Abstract
Objective
To present three cases of young adults with lateral medullary ischaemic events associated with a hypoplastic vertebral artery (VA). All three patients had two additional atherosclerotic or non‐atherosclerotic risk factors for stroke.
Patients and methods
One female, aged 40 years, and two males, aged 38 and 37 years, each with two risk factors for stroke, presented to the emergency department with acute onset of symptoms and findings consistent with lateral medullary syndrome. All three patients underwent emergency CT scan of the brain followed by MRI and magnetic resonance angiography (MRA).
Results
The CT scans were negative in all patients. MRI revealed a lateral medullary lesion in only one patient. All three patients had a hypoplastic VA ipsilateral to the clinical ischaemic event on MRA.
Conclusions
Hypoplasia of VA is not considered a risk factor for stroke as it is a common variant in up to 75% of the general population. However, in our patients, hypoplastic VA coexisted with two risk factors and resulted in stroke. Thus although a hypoplastic VA may not be an uncommon asymptomatic finding, it may contribute to stroke if additional risk factors are present.
The vertebral arteries (VAs) usually originate from their respective subclavian arteries and unite to form the basilar artery after giving rise to the posterior inferior cerebellar arteries. Variations in anatomy of the VAs are relatively frequent, as in 4–15% of the healthy population one VA is atretic with minimal contribution to vertebrobasilar blood flow while lesser degrees of asymmetry are more frequent. The two VAs are of similar size in 25%, while the left is the dominant artery in 50% of the population. The absence of vertebrobasilar insufficiency symptoms in all of these cases suggests that even a pronounced VA asymmetry can be regarded as normal.1,2 The present report of three cases suggests that a hypoplastic VA in association with other risk factors may contribute to an ischaemic brainstem event, even in young patients.
Patients and methods
Patient No 1
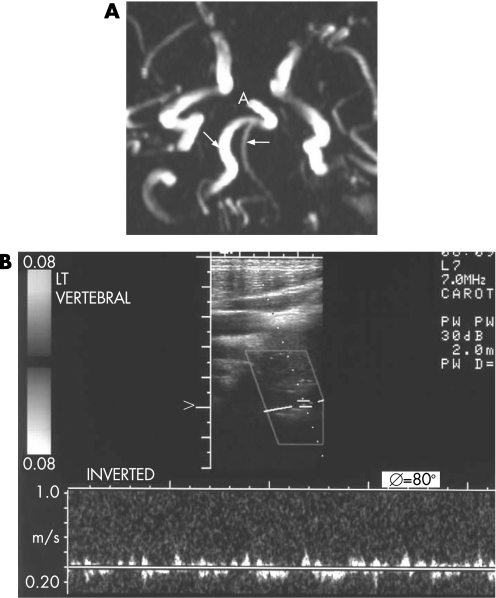
A 37‐year‐old male with no recent history of neck trauma presented to the emergency department complaining of vertigo, nausea, vomiting, diplopia and dysphagia. His past medical history was significant for hypertension and non‐familial dyslipidaemia since the age of 32 years. He reported no cephalalgia or neck pain, and admission arterial blood pressure was 150/100 mm Hg. Neurological examination revealed ipsilateral ataxia of the limbs, ipsilateral facial hypalgesia and thermoanaesthesia, and contralateral trunk and extremity hypalgesia and thermoanaesthesia. In addition, the patient had ipsilateral palatal, pharyngeal and vocal cord paralysis, ipsilateral Horner syndrome, ipsilateral limb ataxia and ipsilateral incomplete VI nerve paresis. A diagnosis of lateral medullary syndrome, known as Wallenberg syndrome, was made. Urgent CT brain scan was unremarkable. The patient's symptoms resolved within 90 min, and the event was considered a brainstem transient ischaemic attack (TIA). The patient underwent MRI and magnetic resonance angiography (MRA). MRI was unremarkable but MRA revealed a hypoplastic VA on the side of the TIA (fig 1A). Subsequently, duplex ultrasonography depicting a peak systolic velocity of 15 cm/s confirmed the presence of the hypoplastic VA (fig 1B), while in the normal VA peak systolic velocity was 45 cm/s. A few weeks later the patient underwent CT angiography showing a hypoplastic VA of similar diameter (not shown). Detailed cardiac, immunological and haematological tests were within normal limits. The patient was treated for secondary stroke prevention with clopidogrel, ramipril and pravastatin.
Figure 1 (A) Magnetic resonance angiography in patient No 1 showing the hypoplastic right vertebral artery compared with the left (arrows). A, basilar artery. (B) Duplex ultrasonography showing very low velocity in the hypoplastic right vertebral artery.
Patient No 2
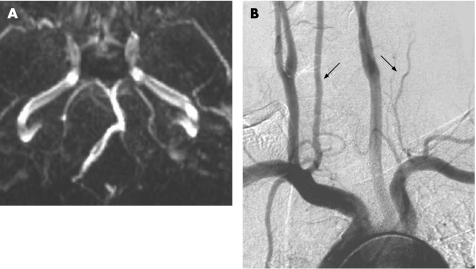
A second male patient, 38 years old, with no history of neck trauma, presented to the emergency department with nausea, diplopia, hoarseness of the voice, dysphagia and gait disturbance. His past medical history was consistent with hypertension since the age of 30 years and autoimmune disease (Crohn's disease), with no history of migraine headaches. Arterial blood pressure on admission was 160/100 mm Hg. Neurological examination showed gaze evoked nystagmus, ipsilateral paresis of the ΙΧ and Χ cranial nerves, ipsilateral ataxia of the limbs, and ipsilateral facial and contralateral body impairment of thermal and pinprick sensations. The patient's symptoms resolved within a few hours, and the event was considered a TIA. CT and MRI scans of the brain were unremarkable. MRA (fig 2A) and intra‐arterial digital subtraction angiography (DSA) (fig 2B) were performed, demonstrating an ipsilateral filamentous VA. Further investigation for additional stroke risk factors was negative. The patient was treated with clopidogrel and ramipril. Follow‐up MRA and CT angiography were performed with the same finding of congenital hypoplastic VA (not shown).
Figure 2 (A) Magnetic resonance angiography in patient No 2 showing the hypoplastic left vertebral artery. (B) Digital subtraction angiography demonstrating the hypoplastic left vertebral artery (black arrows to each vertebral artery).
Patient No 3
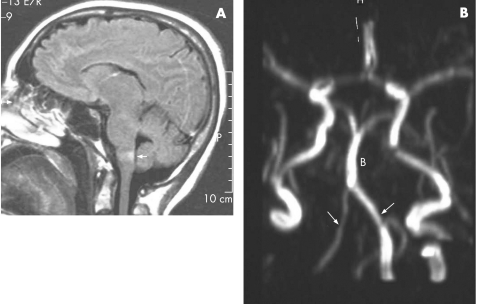
A 40‐year‐old woman, with known hypertension for the past 5 years and migraine since adolescence, presented to the emergency department with nausea and ataxia. On admission she reported no upper body trauma, no cephalalgia and had an arterial blood pressure of 140/100 mm Hg. Neurological examination manifested a lateral medullary syndrome with remarkable ipsilateral ataxia and impaired sensation ipsilaterally in the face and contralaterally in the body, while the pyramidal tracts were intact. MRI depicted a lateral medullary lesion (fig 3A) and MRA showed a pronounced asymmetry between the VAs (fig 3B). A diagnosis of hypoplastic VA with a peak systolic velocity of 15 cm/s ipsilateral to the ischaemic lesion and 40 cm/s contralaterally was confirmed by duplex ultrasonography (not shown). Detailed cardiac, haematological and immunological investigations were negative. Intra‐arterial DSA was not performed because of possible vasospasm in a migrainous patient. The patient was treated with clopidogrel and propranolol for prevention of migraine and hypertension control. Follow‐up MRA depicted the same finding of a hypoplastic VA.
Figure 3 (A) Fluid attenuated inversion recovery imaging of patient No 3 depicting the medullary infarct (arrow). (B) Magnetic resonance angiography showing the hypoplastic right vertebral artery; B, basilar artery.
Discussion
The clinical significance of VA hypoplasia is not yet clearly defined. True congenital hypoplasia is considered when the VA diameter is less than 2–3 mm.3,4 In a recent study, the cut‐off diameter size for hypoplasia was set at 2.2 mm.5 However, asymmetry of the VAs or true hypoplasia is not considered a risk factor for stroke unless associated with stenotic lesions.1
Some years ago, Chaturvedi et al showed that vertebrobasilar hypoplasia may predispose to ischaemic stroke in relatively young patients.6 More recently, Chuang et al suggested that VA stenosis may be a contributing factor for ischaemic stroke in the posterior circulation territories.7 Our cases are consistent with these reports and postulate that VA hypoplasia or asymmetry may play a role in posterior circulation ischaemia, even in young adults.
None of our patients had a recurrent TIA or stroke following the secondary stroke prevention treatment over a 3 year follow‐up period. In addition, none had other ischaemic lesions in other vascular territories except those that corresponded to the posterior inferior cerebellar artery (lateral medulla). On follow‐up MRA, all patients had similar diameters in the hypoplastic VAs, thus ruling out dissection as an underlying mechanism. The benign clinical course of all patients and the presence of flow on follow‐up MRA argued against the presence of severe proximal stenosis to the hypoplastic VAs. These observations support the hypothesis that their vascular events were related to the hypoplastic VA. Two of our patients had TIAs and only one had an MRI documented infarct. Similarly, among Chaturvedi's eight patients, most (6/8) had TIAs and only two had a definite stroke.6 We do not have a reliable explanation for this observation, except perhaps that the low flow state of the hypoplastic VA provoked TIAs rather than infarcts. As thombocytosis was not detected in the patient with Crohn's disease, we assume that for all patients beyond the low flow state, atherosclerosis due to hypertension may have played a contributing role.6 Vasospasm for our migrainous female patient is a possible, but less likely, mechanism as the patient was free of headaches prior to and during the ischaemic event. However, the risk of vasospasm and stroke in patients with migraine is known.8
For accurate diagnosis of VA hypoplasia, the examination of choice remains intra‐arterial DSA.1,9 Recent studies support the use of colour coded duplex ultrasonography as a reliable method for detecting VA hypoplasia, launching VA flow net volume measurements below 100 ml/min as an additional diagnostic criterion.10,11 To our knowledge, data on the use of CT angiography are limited.12 Although the widely used MRA is a reliable method for evaluating the posterior circulation, we confirmed the diagnosis of VA hypoplasia in all cases with a second imaging method.9
In summary, it is likely that an ischaemic event may be caused, even in young patients, when VA hypoplasia coexists with other risk factors for stroke. In our patients, hypoplastic VA coexisted with two additional risk factors and we propose that this combination provoked ischaemic events in our young adult patients.
Abbreviations
DSA - digital subtraction angiography
MRA - magnetic resonance angiography
TIA - transient ischaemic attack
VA - vertebral artery
Footnotes
Competing interests: None.
References
- 1.Cloud G C, Markus H S. Diagnosis and management of vertebral artery stenosis. Q J Med 20039627–34. [DOI] [PubMed] [Google Scholar]
- 2.Tratting S, Hubsch P, Polzeitner D. Color‐coded Doppler imaging of normal vertebral arteries. Stroke 1990211222–1225. [DOI] [PubMed] [Google Scholar]
- 3.Touboul P J, Bousser M G, LaPlane D.et al Duplex scanning of normal vertebral arteries. Stroke 198617921–923. [DOI] [PubMed] [Google Scholar]
- 4.Fischer C M, Gore I, Okabe N.et al Atherosclerosis of the carotid and vertebral arteries—extracranial and intracranial. J Neuropathol Exp Neurol 196524455–476. [Google Scholar]
- 5.Jeng J S, Yip P K. Evaluation of vertebral artery hypoplasia and asymmetry by color‐coded duplex ultrasonography. Ultrasound Med Biol 200430605–609. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi S, Lukovits T G, Chen W.et al Ischemia in the territory of hypoplastic vertebrobasilar system. Neurology 199952980–983. [DOI] [PubMed] [Google Scholar]
- 7.Chuang Y M, Huang Y C, Hu H H.et al Toward a further elucidation: Role of vertebral artery hypoplasia in acute ischemic stoke. Eur Neurol 200655193–197. [DOI] [PubMed] [Google Scholar]
- 8.Tietjen G E. The risk of stroke in patients with migraine and implications for migraine management. CNS Drugs 200518683–692. [DOI] [PubMed] [Google Scholar]
- 9.Tay K Y, U‐King‐Im J M, Triverdi R A.et al Imaging the vertebral artery. Eur Radiol 2005151329–1343. [DOI] [PubMed] [Google Scholar]
- 10.Saito K, Kimura K, Nagatsuka K.et al Vertebral artery occlusion in duplex color‐coded ultrasonography. Stroke 200435168–172. [DOI] [PubMed] [Google Scholar]
- 11.Seidel E, Eicke B M, Tettenborn B.et al Reference values for vertebral artery flow volume by duplex sonography in young and elderly adults. Stroke 1999302692–2696. [DOI] [PubMed] [Google Scholar]
- 12.Sanelli P C, Tong S, Gilberto Gonzalez R.et al Normal variation of vertebral artery on CT angiography and its implications of diagnosis of acquired pathology. J Comput Assist Tomogr 200226462–470. [DOI] [PubMed] [Google Scholar]