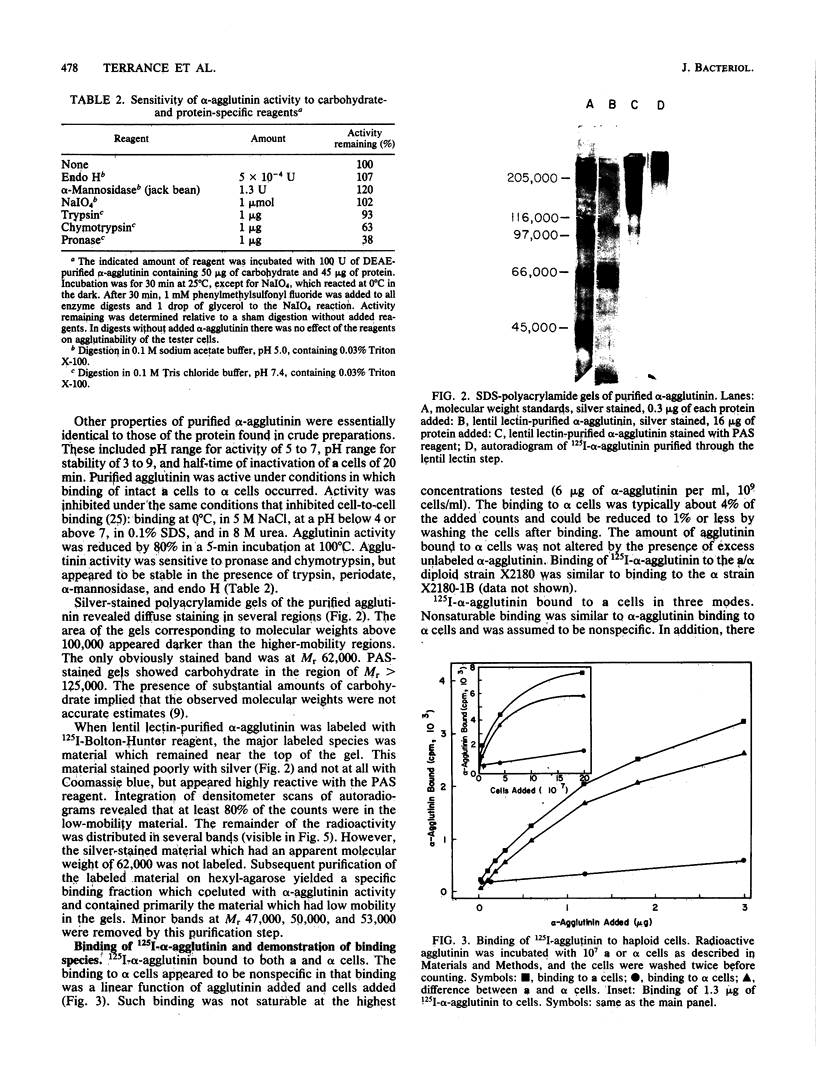
Abstract
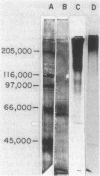
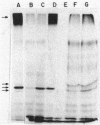
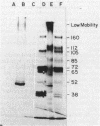
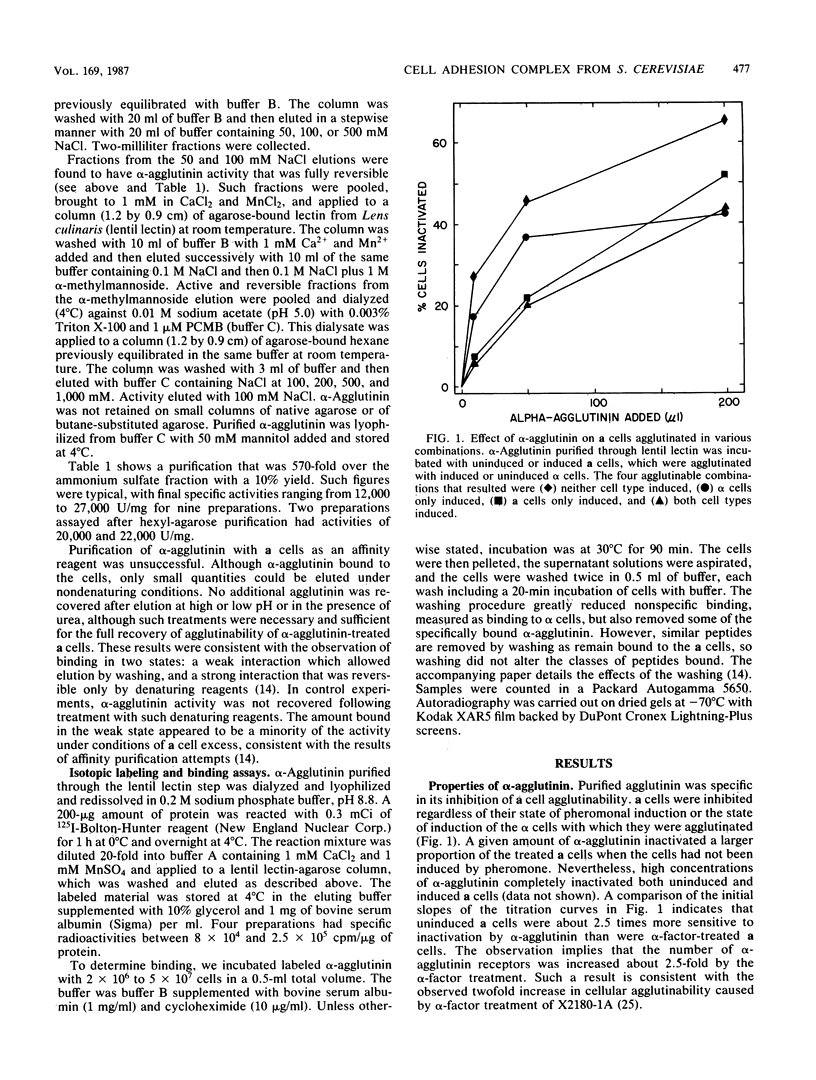
Several glycoproteins which inhibit the agglutinability of Saccharomyces cerevisiae mating type a cells were partially purified from extracts of mating type alpha cells. These proteins, called alpha-agglutinin, were labeled with 125I-Bolton-Hunter reagent. The labeled alpha-agglutinin showed specific binding to a cells. Such specific binding approached saturation with respect to agglutinin or cells and was inhibited in the presence of excess unlabeled alpha-agglutinin. Nonspecific binding was similar in a and alpha cells, was neither saturable nor competable, and was three- to fourfold less than the specific binding to a cells at maximum tested agglutinin concentrations. The major a-specific binding species had a low electrophoretic mobility in sodium dodecyl sulfate-polyacrylamide gels and had an apparent molecular weight of 155,000 by rate zonal centrifugation. Endo-N-acetylglucosaminidase H digestion of the purified glycoprotein complex converted the low-mobility material to four major and several minor bands which were resolved by polyacrylamide gel electrophoresis. All but two minor peptides bound specifically to a cells. Analyses of agglutinin from mnn mutants confirmed the deglycosylation results in suggesting that the N-linked carbohydrate portion of alpha-agglutinin was not necessary for activity.
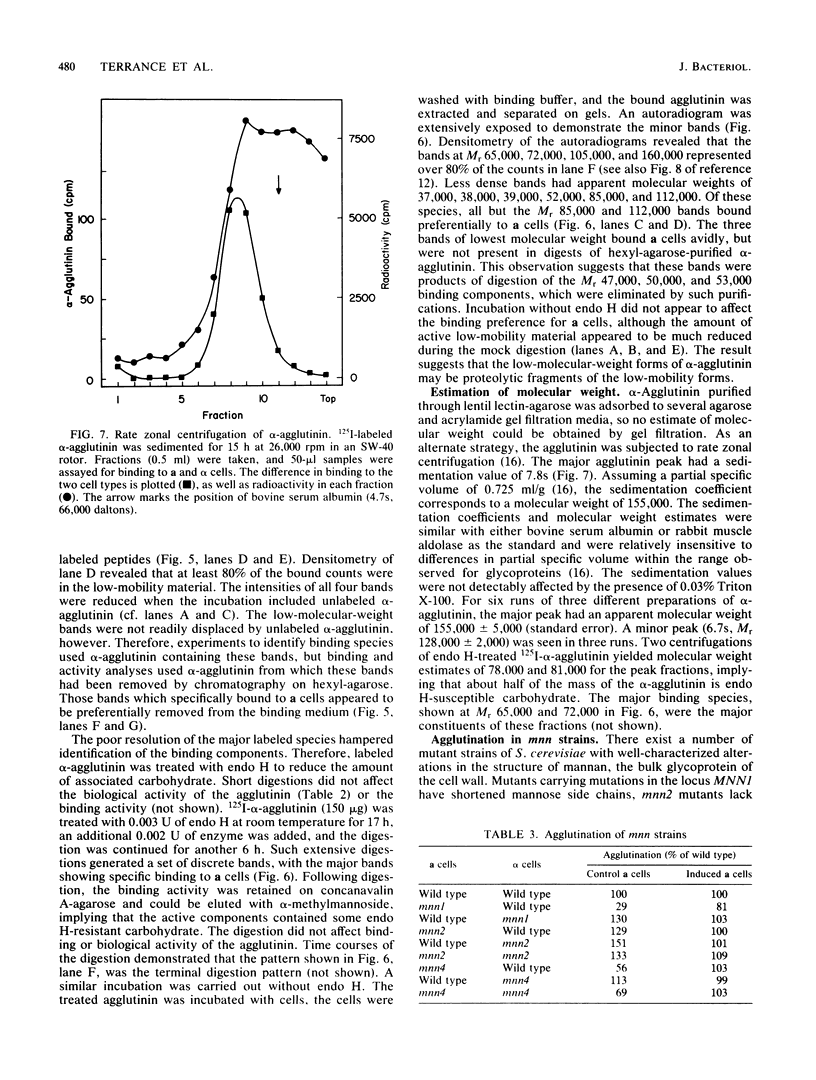
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz R., MacKay V. L., Duntze W. a-Factor from Saccharomyces cerevisiae: partial characterization of a mating hormone produced by cells of mating type a. J Bacteriol. 1977 Nov;132(2):462–472. doi: 10.1128/jb.132.2.462-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Mendonça-Previato L., Ballou C. E. Cell-cell recognition in yeast: purification of Hansenula wingei 21-cell sexual agglutination factor and comparison of the factors from three genera. Proc Natl Acad Sci U S A. 1980 Jan;77(1):318–322. doi: 10.1073/pnas.77.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular basis of mating in the yeast hansenula wingei. Bacteriol Rev. 1968 Sep;32(3):139–163. doi: 10.1128/br.32.3.139-163.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Duntze W., Stötzler D., Bücking-Throm E., Kalbitzer S. Purification and partial characterization of -factor, a mating-type specific inhibitor of cell reproduction from Saccharomyces cerevisiae. Eur J Biochem. 1973 Jun;35(2):357–365. doi: 10.1111/j.1432-1033.1973.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher G., Perry K., Thorner J. Cell-cell recognition in Saccharomyces cerevisiae: regulation of mating-specific adhesion. J Bacteriol. 1978 Jun;134(3):893–901. doi: 10.1128/jb.134.3.893-901.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiya M., Yoshida K., Yanagishima N. The release of sex-specific substances responsible for sexual agglutination from haploid cells of Saccharomyces cerevisiae. Exp Cell Res. 1977 Feb;104(2):263–272. doi: 10.1016/0014-4827(77)90090-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipke P. N., Terrance K., Wu Y. S. Interaction of alpha-agglutinin with Saccharomyces cerevisiae a cells. J Bacteriol. 1987 Feb;169(2):483–488. doi: 10.1128/jb.169.2.483-488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Orlean P., Ammer H., Watzele M., Tanner W. Synthesis of an O-glycosylated cell surface protein induced in yeast by alpha factor. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6263–6266. doi: 10.1073/pnas.83.17.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Ballou C. E. Cell-cell recognition in yeast. Characterization of the sexual agglutination factors from Saccharomyces kluyveri. J Biol Chem. 1983 Mar 25;258(6):3576–3582. [PubMed] [Google Scholar]
- Sakai K., Yanagishima N. Mating reaction in Saccharomyces cerevisiae. II. Hormonal regulation of agglutinability of a type cells. Arch Mikrobiol. 1972;84(3):191–198. doi: 10.1007/BF00425197. [DOI] [PubMed] [Google Scholar]
- Shenbagamurthi P., Baffi R., Khan S. A., Lipke P., Pousman C., Becker J. M., Naider F. Structure-activity relationships in the dodecapeptide alpha factor of Saccharomyces cerevisiae. Biochemistry. 1983 Mar 1;22(5):1298–1304. doi: 10.1021/bi00274a047. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Blair L. C., Thorner J. Cell interactions and regulation of cell type in the yeast Saccharomyces cerevisiae. Annu Rev Microbiol. 1983;37:623–660. doi: 10.1146/annurev.mi.37.100183.003203. [DOI] [PubMed] [Google Scholar]
- Strazdis J. R., MacKay V. L. Reproducible and rapid methods for the isolation and assay of a-factor, a yeast mating hormone. J Bacteriol. 1982 Sep;151(3):1153–1161. doi: 10.1128/jb.151.3.1153-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Association constant of the sex-specific agglutinin in the yeast, Hansenula wingei. Biochemistry. 1970 Jul 7;9(14):2931–2934. doi: 10.1021/bi00816a027. [DOI] [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Sexual agglutination in yeast. VII. Significance of the 1.7S component from reduced 5-agglutinin. Arch Biochem Biophys. 1968 Sep 10;126(3):912–921. doi: 10.1016/0003-9861(68)90485-2. [DOI] [PubMed] [Google Scholar]
- Terrance K., Lipke P. N. Sexual agglutination in Saccharomyces cerevisiae. J Bacteriol. 1981 Dec;148(3):889–896. doi: 10.1128/jb.148.3.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L. E., Pringle J. R. Transient G1 arrest of S. cerevisiae cells of mating type alpha by a factor produced by cells of mating type a. Exp Cell Res. 1974 Nov;89(1):175–187. doi: 10.1016/0014-4827(74)90200-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Yoshida K., Yanagishima N. Isolation and partial characterization of cytoplasmic alpha agglutination substance in the yeast Saccharomyces cerevisiae. FEBS Lett. 1982 Mar 8;139(1):125–129. doi: 10.1016/0014-5793(82)80502-4. [DOI] [PubMed] [Google Scholar]
- Yoshida K., HAGIYA M., Yanagishima N. Isolation and purification of the sexual agglutination substance of mating type a cells in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1085–1094. doi: 10.1016/0006-291x(76)90765-8. [DOI] [PubMed] [Google Scholar]