Abstract
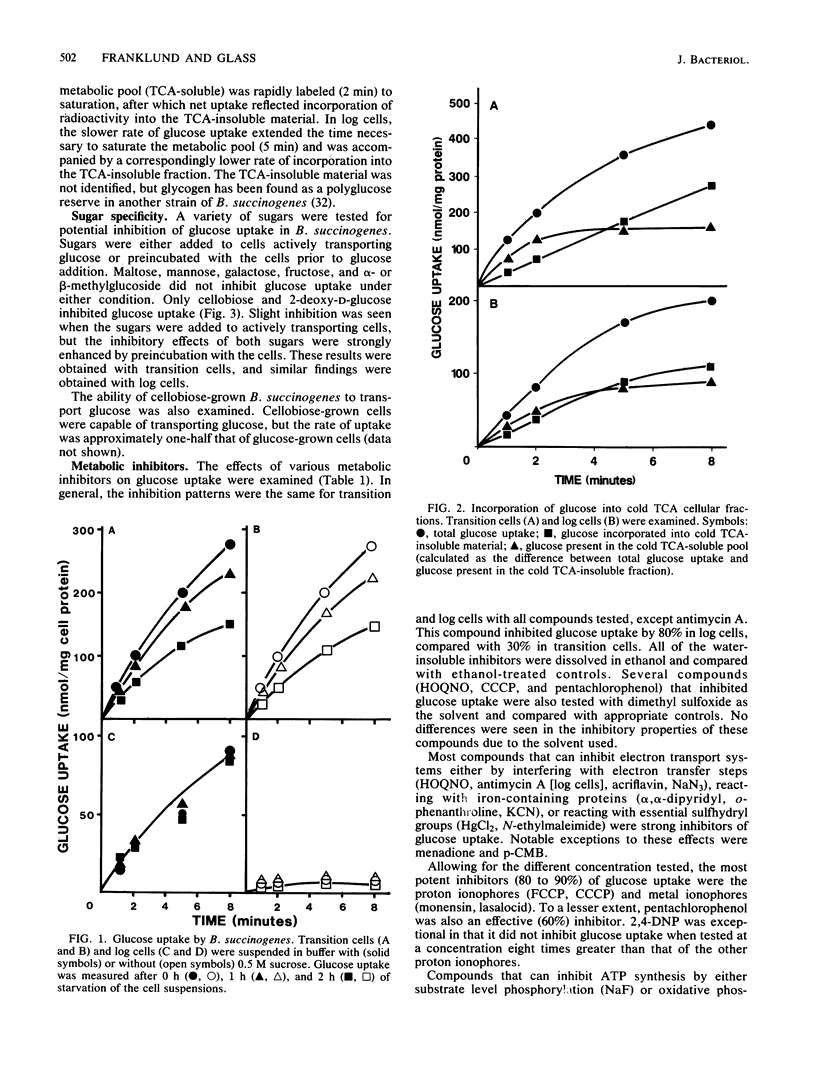
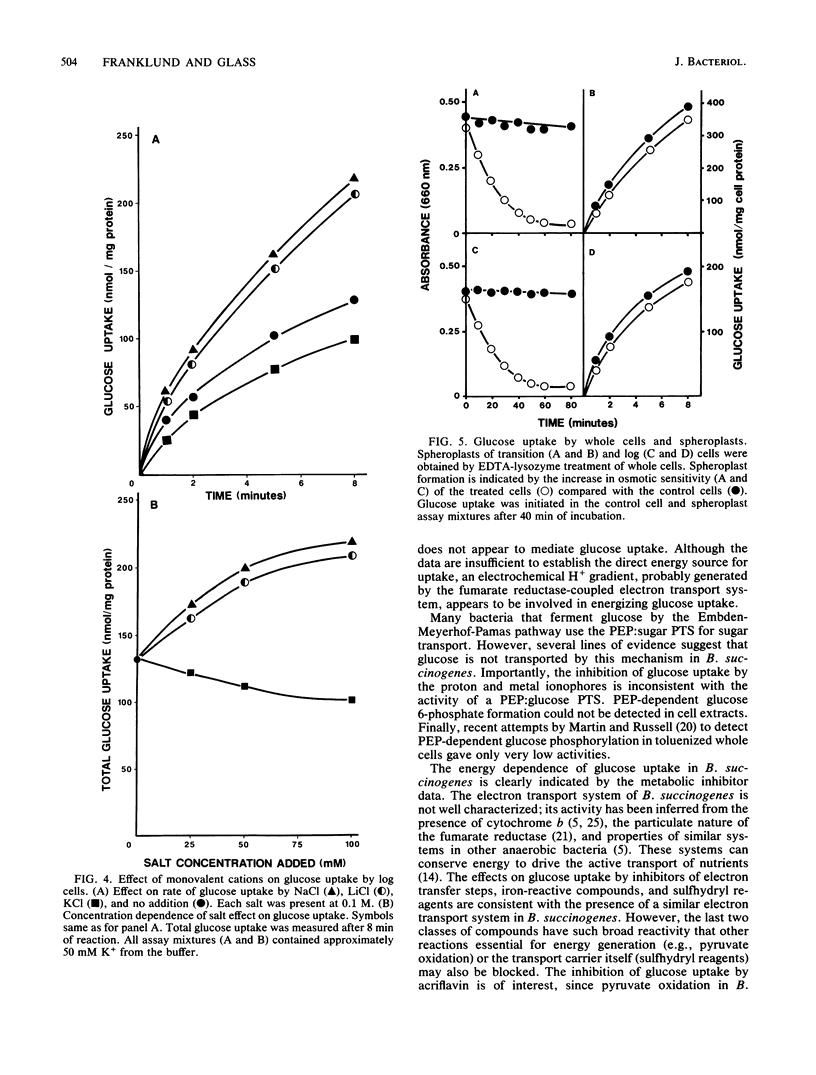
Glucose uptake by Bacteroides succinogenes S85 was measured under conditions that maintained anaerobiosis and osmotic stability. Uptake was inhibited by compounds which interfere with electron transport systems, maintenance of proton or metal ion gradients, or ATP synthesis. The most potent inhibitors were proton and metal ionophores. Oxygen strongly inhibited glucose uptake. Na+ and Li+, but not K+, stimulated glucose uptake. A variety of sugars, including alpha-methylglucoside, did not inhibit glucose uptake. Only cellobiose and 2-deoxy-D-glucose were inhibitory, but neither behaved as a competitive inhibitor. Metabolism of both sugars appeared to be responsible for the inhibition. Cells grown in cellobiose medium transported glucose at one-half the rate of glucose-grown cells. Spheroplasts transported glucose as well as whole cells, indicating glucose uptake is not dependent on a periplasmic glucose-binding protein. Differences in glucose uptake patterns were detected in cells harvested during the transition from the lag to the log phase of growth compared with cells obtained during the log phase. These differences were not due to different mechanisms for glucose uptake in the cell types. Based on the results of this study, B. succinogenes contains a highly specific, active transport system for glucose. Evidence of a phosphoenolpyruvate-glucose phosphotransferase system was not found.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Arcand C. Inorganic and metal-organic growth requirements of the genus Bacteroides. J Bacteriol. 1974 Oct;120(1):322–333. doi: 10.1128/jb.120.1.322-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Preziosi M. C., Caldwell D. R. Some effects of uncouplers and inhibitors on growth and electron transport in rumen bacteria. J Bacteriol. 1979 Aug;139(2):384–392. doi: 10.1128/jb.139.2.384-392.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Lee C. A., Saier M. H., Jr Phosphoenolpyruvate-dependent sugar phosphotransferase activity in Megasphaera elsdenii. Can J Microbiol. 1981 Sep;27(9):949–952. doi: 10.1139/m81-148. [DOI] [PubMed] [Google Scholar]
- Eagon R. G. 2-Deoxyglucose transportation via passive diffusion and its oxidation, not phosphorylation, to 2-deoxygluconic acid by Pseudomonas aeruginosa. Can J Biochem. 1971 May;49(5):606–613. doi: 10.1139/o71-087. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R., Furlong C. E. Reconstitution of binding protein-dependent ribose transport in spheroplasts of Escherichia coli K-12. Arch Biochem Biophys. 1979 Oct 1;197(1):158–162. doi: 10.1016/0003-9861(79)90231-5. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Young J. L., Roadcap R. F., Phibbs P. V., Jr Uptake and incorporation of glucose and mannose by whole cells of Bacteroides thetaiotaomicron. Appl Environ Microbiol. 1977 Nov;34(5):488–494. doi: 10.1128/aem.34.5.488-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Phosphoenolpyruvate-dependent phosphorylation of hexoses by ruminal bacteria: evidence for the phosphotransferase transport system. Appl Environ Microbiol. 1986 Dec;52(6):1348–1352. doi: 10.1128/aem.52.6.1348-1352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L. The pathway of formation of acetate and succinate from pyruvate by Bacteroides succinogenes. Arch Microbiol. 1978 May 30;117(2):145–152. doi: 10.1007/BF00402302. [DOI] [PubMed] [Google Scholar]
- Montgomery L., Macy J. M. Characterization of rat cecum cellulolytic bacteria. Appl Environ Microbiol. 1982 Dec;44(6):1435–1443. doi: 10.1128/aem.44.6.1435-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J Bacteriol. 1982 Jun;150(3):1391–1399. doi: 10.1128/jb.150.3.1391-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P. Deoxyribonucleic acid base composition of certain species of the genus Bacteroides. Can J Microbiol. 1977 Sep;23(9):1252–1256. doi: 10.1139/m77-187. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Effect of dicyclohexylcarbodiimide on growth and membrane-mediated processes in wild type and heptose-deficient mutants of Escherichia coli K-12. J Bacteriol. 1974 Jul;119(1):129–137. doi: 10.1128/jb.119.1.129-137.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R. M. Amino acid uptake systems in Bacteroides ruminicola. Can J Microbiol. 1979 Oct;25(10):1161–1168. doi: 10.1139/m79-180. [DOI] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Fryda S. J., Robinson I. M. Cellulolytic bacteria from pig large intestine. Appl Environ Microbiol. 1984 Jan;47(1):219–221. doi: 10.1128/aem.47.1.219-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein H. G., Gottschalk G. A sodium-stimulated membrane-bound fumarate reductase system in Bacteroides amylophilus. Arch Microbiol. 1985 Nov;143(2):157–162. doi: 10.1007/BF00411041. [DOI] [PubMed] [Google Scholar]



