Abstract
The central compensation of vestibular tonus imbalance due to unilateral peripheral vestibular lesions has been repeatedly documented. Little is known, however, about the central compensation of vestibular tonus imbalance due to central lesions. Dorsolateral medullary infarctions (Wallenberg's syndrome) typically cause a central vestibular tonus imbalance in the roll plane with deviations of perceived verticality and ipsiversive body lateropulsion. The course of normalisation of the tilts of subjective visual vertical (SVV) in 50 patients who had acute Wallenberg's syndrome were retrospectively compared with that in 50 patients with acute vestibular neuritis. The initial displacement of SVV was 9.8° in Wallenberg's syndrome and 7° in vestibular neuritis. The deviation of SVV significantly decreased over time within days to weeks in both groups. This finding shows that the time courses of the central compensation for dorsolateral medullary infarctions and peripheral vestibular lesions are similar.
Both acute peripheral and central vestibular lesions cause vestibular tonus imbalance with respect to ocular motor, perceptual, and postural signs and symptoms (spontaneous nystagmus, ocular torsion and skew deviation; rotatory vertigo, tilt of perceived verticality; lateropulsion and body falls).1,2 Signs and symptoms of an acute unilateral peripheral vestibular loss gradually abate within weeks, even if there is no recovery of peripheral function. This so‐called central vestibular compensation is considered the prototype of brain plasticity. The degree and time course of central vestibular compensation of peripheral lesions have been studied in detail (for review see Brandt et al3 and Curthoys and Halmagyi4), but not for central lesions. This prompted us to retrospectively evaluate the course of normalisation of vestibular tonus imbalance in the roll plane in patients with acute dorsolateral medullary infarctions (Wallenberg's syndrome) in comparison with patients with vestibular neuritis. As posturographic measurement of lateral postural sway was not possible in the acute phase due to uncontrollable falls, the perceptual correlate of the severity of lateropulsion in Wallenberg's syndrome,5 the subjective visual vertical (SVV), was measured over time.
Patients and methods
All patients underwent a standardised neurological, neuro‐ophthalmological and neuro‐otological examination with repeated determinations of SVV, electronystagmography and cranial MRI. The diagnosis of vestibular neuritis was assessed as described in a previous study.6 A dorsolateral medullary infarction was confirmed by cranial MRI in all patients with typical clinical signs of Wallenberg's syndrome. All patients with additional cerebellar or other infarctions were excluded. In all, 50 patients with Wallenberg's syndrome and 50 patients with vestibular neuritis were retrospectively assessed. The two groups differed significantly in gender, but not in age or affected side (table1). The skewed female/male ratio in vestibular neuritis and Wallenberg's syndrome is explained in the legend to table 1.
Table 1 Epidemiological data of the patients.
| Wallenberg' s syndrome | Vestibular neuritis | Total | Difference Wallenberg/neuritis (test) p Value | |
|---|---|---|---|---|
| No of patients | 50 | 50 | 100 | |
| (female:male) | 9:41 | 18:32 | 27:73 | 0.054 (χ2 test) |
| Mean age (years) | 52.6 | 53.1 | 52.8 | 0.860 (t test) |
| SD | 14 | 14.3 | 14.1 | |
| Affected side (left:right) | 24:26 | 26:24 | 50:50 | 0.689 (χ2 test) |
Regarding the high rate of males, it is known that Wallenberg's syndrome occurs more frequently in males; as for the higher frequency of males in the vestibular neuritis group, the inclusion criterion of MRI was causative, since males with vestibular neuritis more often had vascular risk factors, which prompted us to perform an MRI
During the measurement of SVV, patients sat with their chin resting on a fixed pad and looked into a half‐spherical dome 60 cm in diameter, which could be rotated around their line of sight. The surface of the dome extended over the entire visual field and was covered with a random pattern of coloured dots, providing no cues to gravitational orientation. Thirty centimetres in front of the subject was a linear target whose centre was fixed on the shaft of a servomotor. The target could be rotated in the subject's frontal plane. After target and dome were rotated to a randomised offset position, the patients were instructed to align the target with their perceived vertical by using a joystick device. A personal computer recorded the difference between the adjusted orientation and the true spatial vertical, and calculated the average of 10 readjustments. SVV was determined in this way monocularly and binocularly at least twice during the first month after the onset of symptoms.
Statistical analysis
Change over time in SVV was quantified with a linear mixed effect model for the log‐transformed SVV values. A random intercept was introduced to account for the multiple observations per patient. In order to test for a different slope between groups a likelihood ratio test was performed between the models: group+time and group*time. The library non‐linear mixed effects (NLME) of the statistical software package R (www.r‐project.org) was used for calculation.
Results
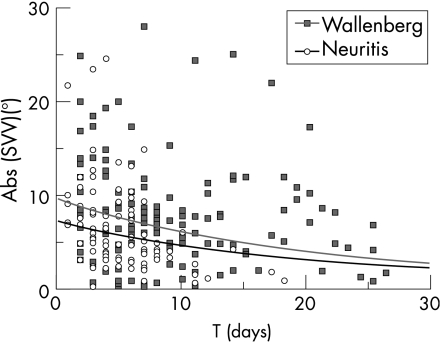
The mixed effect model revealed a significant decay of SVV displacement in the course of time for the entire sample of patients. The analysis showed no significant difference in the change over time between both groups. The daily decay rate was 5.4% (95% CI 3.6% to 7.1%). There was, however, a significant difference between the intercepts of both groups (fig 1). The onset of the vestibular neuritis group was 31% lower compared with the onset of the Wallenberg's syndrome group (95% CI 8.4% to 48%). The variability (standard deviation) of the random intercept was estimated as 0.567 (95% CI 0.458 to 0.703). Single observations and regression curves for both groups are shown as a function of time in fig 1 for binocular determinations of SVV. The data of monocular determinations of SVV gave no additional information.
Figure 1 Subjective visual vertical (SVV) displacement (deviations to the left are flipped to the right side) in the course of time, and regression curves for 50 patients with Wallenberg's syndrome (Wallenberg) and 50 patients with vestibular neuritis (neuritis).
Discussion
Vestibular compensation is an example of the capacity of the central nervous system for plastic adaptive change, as well as a fundamental concept in the study of lesion‐induced neural plasticity.7 The recovery from vestibular lesions is, however, neither a simple nor a single process. It involves multiple processes at various anatomical locations and with different time courses. To analyse the mechanisms of recovery, it is necessary to carefully compare the normalisation of parallel phenomena at the behavioural level, on the one hand, and at the neural level, on the other. In particular, incongruencies in the time course and the magnitude of the changes in behaviour and neural activity clearly indicate that multiple processes of compensation occur in distributed neural networks at different locations and at different times.3,4,8,9 With respect to vestibular neuritis, the central compensatory mechanisms may be biased by recovery of peripheral vestibular function, which is achieved, however, by a minority of the thus afflicted patients. Also, in Wallenberg's syndrome we cannot distinguish compensation from recovery.
The clinical experience gained in managing patients with Wallenberg's syndrome has shown that body lateropulsion gradually disappears within weeks. This was also demonstrated in our retrospective study of the time course during which the associated deviation of SVV normalised. Thus, there is evidence for the central compensation of both peripheral and central (dorsolateral pontomedullary) vestibular lesions. The structures involved and the underlying neural mechanisms, however, may differ with respect to the localisation, especially of the central vestibular nuclear or pathway lesions. An example of this is provided by acquired downbeat or upbeat nystagmus: upbeat nystagmus due to paramedian medullary lesions is usually compensated within days to weeks, whereas downbeat nystagmus, mostly due to bilateral floccular hypofunction,10 usually persists. This illustrates the prominent role played by the vestibulocerebellum in the processes of central vestibular compensation.
The current study does not allow one to draw conclusions about involved structures and mechanisms. It also has the methodological limitation of a retrospective design with varying data acquisition over time. Nevertheless, it at least provides evidence that the time courses of the central compensation of tonus imbalance due to unilateral dorsolateral medullary infarction and peripheral vestibular lesions are similar.
Acknowledgements
We thank Ms J Benson for copyediting the manuscript.
Abbreviations
SVV - subjective visual vertical
Footnotes
Competing interests: None declared.
References
- 1.Halmagyi G M, Curthoys I S, Brandt T.et al Ocular tilt reaction: clinical sign of vestibular lesion. Acta Otolaryngol Suppl 199148147–50. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol 199333292–299. [DOI] [PubMed] [Google Scholar]
- 3.Brandt T, Strupp M, Arbusow V.et al Plasticity of the vestibular system: central compensation and sensory substitution for vestibular deficits. Adv Neurol 199773297–309. [PubMed] [Google Scholar]
- 4.Curthoys I S, Halmagyi G M. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res 1995567–107. [PubMed] [Google Scholar]
- 5.Brandt T, Dieterich M. Vestibular falls. J Vestib Res 199333–14. [PubMed] [Google Scholar]
- 6.Strupp M, Zingler V C, Arbusow V.et al Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. N Engl J Med 2004351354–361. [DOI] [PubMed] [Google Scholar]
- 7.Flohr H, Bienhold H, Abeln W.et al Concepts of vestibular compensation. In: Flohr H, Precht W, eds. Lesion‐induced neuronal plasticity in sensorimotor systems. New York: Springer, 1981153–172.
- 8.Straka H, Dieringer N. Spinal plasticity after hemilabyrinthectomy and its relation to postural recovery in the frog. J Neurophysiol 1995731617–1631. [DOI] [PubMed] [Google Scholar]
- 9.Dieringer N. ‘Vestibular compensation': neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol 19954697–129. [PubMed] [Google Scholar]
- 10.Kalla R, Deutschlander A, Hufner K.et al Detection of floccular hypometabolism in downbeat nystagmus by fMRI. Neurology 200666281–283. [DOI] [PubMed] [Google Scholar]