Abstract
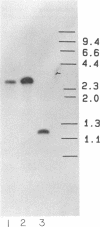
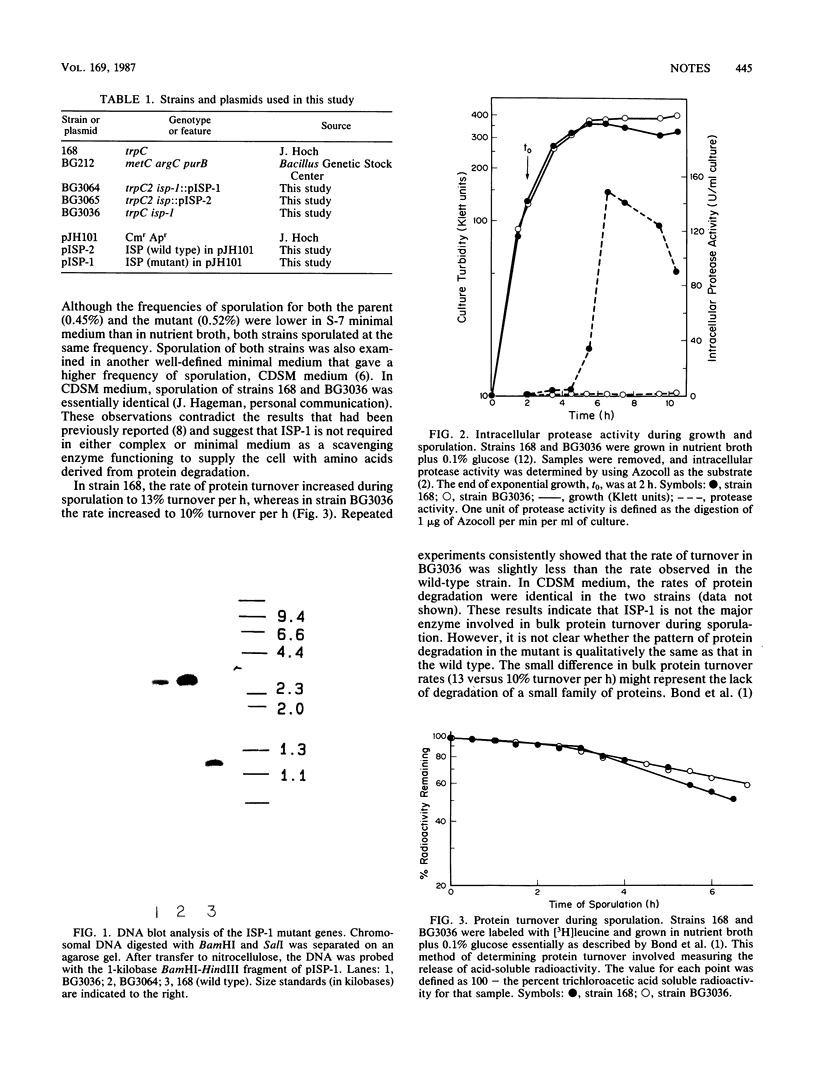
An intracellular serine protease (ISP-1) mutant of Bacillus subtilis was created by introducing a frameshift into the coding region of the cloned gene. Intracellular protease activity in the mutant was very low, yet sporulation in both nutrient broth and minimal medium was normal. The rate of bulk protein turnover in the mutant was slightly slower than that in the wild-type strain. These results suggest that the gene for ISP-1 is not essential and that ISP-1 is not the major enzyme involved in protein turnover during sporulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond R. W., Field A. S., Switzer R. L. Nutritional regulation of degradation of aspartate transcarbamylase and of bulk protein in exponentially growing Bacillus subtilis cells. J Bacteriol. 1983 Jan;153(1):253–258. doi: 10.1128/jb.153.1.253-258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavira R., Jr, Burnett T. J., Hageman J. H. Assaying proteinases with azocoll. Anal Biochem. 1984 Feb;136(2):446–450. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. B., Vasantha N., Freese E. Induction of sporulation in developmental mutants of Bacillus subtilis. Mol Gen Genet. 1979 Feb 16;170(1):67–74. doi: 10.1007/BF00268581. [DOI] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. H., Shankweiler G. W., Wall P. R., Franich K., McCowan G. W., Cauble S. M., Grajeda J., Quinones C. Single, chemically defined sporulation medium for Bacillus subtilis: growth, sporulation, and extracellular protease production. J Bacteriol. 1984 Oct;160(1):438–441. doi: 10.1128/jb.160.1.438-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan P., Keryer E., Szulmajster J. Characterization of a thermosensitive sporulation mutant of Bacillus subtilis affected in the structural gene of an intracellular protease. Eur J Biochem. 1979 Aug 1;98(2):353–362. doi: 10.1111/j.1432-1033.1979.tb13194.x. [DOI] [PubMed] [Google Scholar]
- Koide Y., Nakamura A., Uozumi T., Beppu T. Cloning and sequencing of the major intracellular serine protease gene of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):110–116. doi: 10.1128/jb.167.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Switzer R. L. Proteolysis in bacterial sporulation. Curr Top Cell Regul. 1980;16:163–224. doi: 10.1016/b978-0-12-152816-4.50010-8. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Sastry K. J., Srivastava O. P., Millet J., FitzJames P. C., Aronson A. I. Characterization of Bacillus subtilis mutants with a temperature-sensitive intracellular protease. J Bacteriol. 1983 Jan;153(1):511–519. doi: 10.1128/jb.153.1.511-519.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an In vitro-derived deletion mutation. J Bacteriol. 1984 May;158(2):411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Ermakova L. M., Baratova L. A., Belyanova L. P., Stepanov V. M. Intracellular serine protease of Bacillus subtilis: sequence homology with extracellular subtilisins. J Bacteriol. 1978 Mar;133(3):1401–1411. doi: 10.1128/jb.133.3.1401-1411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. Y., Ferrari E., Henner D. J. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984 Oct;160(1):15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. The mechanism of insertion of a segment of heterologous DNA into the chromosome of Bacillus subtilis. J Gen Microbiol. 1983 May;129(5):1497–1512. doi: 10.1099/00221287-129-5-1497. [DOI] [PubMed] [Google Scholar]