Abstract
Background
Anti‐aquaporin 4 (AQP4) antibodies were found in patients with neuromyelitis optica (NMO) and Japanese optic–spinal multiple sclerosis (OSMS).
Objective
To review the clinical features and investigate anti‐AQP4 antibodies of Japanese patients with multiple sclerosis (MS), with or without long spinal cord lesions (LCL).
Methods
Anti‐AQP4 antibodies were examined in the sera of 128 consecutive Japanese patients by the immunofluorescence method using AQP4 transfected cells.
Results
The 45 LCL‐MS patients included 28 with a long spinal cord lesion extending contiguously over three vertebral segments on sagittal T2 weighted images (long T2 lesion) and 17 with segmental cord atrophy extending more than three vertebral segments. We identified 25 patients with anti‐AQP4 antibody with LCL and anti‐AQP4 antibody. Anti‐AQP4 antibody was found in 12/17 (70.6%) LCL‐MS patients with segmental cord atrophy, and in 13/28 (46.4%) LCL‐MS patients without segmental long cord atrophy (p = 0.135, Fisher's exact test). Seropositive MS patients with LCL had more relapses than seronegative patients (p = 0.0004, Mann–Whitney U test). 9 patients with OSMS were negative for anti‐AQP4 antibody who did not show LCL.
Conclusion
These results suggest that an anti‐AQP4 antibody is found not only in MS patients with long T2 lesions but also in patients with segmental cord atrophy extending more than three vertebral segments. It is a marker of LCL‐MS showing frequent exacerbations. Japanese OSMS cases comprised those that were identical to NMO cases and those that were more closely related to classic MS.
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system. Japanese MS patients have been classified into two phenotypes: classic MS (CMS) and optic–spinal MS (OSMS).1 OSMS has been recognised since the 1950s.2 Patients with OSMS have symptoms and MRI findings in which the main lesions are confined to the optic nerve and spinal cord. In patients with OSMS, there is a higher female/male ratio; neuropathologically necrotic lesions; pleocytosis with a predominance of polymorphonuclear cells and a low frequency of oligoclonal IgG bands in CSF; a high incidence of autoantibodies in sera; long spinal cord lesions (LCL) extending more than three vertebral segments in MRI scans; and an association with a human leucocyte antigen class II allele (DPB1*0502).3
Neuromyelitis optica (NMO) has been described as Devic disease, but its clinical definition has frequently been revised.4 LCL extending contiguously over three vertebral segments on sagittal T2 weighted images (long T2 lesion) is a disease marker. Current NMO criteria include unilateral optic neuritis, no restriction on onset of optic neuritis and myelitis, relapsing course and brain involvement.4,5 Recent NMO criteria stress the presence of both brain MRI abnormalities that do not meet diagnostic criteria for MS and NMO‐IgG,4 a highly specific biomarker of NMO,6 and its target antigen is the aquaporin 4 (AQP4) water channel.7
The incidence of NMO‐IgG seropositivity in Japanese patients with OSMS (6/11 cases, 54%) was similar to that in NMO (33/45, 73%), and OSMS was thought to be the same disease.6 However, in that study,6 the Japanese OSMS patients had been selected using 1999 NMO criteria (Fujihara K, personal communication) that are not the same as the clinical definition of OSMS widely used in Japan.8 Their recent report showed that serum NMO‐IgG was found in 12 of 19 patients with OSMS (63%).9 We established an AQP4 antibody assay system and identified nine seropositive MS patients with LCL‐MS.10
Patients and methods
A total of 128 consecutive Japanese patients (36 men, 92 women), aged 21–75 years, who had definite relapsing–remitting MS clinically, according to McDonald's criteria, were considered for enrolment in our study. Patients with primary progressive MS, spinal MS and relapsing optic neuritis were excluded. Time since onset was 1–35 years. Kurtzke's Expanded Disability Status Scale (EDSS) score11 was used for the clinical ratings.
Patients who showed selective involvement of the spinal cord and optic nerves but no clinical signs indicative of cerebrum, cerebellum or brainstem involvement were classified as having OSMS, while those with estimated lesions in the cerebrum, cerebellum or brainstem were defined as CMS.1 Patients with a long cord lesion on spinal cord MRI (long T2 lesion or segmental cord atrophy) extending over three vertebral segments were designated as having LCL‐MS.
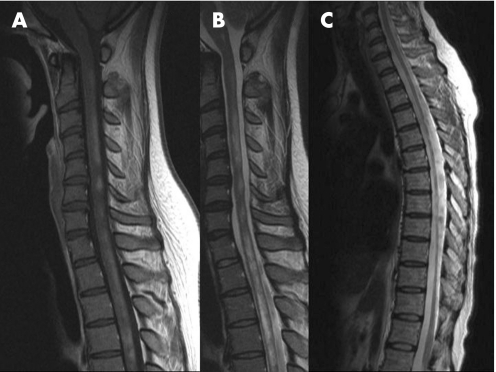
Figure 1 MRI findings of patients with long spinal cord lesions–multiple sclerosis (LCL‐MS). The patient shows discontinuous enhanced lesions from C1 to Th5 (A) and discontinuous T2 high intensity lesions in the cervical segment and T2 high intensity lesions from Th1 to Th5 (long T2 lesion) (B). Another patient shows segmental spinal cord atrophy from C7 to Th11 (C).
Anti‐AQP4 antibody measurement
Anti‐AQP4 IgG antibody in MS patient sera was measured using an indirect immunofluorescence method reported previously10 with HEK‐293 cells transfected with an AQP4 expression vector containing full length cDNA of human AQP4. Sera were obtained during the clinically stable stage. Anti‐AQP4 antibody was measured by one of the authors (KT) who was blind to all clinical information, including clinical phenotype.
The statistical significance of differences between the two groups were determined using the Mann–Whitney U test or Fisher's exact test for difference in percentage anti‐AQP4 antibodies between patients with and without segmental long spinal cord atrophy.
This study was approved by the Medical Ethics Committee of Utano National Hospital. All participants gave written informed consent.
Results
The 45 LCL‐MS patients (five men and 40 women) included 28 with long T2 lesions (four men and 24 women) and 17 with segmental cord atrophy extending more than three vertebral segments (two men and 15 women) (fig 1). Anti‐AQP4 antibodies were found in 12/17 (70.6%) LCL‐MS patients with segmental cord atrophy and in 13/28 (46.4%) LCL‐MS patients without segmental long cord atrophy (p = 0.135, Fisher's exact test) (table 1). Excluding those with LCL‐MS, patients did not have anti‐AQP4 antibodies. There was a relationship between blindness and seropositivity against AQP4 (p<0.0005) (table 2). Eleven of 128 patients were blind, nine of whom had LCL‐MS. Seven of the nine blind patients had LCL and anti‐AQP4 antibodies.
Table 1 Number of seropositive patients with multiple sclerosis.
| n | No of anti‐AQP4 antibody positive (%) | |
|---|---|---|
| Total LCL‐MS | 45 | 25 (55.6%) |
| With segmental cord atrophy | 17 | 12 (70.6%) |
| Without segmental cord atrophy | 28 | 13 (46.4%) |
| OSMS | 22 | 2 (9.1%) |
| Including LCL‐MS | 3 | 2 (66.7%) |
| CMS | 64 | 0 (0%) |
| Less than 5 y after onset | 29 | 4 (13.8%) |
| Including LCL‐MS | 9 | 4 (44.4%) |
| OSMS | 9 | 0 (0%) |
AQP4, aquaporin 4; CMS, classic multiple sclerosis—patients with estimated lesions in the cerebrum, cerebellum or brainstem; LCL‐MS, long spinal cord lesions‐multiple sclerosis—patients with a long spinal cord lesion on MRI (long T2 lesion or segmental cord atrophy) extending over three vertebral segments; OSMS, optic–spinal multiple sclerosis—patients with selective involvement of the spinal cord and optic nerves but no clinical signs indicative of cerebrum, cerebellum or brainstem involvement;
The 128 patients include 45 patients with LCL‐MS, 12 with OSMS and 71 with CMS.
Table 2 Relationship between blindness and seropositivity against aquaporin 4 (p<0.0005).
| Blindness | ||
|---|---|---|
| + | − | |
| Anti‐AQP4 antibody | ||
| Positive | 7 | 16 |
| Negative | 4 | 101 |
The number of relapses in 22 patients with LCL‐MS and anti‐AQP4 antibodies (0–7; mean 3.64) was greater than in 18 seronegative patients with LCL‐MS (0–6; 1.39) in the year before determination of antibodies (p = 0.0004). Time from onset for seropositive patients (mean 14.3) was not different from that of seronegative patients (9.1) (p = 0.1518). There was no other difference in clinical phenotype between patients with and without anti‐AQP4 antibodies.
We found nine OSMS patients who had a 5 year minimum follow‐up period after onset and did not have LCL or anti‐AQP4 antibodies (OSMS(−)). Age of OSMS(−) onset ranged from 7 to 51 years (mean 27.0), which did not differ from that of LCL‐MS cases (2–62 years; 35.2) (p = 0.1281). The period after onset of OSMS (−) was 5–24 years (mean 11.4), which did not differ from that of LCL‐MS cases (1–30 years; 12.0) (p = 0.6685). Kurtzke's EDSS for OSMS(−) (0–4.0; mean 1.50), however, was lower than that for LCL‐MS (0–9.5; 6.63) (p<0.0001). At disease onset, one of three patients had asymptomatic subcortical white matter lesions on brain MRI, while the other two had normal brain MRI findings. However, most patients who had optic neuritis as the first event did not undergo MRI. Patients with OSMS(−) had no clinical manifestations except for symptoms or signs caused by lesion of the optic nerves or spinal cord. All showed asymptomatic brain lesions on T2 weighted brain MRI scans.
Discussion
Anti‐AQP4 antibody is a good diagnostic biomarker for LCL‐MS as 25 of 45 LCL‐MS patients (56%) had anti‐AQP4 antibodies but patients who did not have LCL‐MS did not have antibodies.
The Mayo Clinic Group has reported that long T2 lesions was a disease marker of NMO4 but they did not mention spinal cord atrophy, which would also be a marker of NMO. Segmental cord atrophy seems to be caused by severe necrotic lesions in a similar way as long T2 lesions on spinal cord MRI in patients with NMO. The percentage of anti‐AQP4 antibodies in patients with segmental long spinal atrophy was not different from that in patients with long T2 lesions, which also supports the hypothesis that the pathomechanism of segmental cord atrophy is similar to that of long T2 lesions.
Similar findings of LCL on spinal cord MRI and seropositivity against AQP4 in NMO and OSMS seem to indicate that these diseases fall into the same clinical spectrum. Some Japanese OSMS patients, however, did not fulfil NMO criteria4 because they did not have LCL or anti‐AQP4 antibodies. Patients with CMS may present with only optic neuritis and myelitis for the first few years after onset, whereas the diagnosis of OSMS requires a 5 year follow‐up period after onset.3 Several patients with OSMS differed from those with NMO, with the disease being clinically restricted to the optic nerves and spinal cord for a period of 5–24 years after the first clinical event. This may be the same clinical phenotype as the benign form of OSMS.3 All of our patients with OSMS(−) had brain lesions but no brain symptoms or signs. Moreover, they had lower EDSS scores than patients with LCL‐MS, indicating that the higher LCL‐MS scores may have been caused by LCL.
The role of anti‐AQP4 antibody as the primary cause of tissue destruction has not been demonstrated and there were more relapses in patients with anti‐AQP4 antibodies than in seronegative patients. This does not exclude the possibility of a secondary immune response to damage. However, NMO IgG is a known marker of relapse after myelitis with LCL.12 This suggest that anti‐AQP4 antibody/NMO IgG is not the secondary product of tissue destruction, rather it causes necrotic tissue damage and is a marker of a condition related to frequent exacerbation.
Abbreviations
AQP4 - aquaporin 4
CMS - classic multiple sclerosis
EDSS - Expanded Disability Status Scale
LCL - long spinal cord lesions
MS - multiple sclerosis
NMO - neuromyelitis optica
OSMS - optic–spinal multiple sclerosis
Footnotes
This work was supported in part by a Neuroimmunological Disease Research Committee grant from the Ministry of Health, Labour and Welfare, Japan.
Competing interests: Researchers from Mayo MS group. Professor Kira, Kyushu University, Japan.
References
- 1.Saida T, Tashiro K, Itoyama Y.et al Interferon Beta‐1b Multiple Sclerosis Study Group of Japan. Interferon beta‐1b is effective in Japanese RRMS patients: a randomized, multicenter study, Neurology 200564621–630. [DOI] [PubMed] [Google Scholar]
- 2.Okinaka S, Tsubaki T, Kuroiwa Y.et al Multiple sclerosis and allied diseases in Japan: clinical characteristics. Neurology 19588756–763. [DOI] [PubMed] [Google Scholar]
- 3.Misu T, Fujihara K, Nakashima I.et al Pure optic‐spinal form of multiple sclerosis in Japan. Brain 20021252460–2468. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk D M, Lennon V A, Pittock S J.et al Revised diagnostic criteria for neuromyelitis optica. Neurology 2006661485–1489. [DOI] [PubMed] [Google Scholar]
- 5.Pittock S J, Lennon V A, Krecke K.et al Brain abnormalities in neuromyelitis optica. Arch Neurol 200663390–396. [DOI] [PubMed] [Google Scholar]
- 6.Lennon V A, Wingerchuk D M, Kryzer T J.et al A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 20043642106–2112. [DOI] [PubMed] [Google Scholar]
- 7.Lennon V A, Kryzer T J, Pittock S J.et al IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med 2005202473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kira J, Kanai T, Nishimura Y.et al Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol 199640569–574. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima I, Fujihara K, Miyazawa I.et al Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO‐IgG. J Neurol Neurosurg Psychiatry 2006771073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka K, Tani T, Tanaka M.et al Anti‐aquaporin 4 antibody in Japanese opticospinal multiple sclerosis. Mult Scler in press
- 11.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: An expected disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 12.Weinshenker B G, Wingerchuk D M, Vukusic S.et al Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 200659566–569. [DOI] [PubMed] [Google Scholar]