Abstract
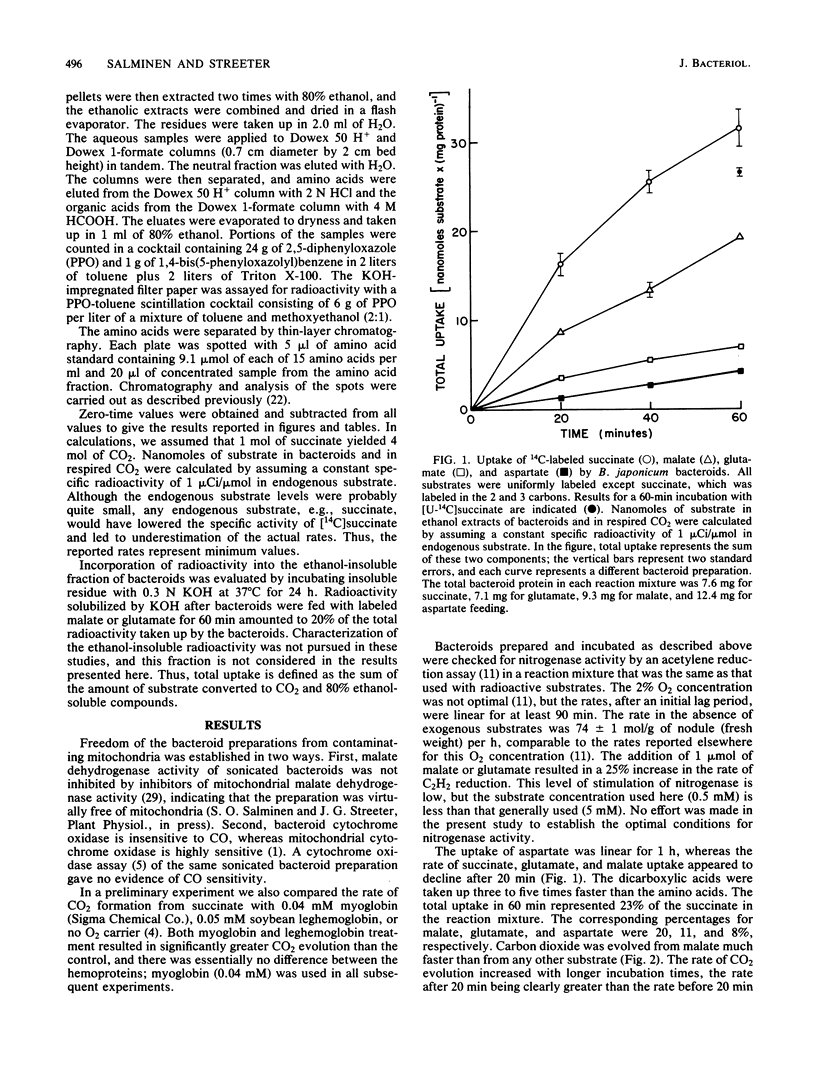
Bradyrhizobium japonicum bacteroids were isolated anaerobically and supplied with 14C-labeled succinate, malate, aspartate, or glutamate for periods of up to 60 min in the presence of myoglobin to control the O2 concentration. Succinate and malate were absorbed about twice as rapidly as glutamate and aspartate. Conversion of substrate to CO2 was most rapid for malate, followed by succinate, glutamate, and aspartate. When CO2 production was expressed as a proportion of total carbon taken up, malate was still the most rapidly respired substrate, with 68% of the label absorbed converted to CO2. The comparable values for succinate, glutamate, and aspartate were 37, 50, and 38%, respectively. Considering the fate of labeled substrate not respired, greater than 95% of absorbed glutamate remained as glutamate in the bacteroids. In contrast, from 39 to 66% of the absorbed succinate, malate, or aspartate was converted to glutamate. An increase in the rate of CO2 formation from labeled substrates after 20 min appeared to coincide with a maximum accumulation of label in glutamate. The results indicate the presence of a substantial glutamate pool in bacteroids and the involvement of glutamate in the respiratory metabolism of bacteroids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Barnes S. J., Weitzman P. D. Organization of citric acid cycle enzymes into a multienzyme cluster. FEBS Lett. 1986 Jun 9;201(2):267–270. doi: 10.1016/0014-5793(86)80621-4. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Systems utilizing oxygenated leghemoglobin and myoglobin as sources of free dissolved O2 at low concentrations for experiments with bacteria. Anal Biochem. 1979 Jul 1;96(1):165–174. doi: 10.1016/0003-2697(79)90569-4. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Datta A., Merz J. M., Spivey H. O. Substrate channeling of oxalacetate in solid-state complexes of malate dehydrogenase and citrate synthase. J Biol Chem. 1985 Dec 5;260(28):15008–15012. [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol. 1983 Jun;154(3):1403–1413. doi: 10.1128/jb.154.3.1403-1413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane M., Meade J., Manian S. S., O'Gara F. Expression and regulation of the Escherichia coli glutamate dehydrogenase gene (gdh) in Rhizobium japonicum. Arch Microbiol. 1986 Feb;144(1):29–34. doi: 10.1007/BF00454952. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Salminen S. O. Uptake hydrogenase activity and ATP formation in Rhizobium leguminosarum bacteroids. J Bacteriol. 1982 Aug;151(2):989–995. doi: 10.1128/jb.151.2.989-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Larue T. A. Utilization of aldehydes and alcohols by soybean bacteroids. Plant Physiol. 1981 Aug;68(2):489–493. doi: 10.1104/pp.68.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Evaluation of active versus passive uptake of metabolites by Rhizobium japonicum bacteroids. J Bacteriol. 1984 Jul;159(1):47–52. doi: 10.1128/jb.159.1.47-52.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Srere P. A. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985 Sep 5;260(19):10800–10805. [PubMed] [Google Scholar]
- Ronson C. W., Lyttleton P., Robertson J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4284–4288. doi: 10.1073/pnas.78.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall I., Cole M. Organic Acid Metabolism by Isolated Rhizobium japonicum Bacteroids. Plant Physiol. 1978 May;61(5):787–790. doi: 10.1104/pp.61.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Tubb R. S. Regulation of nitrogen fixation in Rhizobium sp. Appl Environ Microbiol. 1976 Oct;32(4):483–488. doi: 10.1128/aem.32.4.483-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



