Fabry disease (FD, OMIM 301 500) is a lysosomal storage disorder caused by an X‐linked inborn error of glycosphingolipid catabolism, resulting from deficient activity of α‐galactosidase A. It leads to accumulation of globotriaosylceramide (Gb3) in various organs. Manifestations of FD occur mostly in affected hemizygous males but also in heterozygous (carrier) females.1,2
Neurological complications in FD include CNS involvement, acroparesthesia, peripheral neuropathy, cranial nerve palsies with predominant VIIIth nerve involvement, and autonomic dysfunction.
Because a specific treatment has recently emerged, the diagnosis of FD may have a strong practical impact. Yet, enzyme replacement therapy has no proven efficacy on CNS Fabry manifestations.
We report on a woman who suffered from chronic meningitis which revealed heterozygous FD.
Case report
A 25‐year‐old woman was referred because of headache. Since the age of 12 years, the patient had complained of recurrent episodes of paresthesia in her four limbs, over 7–15 days, and sometimes leading to syncope. Headache started in November 2003, lasting 2–3 h everyday. In March 2004, the patient was hospitalised because her headache was unusually severe. At physical examination, hypoesthesia was affecting the left part of the body with respect to the face. C reactive protein blood level was 4.7 mg/dl (normal <1). CSF analysis showed 8 elements/μl. Brain and cervical medulla MRI was normal.
Throbbing headache recurred in October 2004. Body temperature was 38.5°C. She complained of sicca syndrome. There was persistent hypoesthesia of the left part of the body. Numerous telangiectasia were disclosed on the trunk. C reactive protein level was 2.7 mg/dl. Creatinine blood level was normal. Microalbuminuria was slightly elevated at 44 mg/dl (normal <15). A second lumbar puncture was then performed showing 76 elements/μl with lymphocytes (44%) and granulocytes (43%). Testing for infectious agents and serum autoantibodies was negative.
Audiogram found a bilateral sensorineural hearing loss of 20 dB on 1000–8000 Hz frequencies. Ultrasound study of the extracranial and intracranial supra‐aortic arteries was normal. Brain MRI was considered normal. Total body CT scan, bronchoalveolar lavage, and bronchial and salivary gland biopsy specimen analysis were normal.
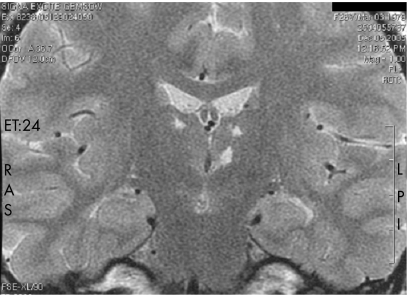
Taking into consideration chronic meningitis and the fact that the patient originated from an area endemic for tuberculosis, antituberculous treatment was started in October 2004. In May 2005, the patient was hospitalised again. CSF analysis showed 31 elements/μl with 72% of lymphocytes and 14% of granulocytes. A third brain MRI disclosed an abnormal signal in both thalami (fig 1). Past familial history was then more thoroughly addressed. Her younger sister had also suffered from recurrent pain in the four limbs in her adolescence. Their father had been on haemodialysis since the age of 42 years and died at 47 years, with no further data on the cause of renal disease or death.
Figure 1 Brain MRI. T2 weighted coronal section shows bithalamic small infarcts, as fluid T2 hyperintensities, of the dorsolateral and left centromedial nuclei.
FD was considered. Cornea verticillata was disclosed on a slit lamp ophthalmological examination. Globotriaosylceramide dosage in urine was highly elevated at 41.2 nmol/mmol of creatinine (n<6). The leucocyte specific activity of alpha‐galactosidase A was decreased at 54 nmol/h/mg of protein (n in controls = 95). Analysis of the gene encoding alpha‐galactosidase A found a deletion (c123delC) in exon 1.
The patient was then treated with acetylsalicylic acid 250 mg/day, gabapentine 300 mg three times a day and paroxetine 20 mg/day. Enzyme replacement therapy for FD was begun in October 2005. In June 2006, after 9 months of enzyme replacement therapy, there was no improvement in CSF analysis (40 elements/μl with 50% of lymphocytes and 37% of granulocytes) and the patient suffered from definite intracranial hypertension (recurrent headache, blurred vision, bilateral papilloedema, high CSF pressure with unchanged MRI). Intracranial hypertension resolved completely and CSF analysis improved (9 elements/μl) after 2 weeks of high dose steroid treatment.
Discussion
In our female patient, highly misleading features, such as fever of unknown origin, high C reactive protein level and chronic meningitis were related to FD because (a) specific ongoing CNS involvement was demonstrated, with bilateral thalamic infarcts, (b) no other cause was elicited after a thorough investigation panel and (c) such inflammatory features have already been reported in male patients with FD.3,4
One of these patients had fever of unknown origin at onset3 and (as in our female patient) was treated with antituberculous therapy before the diagnosis of FD. Of note, a transient improvement in meningitis was also observed in two cases receiving corticosteroid therapy alone.3,4
The pathophysiology of cerebrovascular involvement in FD, including dolichoectasia of the large arteries and progressive occlusion of the small arteries, is poorly understood. Cardioembolic events may also complicate specific hypertrophic cardiomyopathy, valvulopathies and arrhythmia. Accelerated atherosclerosis related to end stage renal failure may occur. The fact that a genetic disorder such as FD may be associated with spontaneous wax and wane fever and inflammation is intriguing. The mechanism of inflammation in FD may reside beyond the schematic “vascular deposits” model. Interestingly, among the genetic modifiers addressed in FD, some are implicated in inflammation, thrombosis or both.5
FD might to some extent belong to the genetic autoinflammatory disorders spectrum. Diseases such as familial Mediterranean fever (FMF, OMIM 249 100) and tumour necrosis factor receptor superfamily 1A associated periodic syndrome (TRAPS, OMIM 142 680) may cause periodic fever and recurrent CNS inflammation.
In conclusion, FD may cause steroid responsive CNS inflammation in both men and women and should be included in the list of the causes of chronic meningitis.
Acknowledgements
We wish to acknowledge Catherine Caillaud, MD, PhD, and Roselyne Froissart, MD, PhD for their technical assistance.
Footnotes
Competing interests: Dr Lidove has received support from TKT Europe AB (Shire Human Genetics, Basingstoke, UK), Actelion Pharmaceuticals Ltd and travel fees from Genzyme Corporation; Professor Papo has received support from Genzyme Corporation; Dr Chauveheid, Dr Benoist, Dr Alexandra and Dr Klein have no conflicts of interest to declare.
References
- 1.MacDermot K D, Holmes A, Miners A H. Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 200138750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDermot K D, Holmes A, Miners A H. Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 200138769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubost J J, Viallard I L, Sauvezie B. Chronic meningitis in Fabry's disease. J Neurol Neurosurg Psychiatry 198548714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyama E, Ueno N, Uchino M.et al Headache associated with aseptic meningeal reaction as clinical onset of Fabry's disease. Headache 199535498–501. [DOI] [PubMed] [Google Scholar]
- 5.Altarescu G, Moore D F, Schiffmann R. Effect of genetic modifiers on cerebral lesions in Fabry disease. Neurology 2005642148–2150. [DOI] [PubMed] [Google Scholar]