling, could not be excluded, monopolar stimulation at the most rostral contact point 3 was started.
A CT scan was performed and co‐registered with the preoperative MRI using the ImageMerge module of Surgiplan (Elekta, Stockholm, Sweden) to verify the position of the electrodes. On the left, the deepest contact (0) was 2 mm higher than the target. On the right, the deepest contact (0) was at target, within the limits of precision of this fusion technique.
One month later, with stimulation at contact 3, his wife reported that the patient had been buying scratch cards although his allowance had been restricted. The results on standard neuropsychological testing were stable but performance on the IGT and on the go/no go task had deteriorated (table 1, FU 5).
Eventually, pergolide was tapered and stopped. The urge to gamble completely disappeared 2 days after the last dose. The patient was able to sit in a café with spare money in his pocket ignoring the slot machine. He regained his normal interest in family and hobbies. Emotional lability and vivid dreaming did not improve. There were no changes on standard neuropsychological testing. IGT or go/no go task performance did not improve (table 1, FU 6).
Because of ongoing marital discord and severe financial problems, the patient was referred to social services for counselling.
Discussion
Our patient developed pathological gambling shortly after bilateral STN stimulation despite a reduction in dopamine agonist medication. Slight cognitive decline and emotional lability were also present. Pathological gambling resolved suddenly after discontinuation of pergolide but the stimulation parameters had also been changed to a more rostral (ie, dorsal active) contact point.
The association between pathological gambling and the use of dopamine agonists has been described previously.1,12 However, in these studies, PD patients developed this behaviour disorder after the introduction or after an increase in the dopamine agonist and not after reducing the daily dose. Moreover, pathological gambling after deep brain stimulation (DBS) STN has recently been reported in five of 39 PD patients, despite a reduction or discontinuation of the dopamine agonists.12 This suggests the influence of chronic STN stimulation on the development of pathological gambling in PD. Stimulation seems to sensitise the brain to the behavioural side effects of dopamine agonists, especially in patients with a history of addictive behaviours, as was the case in our patient. This explanation is in contrast with that given in a recent study8 which postulated that desensitisation of the limbic dopaminergic system after DBS STN and reduction of medication led to improvement of pre‐existing pathological gambling. However, the study also stated that pathological gambling in patients with active symptoms may worsen after DBS STN.
Impaired decision making has been reported in pathological gambling and in PD.11,13 In pathological gambling research, diminished self‐regulation, a neurocognitive function related to decision making, has been associated with risk of developing gambling problems later in life.14 In PD, disadvantageous decision making was highly correlated with executive dysfunction and has been associated with decreased dopaminergic transmission in the frontostriatal loops.13 In our patient, worse performance on decision making tasks was seen with stimulation of the most dorsal contact compared with the ventral contact, both with and without medication. This implies that bilateral STN stimulation directly influences decision making. A previous report15 did not find any effects of bilateral STN stimulation on a similar gambling task. However, the study compared performance between on and off stimulation, with stimulation switched off for only 1 h.
Impaired decision making does not seem to be directly related to pathological gambling because the performance of our patient on the IGT was normal and performance on the go/no go task did not improve when the pathological gambling disappeared. Perhaps the IGT is not as ecologically valid as it is claimed to be, as it does not predict real world behaviour.
We conclude that pathological gambling may be induced by bilateral STN stimulation. Because of the known association between dopamine agonists and pathological gambling in PD, discontinuation of the dopamine agonist seems to be the first treatment option before changing stimulation parameters. Because of the devastating effect of pathological gambling, physicians or neurologists should inform patients and their family about this risk of dopamine agonists and bilateral STN stimulation.
Abbreviations
DBS - deep brain stimulation
IGT - Iowa Gambling Task
MCP - mid‐commissural point
PD - Parkinson's disease
STN - subthalamic nucleus
Footnotes
Competing interests: AEG receives salary support from a New Investigator grant from the National Center for Responsible Gambling, as provided by the Institute for Research on Pathological Gambling and Related Disorders (Harvard Medical School's Division on Addictions). EMJF received a reimbursement for a lecture from Medtronic ltd. (Minneapolis). PRS received reimbursement from Medtronic Ltd for attending two conferences. JDS acts as an independent consultant for Medtronic Ltd. He has received travel grants from Medtronic Ltd.
References
- 1.Weintraub D, Siderowf A D, Potenza M N.et al Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 200663969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pontone G, Williams J R, Bassett S S.et al Clinical features associated with impulse control disorders in Parkinson disease. Neurology 2006671258–1261. [DOI] [PubMed] [Google Scholar]
- 3.Molina J A, Sainz‐Artiga M J, Fraile A.et al Pathologic gambling in Parkinson's disease: a behavioral manifestation of pharmacologic treatment? Mov Disord 200015869–872. [DOI] [PubMed] [Google Scholar]
- 4.Houeto J L, Mesnage V, Mallet L.et al Behavioural disorders, Parkinson's disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 200272701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berney A, Vingerhoets F, Perrin A.et al Effect on mood of subthalamic DBS for Parkinson's disease. Neurology 2002591427–1429. [DOI] [PubMed] [Google Scholar]
- 6.Kulisevsky J, Berthier M L, Gironell A.et al Mania following deep brain stimulation for Parkinson's disease. Neurology 2002591421–1424. [DOI] [PubMed] [Google Scholar]
- 7.Morgan J C, diDonato C J, Jenkins P D.et al Self‐stimulatory behavior associated with deep brain stimulation in Parkinson's disease. Mov Disord 200621283–285. [DOI] [PubMed] [Google Scholar]
- 8.Ardouin C, Voon V, Worbe Y.et al Pathological gambling in Parkinson's disease improves on chronic subthalamic nucleus stimulation. Mov Disord 2006211941–1946. [DOI] [PubMed] [Google Scholar]
- 9.Bandini F, Primavera A, Pizzorno M.et al Using STN DBS and medication reduction as a strategy to treat pathological gambling in Parkinson's disease. Parkinsonism Relat Disord. In press [DOI] [PubMed]
- 10.Bechara A, Damasio A R, Damasio H.et al Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994507–15. [DOI] [PubMed] [Google Scholar]
- 11.Goudriaan A E, Oosterlaan J, de B E.et al Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res 200523137–151. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Bharmal A, Suchowersky O. Gambling and Parkinson disease. Arch Neurol 200663298. [DOI] [PubMed] [Google Scholar]
- 13.Brand M, Labudda K, Kalbe E.et al Decision‐making impairments in patients with Parkinson's disease. Behav Neurol 20041577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitaro F, Arseneault L, Tremblay R E. Impulsivity predicts problem gambling in low SES adolescent males. Addiction 199994565–575. [DOI] [PubMed] [Google Scholar]
- 15.Czernecki V, Pillon B, Houeto J L.et al Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson's disease? J Neurol Neurosurg Psychiatry 200576775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]