Abstract
An analytical method was developed and validated for the quantitative determination of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; NSC707545), a novel heat shock 90 inhibitor, in human plasma. Calibration curves were linear in the concentration range of 1 to 500 ng/ml. Sample pretreatment involved a liquid-liquid extraction of 0.2 ml aliquots of plasma with ethyl acetate. 17-DMAG and the internal standard, beclomethasone, were separated on a Zorbax SB C18 column (75 × 2.1 mm, 3.5 μm), using a mobile phase composed of methanol and 0.2% formic acid (55:45, v/v). The column effluent was monitored by mass spectrometry with electrospray ionization. For the quality control samples at four different concentrations that were analyzed in quintuplicate, on four separate occasions, the accuracy and precision ranged from 93.8 to 99.5% and 1.4 to 3.3%, respectively. The assay modifications significantly improve upon our original, validated method. The developed method was subsequently applied to study the pharmacokinetics of 17-DMAG in a group of 23 patients.
1. Introduction
The molecular chaperone heat shock protein 90 (Hsp90) is an exciting cancer target since it plays a major role in the folding and activation of several signaling proteins that promote the growth and survival of tumor cells [1,2]. Inhibition of Hsp90 results in degradation of a wide range of oncogenic ‘client’ proteins, which include ErbB2, c-Raf-1, Akt/PKB, CDK4, Polo-1 kinase, Met, mutant p53, and hTERT [1]. As a result, it causes simultaneous blockage of multiple cancer-causing pathways, and ultimately, the death of the malignant cells. Extensive efforts towards the development of Hsp90 inhibitors resulted in the identification of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; NSC707545; Fig. 1) as a promising agent and multiple Phase I clinical trials were subsequently initiated [3].
Fig. 1.
Structure of 17-DMAG (A), and internal standard, beclomethasone (B).
An analytical method was previously developed and validated in our lab, for the quantitation of 17-DMAG in human plasma in support of a phase I clinical trial [3-5]. However, the lack of internal standard became a major concern in daily practice, since the assay was being used long-term. Furthermore, while the original assay was suitable for the analysis of samples following very low doses of the drug, this was not true at higher doses. As drug exposure increased, due to the rapid dose escalation design of the study, the original upper limit of quantitation (ULOQ) of previously developed assay, 100 ng/mL, was no longer sufficient. Attempts of extending previous assay range failed without the application of an internal standard. In addition, the large sample volume required (0.5 mL), limited the possibility of re-analysis. Due to these major limitations, we refined the method and report here an improved analytical method for determination of 17-DMAG concentration in human plasma based on liquid chromatography coupled with single-quadrupole mass-spectrometric detection.
2. Experimental
2.1. Chemicals
17-DMAG was supplied by Pharmaceutical Management Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, NCI (Bethesda, MD, USA). Internal standard, beclomethasone (99%), was purchased from Sigma-Aldrich (St. Louis, MO, USA). Formic acid (98%) was obtained from Fluka (through Sigma-Aldrich, St. Louis, MO, USA). Ethyl acetate (Fisher Scientific, Fairlawn, NJ, USA) and methanol (J.T. Baker, Phillipsburg, NJ, USA) are of HPLC grade. Deionized water was generated with a Hydro-Reverse Osmosis system (Durham, NC, USA) connected to a Milli-Q UV Plus purifying system (Billerica, MA, USA). Drug-free heparinized human plasma was obtained from the National Institutes of Health Clinical Center Blood Bank (Bethesda, MD, USA).
2.2. Preparation of stock solutions and standards
Master stock solutions of 17-DMAG were prepared by dissolving the drug in DMSO at a concentration of 1 mg/mL and stored in glass tubes at -20 °C. From the master stock solution, a working solution containing 100 ug/mL of drug in methanol was prepared each week and stored at -20 °C between uses. Serial dilutions were prepared from this working solution for the preparation of calibration and quality control (QC) samples. A master stock of the internal standard, beclomethasone, was prepared at a concentration of 1 mg/mL in absolute ethanol. From the master stock, a working solution of the internal standard was prepared by dilution to 20 ng/mL in ethyl acetate, the extraction solvent. Both the master and working internal standard solutions were stored at -20 °C.
With each analytical run, calibration standards in drug-free human heparinized plasma were freshly prepared in duplicate at 17-DMAG concentrations of 1, 2, 5, 10, 50, 100 and 500 ng/mL, such that the total amount of methanol added was identical in each sample (5%). QC samples were prepared in batch at concentrations of 3, 200, 400 and 2000 ng/mL, by adding plasma to the required amount of working solution in a volumetric flask. After vortexing to ensure complete mixing, these QC samples were subdivided into 1.2 mL aliquots (300 μL for 2000 ng/mL QC samples) in cryo vials and stored at -20 °C. Together with patient samples, a portion of the QC samples were stored at -80 °C for the assessment of long term stability.
2.3. Sample preparation
Samples were prepared by spiking 190 μL of blank human plasma in a 5 ml disposable glass centrifuge tube (Kimble, Vineland, NJ, USA) with 10 μL of the appropriate 17-DMAG working solution for a total volume of 200 μL. Patient samples were allowed to thaw at room temperate, vortex-mixed for 20s to ensure uniformity, and a volume of 200 μL of each sample was aliquotted into each glass tube. QC samples were also thawed at room temperature, vortex-mixed and then aliquotted out into each glass tube. To each tube, 800 μL of ethyl acetate containing 20 ng/mL internal standard was added, followed by an additional 1mL of ethyl acetate. Tubes were then capped and vortex-mixed for 5 min, followed by centrifugation for 10 min at 1303 × g. The clear supernatant was transferred to a clean glass drying tube and evaporated to dryness under desiccated air in a water bath at 40 °C in a Zymark TurboVap LV (Hopkinton, MA, USA). The residue was reconstituted in 80 μL of a mixture of methanol/0.2% formic acid (55:45, v:v), and vortex-mixed for 15 s. Finally, each solution was transferred to a glass vial for injection. A volume of 25 μL of this solution was then injected into the chromatographic system.
2.4. Equipment
The experiments were conducted on an Agilent 1100 system (Agilent Technology, Palo Alto, CA, USA) which included a G1312 binary pump, a G1329 refrigerated autosampler, a mobile phase vacuum degassing unit, and a temperature-controlled column compartment, coupled with a single-quadrupole mass spectrometric (MS) detector (Agilent 1100 MSD) equipped with an electrospray ionization source. The autosampler was maintained at 4 °C and the column was at 40 °C. An Agilent ZORBAX SB-C18 column (75 × 2.1 mm I.D.) packed with 3.5-μm packing material was employed. Samples were eluted isocratically at a flow rate of 300 μL/min, using a mobile phase composed of methanol and 0.2% formic acid (55:45, v/v). The MS conditions were as follows: fragmentor, 95 V; gain, 2; drying gas flow, 11 L/min; nebulizing gas pressure 45 psi; drying gas temperature, 350 °C; and capillary voltage 4000 V. Selected-ion monitoring was accomplished at m/z 617.3 for 17-DMAG, and m/z 409.2 for the internal standard. The chromatographic data were acquired and analyzed using the Chemstation software package (Agilent).
2.5. Validation procedures
Validation of the method with respect to accuracy and precision was carried out according to procedures reported in detail previously [6]. Calibration standards of 1, 2, 5, 10, 50, 100 and 500 ng/mL were prepared freshly by mixing the working standard solutions of 17-DMAG and drug-free human plasma. Pools of QC samples were prepared at 3, 200, 400 and 2000 ng/ml concentrations before the validation process began and stored at -20 °C. QC samples at each concentration were thawed at room temperature and analyzed daily in quintuplicate each in aliquots of 200 μL. Each of the 2000 ng/ml QC samples was diluted 5-fold by adding 160 μL blank plasma to 40 μL of the spiked plasma, prior to addition of the IS or extraction, to test the accuracy and reproducibility of diluting samples which upon initial analysis are found to exceed the ULOQ. Validation runs included blank (zero concentration) and internal standard only samples, along with calibration and QC samples.
Calibration curves were calculated by least-squares linear regression analysis of the peak area ratio of 17-DMAG and the internal standard versus the drug concentration of the nominal standard. The regression parameters of slope, intercept and correlation coefficient were calculated using a weighting factor of 1/x. The linearity was evaluated by comparing the correlation coefficient (r2), residuals and errors between theoretical and back calculated concentrations of calibration standard samples. The zero concentration (blank), and internal standard only sample were used to visually verify the purity of the reagents and the lack of other potentially interfering substances, but were not considered for the regression analysis of standards. This calibration curve was then used to calculate measured QC concentrations, and that of unknown samples, by interpolation.
The lower limit of quantitation (LLOQ) of the assay was assessed by determining the concentration of 17-DMAG at which the values for precision and accuracy were less than 20%. At least five different lots of plasma were used and resulted in accurate and reproducible measurement at the concentration of LLOQ, based on the deviation from nominal concentration.
The accuracy and precision of the assay were assessed by the mean relative percentage deviation (DEV) from the nominal concentrations and the within-run and between-run precision, calculated according to previously published equations [7]. Estimates of the between-run precision were obtained by one-way analysis of variance (ANOVA) using the run day as the classification variable. The between-groups mean square (MSbet), the within-groups mean square (MSwit), and the grand mean (GM) of the observed concentrations across runs were calculated using Microsoft Excel 2003 (Redmond, WA, USA).
The extraction efficiency for 17-DMAG in human plasma, expressed as a percentage, was determined at 10 and 400 ng/mL, in five replicates, by comparing samples spiked to plasma and mobile phase. Different lots of plasma were used to prepare QC samples and calibration curves that were analyzed on the same day, in order to assess relative matrix effect. Ion suppression or crossover of drug and IS was examined by comparing samples spiked with only IS, only 17-DMAG at the ULOD (500ng/mL), or both.
Stability of the drug in the reconstitution solution was assessed by reinjection of calibrator and QC samples after remaining in the autosampler for 24h following initial injection. The stability of 17-DMAG in human plasma was evaluated following three freeze-thaw cycles, using QC samples at concentrations of 3 and 400 ng/mL, in quintuplicate. Long term stability of 17-DMAG in human plasma was evaluated by analysis of five replicates each at 3, 400 and 2000 ng/mL, after 3.5 months storage at -80°C.
3. Results and Discussion
3.1. Specificity
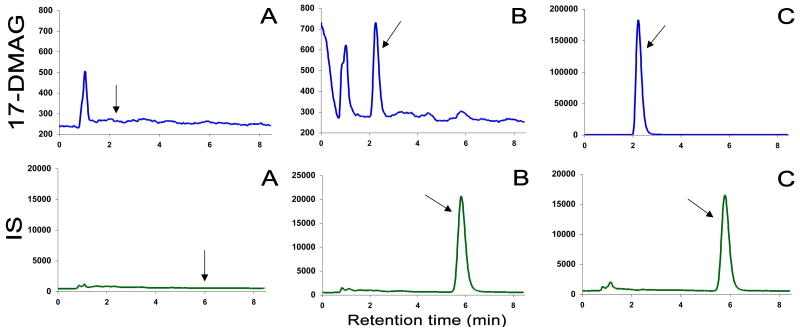
Due to the previously reported [3] difficulty in finding a suitable internal standard with similar chemical structure and properties, a wider variety of different compounds were tested. Finally, beclomethasone (Fig. 1) was chosen. Fig. 2. displays typical chromatograms of an extract of a blank human plasma sample (A), and an extract of a plasma sample spiked with IS and 17-DMAG at a concentration of 1 ng/mL(B). The selectivity of the analysis is shown by symmetrical resolution of the peaks, with no interference around the retention time of the analyte in drug-free plasma obtained from six different individuals. Overall chromatographic run time was established at 8.5 min with 17-DMAG eluting at tR=2.20 min and internal standard, beclomethasone, at tR=5.85 min.
Fig. 2.
Chromatograms from reversed-phase HPLC of blank human plasma (A); a human plasma spiked with beclomethasone (IS) and 1 ng/mL (LLOQ) 17-DMAG, (B); and a patient sample 1.5 hours after the start of infusion of 21 mg/m2 17-DMAG (470.7 ng/mL) spiked with IS, (C).
3.2. Validation characteristics
The calculated detector response of 17-DMAG versus the nominal concentration displayed a linear relationship in the tested range of 1-500 ng/mL. However, variance increased proportionally with drug concentration. A weighting factor was applied, inversely proportional to the variance at the given concentration level (1/[nominal 17-DMAG concentration]). Using least-squares linear-regression, a mean (± standard deviation) correlation coefficient of 0.9995±0.00027 (range, 0.9992-0.9997) was obtained.
In blank human plasma spiked with 17-DMAG at a concentration of 1 ng/mL, all of the 8 calibration samples run on four separate days were within required ±20% deviation of the nominal value. The mean percentage deviation from nominal value for these 8 samples was 9.37% (Table 1), together with within and between-run variability of 3.73% and 1.45% (not shown in the table), respectively. Samples at 1 ng/mL prepared on the same day from different lots of plasma were back calculated from the same calibration curve which gave the following results: 1.03, 0.95, 0.87, 1.01, 1.00 and 0.99 ng/mL, all within 15% deviation of the nominal value. Based on these results, the lower limit of quantitation for 17-DMAG was established at 1 ng/mL.
Table 1.
Back-calculated concentrations from calibration curves run in duplicate on four occasions
| Nominal
(ng/mL) |
GM
(ng/mL) |
S.D.
(ng/mL) |
DEV(%) | R.S.D.(%) | n |
|---|---|---|---|---|---|
| 1.00 | 1.09 | 0.041 | 9.37 | 3.75 | 8 |
| 2.00 | 2.03 | 0.101 | 1.57 | 4.95 | 8 |
| 5.00 | 4.92 | 0.228 | -1.66 | 4.63 | 8 |
| 10.0 | 9.59 | 0.494 | -4.15 | 5.15 | 8 |
| 50.0 | 48.49 | 2.410 | -3.02 | 4.97 | 8 |
| 100 | 96.89 | 2.736 | -3.11 | 2.82 | 8 |
| 500 | 504.99 | 10.605 | 1.00 | 2.10 | 8 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; R.S.D., relative standard deviation; n, total number of replicate observations within the validation runs.
Validation data for the analytical method in terms of accuracy and precision are summarized in Table 1 and 2. Table 1 displays the data calculated from duplicate calibration curves on four separate days. Shown in Table 2 is data from the QC samples that were run in quintuplicate at each concentration, on each of these four days. Values were back-calculated using the calibration curve from the same run. The assay was found to be accurate, within 6.2% at all four concentrations, and precise with within-run and between-run precision error of less than 3.3%.
Table 2.
Assessment of accuracy and precision from quality control samplesa
| Nominal
(ng/mL) |
GM
(ng/mL) |
S.D.
(ng/mL) |
DEV
(%) |
BRP
(%) |
WRP
(%) |
n |
|---|---|---|---|---|---|---|
| 3.00 | 2.99 | 0.10 | -0.49 | 1.72 | 2.64 | 20 |
| 200 | 190.70 | 4.76 | -4.65 | 1.36 | 2.19 | 20 |
| 400 | 378.91 | 13.43 | -5.27 | 1.68 | 3.22 | 20 |
| 2000 | 1876.8 | 75.90 | -6.16 | 3.31 | 2.78 | 20 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; WRP, within-run precision; BRP, between-run precision; n, total number of replicate observations within the validation runs.
Five samples at each concentration were run on four different occasions.
The mean overall extraction efficiency for 17-DMAG was approximately 77.7%, independent of the spiked concentration. QC samples prepared using different lot of plasma from the calibration curve did not show additional plasma to plasma variation. This result is also consistent with the slopes of calibration curves obtained from different lots of plasma with a mean (± of standard deviation) of 0.17020 ± 0.000773 (range, 0.01608-0.01782).
Possible crossover interference was also evaluated by comparing the direct instrument responses to the following three sets of experiments in triplicate: samples spiked with IS only, 500 ng/ml 17-DMAG only, or both. No interference between 17-DMAG and the IS was found, as the response of each analyte did not vary with the addition of the other.
3.3. Stability
The reinjection measurements were consistent with the initial run, allowing samples to be reanalyzed on the following day when necessary (for example, in the case of machine failure). For the freeze-thaw stability test, back calculated values at both 3 and 400 ng/mL after each freeze-thaw cycle were within 10% of the nominal values, indicating no degradation. Samples stored at -80°C at 3, 400 and 2000 ng/mL did not show degradation after 3.5 months.
4. Application
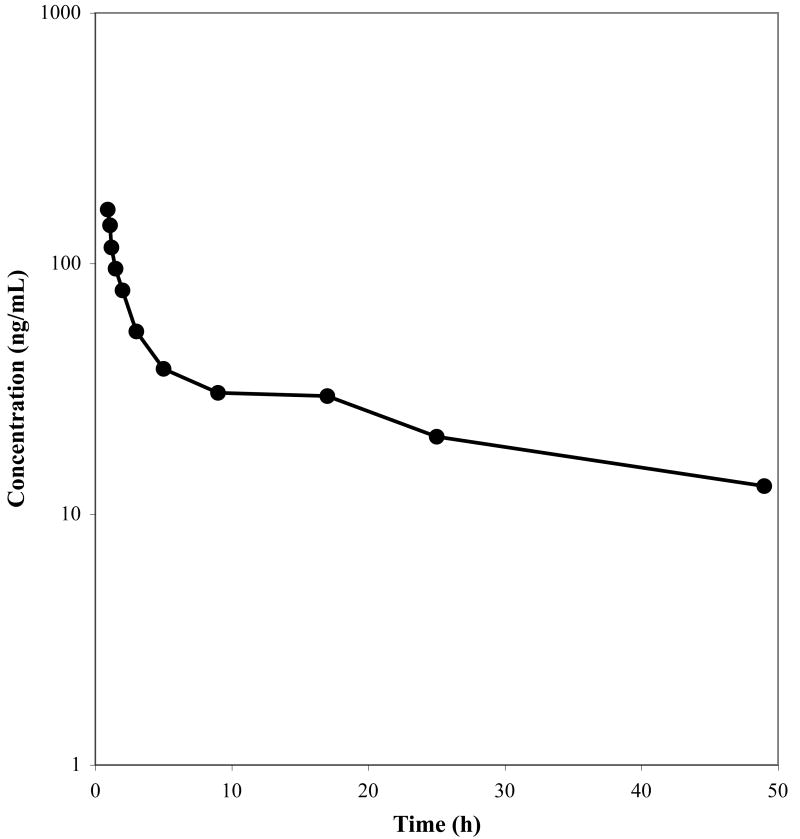
The validated method was then applied to study the pharmacokinetics of 17-DMAG in patients on a Phase I dose escalation study. Fig. 3 shows the observed plasma concentration time profile of a patient that received 21 mg/m2 of 17-DMAG as a continuous 1 hr infusion. A typical chromatogram of a patient sample is presented in Fig. 2 (C) with a concentration of 470.7 ng/mL.
Fig. 3.
Concentration-time profile for a representative individual, that received 21 mg/m2 of 17-DMAG as a 1 hr infusion.
5. Conclusion
In conclusion, a novel chromatographic method with mass-spectrometric detection has been developed and validated for the quantitative determination of 17-DMAG in human plasma. This method is specific, accurate and precise, and can be easily implemented into routine practice. Compared to our previously published LC/MS method [3], the addition of IS greatly reduced assay variability, allowing for the reduction of plasma volume from 500 μL to 200 μL, without a decrease in reliability. Additionally, validation of sample dilution extended the assay range up to 2500 ng/mL, as necessary for measurement of drug following administration of higher doses. This method reached 1ng/mL LLOQ, slightly lower than previous published LC/MS/MS method (1.89 nM, corresponding to 1.16 ng/mL) which used 250 μL plasma [8].
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.* The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
ER Gardner
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Workman P. Trends Mol Med. 2004;10:47. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Xiao L, Lu X, Ruden DM. Mini Rev Med Chem. 2006;6:1137. doi: 10.2174/138955706778560166. [DOI] [PubMed] [Google Scholar]
- 3.Hwang K, Scripture CD, Gutierrez M, Kummar S, Figg WD, Sparreboom A. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:35. doi: 10.1016/j.jchromb.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Lancet J, Gojo I, Baer M, Burton M, Klein M, Nowadly C, Gorre M, Zhong Z, Johnson RG, Hannah AL. 2006 ASCO Annual Meeting; Abstract No. 2081. [Google Scholar]
- 5.Cartwright EP, Kummar S, Muir CA, Ivy P, Conley BA, Scripture CD, Figg WD, Murgo AJ, Doroshow JH, Gutierrez ME. 2006 ASCO Annual Meeting; Abstract No. 13148. [Google Scholar]
- 6.Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD, et al. Eur J Drug Metab Pharmacokinet. 1991;16:249. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 7.Lepper ER, Hicks JK, Verweij J, Zhai S, Figg WD, Sparreboom A. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:305. doi: 10.1016/j.jchromb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Farre J, Asad Y, Pacey S, Workman P, Raynaud FI. Rapid Commun Mass Spectrom. 2006;20:2845. doi: 10.1002/rcm.2668. [DOI] [PubMed] [Google Scholar]