Summary
Ribosome binding factor A (RbfA) is a bacterial cold-shock response protein, required for an efficient processing of the 5′end of the 16S ribosomal RNA (rRNA) during assembly of the small (30S) ribosomal subunit. Here we present a crystal structure of Thermus thermophilus RbfA and a three-dimensional cryo-electron microscopic (EM) map of the T. thermophilus 30S·RbfA complex. RbfA binds to the 30S subunit in a position overlapping the binding sites of the A- and P-site tRNAs, and RbfA’s functionally important C-terminus extends toward the 5′ end of the 16S rRNA. In the presence of RbfA, a portion of the 16S rRNA encompassing helix 44, which is known to be directly involved in mRNA decoding and tRNA binding, is displaced. These results shed light on the role played by RbfA during maturation of the 30S subunit, and also indicate how RbfA provides cells with a translational advantage under conditions of cold shock.
Introduction
Ribosomes are complex macromolecular machines, which are involved in translating an organism’s genetic information into polypeptides (reviewed by Ramakrishnan, 2002). All ribosomes consist of two unequally sized subunits, each composed of both ribosomal RNA (rRNA) and ribosomal protein (r-protein) molecules. The small (30S) subunit plays a direct role in decoding of the genetic message (Ogle et al., 2003); in bacteria, such as Escherichia coli, the small subunit is composed of one 16S rRNA molecule and 21 r-proteins (designated S1–S21) (Wittmann-Liebold, 1986). In vitro, the 30S subunit can be assembled from only its rRNA and r-protein components (Traub and Nomura, 1968, Culver and Noller, 2000); however, the process requires non-physiological conditions, namely, high magnesium ion and salt concentrations (Traub and Nomura, 1968). In vivo, however, maturation of rRNAs and assembly of the r-proteins into a functional ribosome appear to be highly complex processes (Culver, 2003), involving multiple accessory factors (Williamson, 2003). While the involvement of many protein factors in ribosomal assembly has been well characterized, including modification enzymes, such as methylases and pseudouridinylases (Decatur and Fournier, 2002), RNA helicases (Iost and Dreyfus, 2006) and molecular chaperones (Alix and Nierhaus, 2003; Maki et al., 2002), there appear to be a number of additional protein factors, the exact roles of which remain to be defined (reviewed by Wilson and Nierhaus, 2007). Protein factors implicated in 30S maturation include the highly conserved GTPase Era (Sharma et al 2005), the PRC β-barrel protein RimM (Bylund et al., 1998), the ribosome-activated GTPase RsgA (also called YjeQ; Daigle and Brown, 2004; Himeno et al., 2004), and the ribosome binding factor A (RbfA), the focus of our study.
RbfA is a small protein (10.9 kDa in T. thermophilus) required for efficient processing of the 16S rRNA (Bylund et al., 1998; Xia et al., 2003). RbfA binds to the 30S subunit, but not to the large (50S) subunit, nor to the 70S ribosome (Dammel and Noller, 1995; Xia et al., 2003). NMR (Huang et al., 2003; Rubin et al., 2003) and X-ray crystallographic (PDB ID 1JOS) structures of RbfA obtained from various bacterial species reveal a single-domain protein with a type-II KH-domain fold topology, characteristic of a nucleic acid-binding protein family. Consistent with a role in ribosome biogenesis, deletion of the rbfA gene (Δ rbfA) causes a decrease in the quantity of 70S ribosomes and polysomes, and a concomitant increase in 30S and 50S ribosomal subunits (Dammel and Noller, 1995; Jones and Inouye, 1996; Bylund et al., 1998). Furthermore, ΔrbfA mutants display an accumulation of 17S rRNA, a precursor to the 16S rRNA (Bylund et al., 1998; Inoue et al., 2003). RbfA was originally identified as a multi-copy suppressor of the cold sensitivity of a C23U mutation at the 5′-terminal helix (h1) of the 16S rRNA (Dammel and Noller, 1993, 1995). The C23U mutation is predicted to significantly weaken the helix, enabling formation of an alternative helix through basepairing with nucleotides located in the upstream region of the precursor 17S rRNA (See Supplemental Figure S1A, B). Indeed, additional suppressors of the C23U mutation were identified within the 16S rRNA that would appear to push the equilibrium back toward formation of h1 (Dammel and Noller, 1995). The cold sensitivity of the C23U mutant and ΔrbfA strains suggests the existence of an energy barrier to the formation of the canonical h1, which is provided by high-temperature at the permissive temperatures of these strains, and by RbfA in the case of the C23U mutant strain at cold-shock temperatures. Thus, part of the role of RbfA could be to facilitate correct folding and maturation of h1 at the 5′ end of the 16S rRNA, which is particularly important under cold-shock conditions.
Cold shock results in an increase in the level of non-translating ribosomes and produces a temporary cessation of bacterial growth; growth is then restored through the action of a set of cold shock response proteins (Jones and Inouye, 1994; Graumann et al., 1996; Datta and Bhadra, 2003). The role of RbfA as a cold-shock protein has been well documented. In E. coli, RbfA is encoded in an operon together with the cold-shock protein NusA (Dammel and Noller, 1995). RbfA is expressed constitutively under normal growth conditions; however, the expression level rapidly increases upon cold shock, due to an up-regulation of the transcription of the rbfA mRNA (Jones and Inouye, 1996), resulting in a several-fold increase in the amount of 30S-bound RbfA (Xia et al., 2003). The elevated levels of RbfA under cold-shock conditions are necessary to overcome the translational block at the reduced temperature, presumably by facilitating rapid maturation of the 30S subunits. This role is in contrast to the action of the cold-shock protein pY; the latter has been proposed to stabilize 70S ribosomes against dissociation, and thus protect them from degradation, by binding to them (Vila-Sanjurjo et al., 2004).
Here we report a crystal structure of T. thermophilus RbfA and a cryo-EM structure of a T. thermophilus 30S·RbfA complex at resolutions of 1.84 Å and 12.5 Å, respectively. Our analysis shows that RbfA binds at the junction of the head and body i.e. at the neck region of the 30S subunit, with the C-terminus of RbfA approaching helix 1 located at the 5′ end of the 16S rRNA. This strategic location of RbfA on the 30S subunit, and interaction of RbfA with multiple rRNA helices and r-proteins, is suggestive of an important role in a late step in maturation of the 30S subunit. In addition, we find that the presence of the RbfA maintains the decoding region of the 30S subunit in a conformation unsuitable for the subunit’s participation in protein synthesis. Specifically, RbfA appears to dramatically alter the position and conformation of helix 44, a functionally important segment of the 16S rRNA that is known to be directly involved in mRNA decoding and in the formation of two of the intersubunit bridges, B2a and B3 (Gabashvili et al., 2000; Yusupov et al., 2001). Our results not only provide insight into the role of RbfA during maturation of the 30S subunit, but they also suggest how RbfA confers a translational advantage to cells under conditions of cold shock.
Results and Discussions
Crystal Structure of the Thermus thermophilus RbfA
The crystal structure of RbfA from T. thermophilus (Tth) was determined at 1.84 Å resolution is shown in Figure 1A, and crystallographic and refinement data are provided in Table 1. The asymmetric unit contains two molecules (A and B), and 90 (4–94) and 89 (3–92) out of the 95 residues comprising Tth RbfA, could be unambiguously modeled, respectively. The refined models of the two molecules can be superimposed with a root-mean-square deviation (r.m.s.d.) of 0.53 Å for the main chain atoms. The structure shows a single KH-domain containing three α-helices (α1 to α3) and three β-strands (β1 to β3) with an αββααβ topology (Figure 1B). The α2 and α3 helices are arranged to form a helix-kink-helix (hkh) at the strictly conserved Ala65 with a CH-π interaction between Leu62 (α2) and Phe87 (β3) (Figure 1B). It is possible that the kink serves to expand the space for an interaction and to alter the direction of basic residues on α3. The electrostatic surface potential reveals highly positively charged regions, which encompass three loops between β1-β2, β2-α2, and α3-β3, and the surface of α2-α3 (Figure 1C). A highly conserved sequence 25DPRL28 (29DPRL32 in E. coli) forms a 310 helix both in molecules A and B, as seen in the E. coli structure (see alignment in Supplemental Figure S2; Huang et al., 2003). While Asp25 and Arg27 form an electrostatic interaction in molecule A, the side chain direction of Arg27 is different and the interaction is not observed in molecule B. The loop between α3-β3, where the well-conserved Arg78 (Arg88 in E. coli) is located, also shows a notable difference in the two molecules. These conserved regions are likely to possess functional importance due to the structural flexibility.
Figure 1. Crystal structure of Tth RbfA and its comparison with known atomic structures of RbfA from other species.

(A) Stereo representation of the Tth RbfA is shown in cartoon (PDB code: 2DYJ). (B) An enlarged view of the helix-kink-helix motif (cyan) with all residues in stick. Residues involved in notable interactions, Asp25 and Arg27 on the 310 helix (magenta) and the conserved Phe87, are also shown in stick. (C) Stereo representation of Tth RbfA in the surface potential prepared by using APBS tools built in PyMOL. (D) Superposition of structures of RbfA from T. Thermophilus (molecules A and B in the asymmetric unit are shown in dark and light blue colors, respectively), H. influenzae (green, 1JOS) and M. pneumoniae (orange, 1PA4). In all panels, N, C, and hkh mean N-terminus, C-terminus and helix-kink-helix motif, respectively.
Table 1.
X-ray crystallographic structure determination statistics
|
Crystal characteristics
| |
| Space group | P1 21 1 |
| Unit-cell parameters | a = 28.43, b = 65.84, c = 43.17 β = 96.18 |
| Molecules/Asymmetric unit | 2 |
|
| |
|
Data collection
| |
| Wavelength (Å) | 1.0000 |
| Resolution (Å)a | 1.84 (1.96-1.84) |
| Unique reflections | 13813 (1383) |
| Completeness (%)a | 100 (100) |
| I/σ(I)a | 24.4 (3.22) |
| Redundancy | 3.7 (3.7) |
| Rsyma,b | 0.061 (0.312) |
|
| |
|
Refinement
| |
| Resolution range (Å) | 43.03-1.84 |
| Reflections | 13796 |
| Rcryst/Rfreec | 0.199/0.245 |
| Rms deviation | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.30 |
| Dihedral angles (°) | 21.50 |
| Improper (°) | 0.79 |
| Average B factor | 25.6 |
Numbers in parentheses are for the highest resolution shell.
Rsym=ΣhΣi|Ii(h)−<I(h)>|/ΣhΣI(h)
Rcryst=Σh||Fobs|−|Fcalc||/Σh|Fobs|. Rfree is Rcryst calculated with only the test set (5%) of reflections.
Comparison of the two Tth RbfA structures with previously known RbfA structures reveals that, despite the low sequence conservation (20%), the overall topologies are remarkably similar (Figures 1D). Tth RbfA is shorter than other known RbfA proteins, being ~95 amino acids (aa) rather than the usual 111–150 aa (with E. coli having 133 aa), and the residues appear to be lacking from the C-terminal end (Supplemental Figure S2). However, significant differences between the structures are clearly evident, especially when comparing the highly flexible loop regions, as well as the N- and C-terminal ends (Figure 1D).
Cryo-EM Structure of the 30S·RbfA Complex
After ascertaining that Tth RbfA binds to the mature T. thermophilus 30S subunit (Figure 2A), we obtained a 3D cryo-EM map of the Tth 30S·RbfA complex at 12.5 Å resolution, according to the FSC criterion with a 0.5 cutoff (Bottcher, et al., 1997; Malhotra et al., 1998), or at 8.0 Å according to the 3 σ cutoff (Orlova et al., 1998). The map shows all of the recognizable features of the 30S subunit, namely, head with beak, body with spur, platform, and helix 44 (h44) of the 16S rRNA (Figure 2B). A complex mass of extra density, with two protruding cylindrical features on the subunit interface side was directly visible within the cryo-EM map of the 30S·RbfA complex, located between the head and body of the 30S subunit. In order to interpret this density, we docked the X-ray crystallographic structure of the 30S subunit (Wimberly et al., 2000; PDB1J5E) into the cryo-EM map of the 30S·RbfA complex. For an optimum fit, the X-ray coordinates were divided into four structural domains (head, body, platform, and the 3′-minor domain encompassing h44 and h45 of the 16S rRNA), and then each domain was fitted individually as a rigid body. Subsequently, the fitted coordinates were filtered to match the resolution of the cryo-EM map, and then subtracted from the cryo-EM map of the 30S·RbfA complex. This process allowed the boundaries of the extra mass of density in the 30S·RbfA cryo-EM map to be delineated (Figure 2B).
Figure 2. Binding of RbfA to the 30S ribosomal subunit.
(A) SDS-PAGE results showing the binding of RbfA to the 30S subunit. T. thermophilus 30S subunits were incubated alone (lanes 2–4), or with increasing concentrations of RbfA (10-fold (lanes 5–7), 20-fold (lanes 8–10) and 40-fold (lanes 11–13) molar excess over 30S subunit), before being centrifuged through a 10% sucrose cushion (see Experimental Procedures). As a control, reactions were also performed with RbfA in the absence of 30S subunits (lanes 15–20). For each condition, aliquots of the initial (pre) reaction, supernatant (S), and pellet (P) were subjected to 20% SDS-PAGE and stained with Coomassie blue. RbfA pellets only in the presence of the 30S subunit, and the stoichiometry of binding increases with increasing initial excess of RbfA over 30S subunit. Lanes 1 and 14 are marker lanes, with 14, 20 and 33 kDa bands indicated. (B) Cryo-EM map showing extra mass of density that encompasses the binding position of RbfA (red) on the T. thermophilus 30S subunit (yellow).
Volumetric calculations indicate that the extra density encloses a space (~922 voxels ≈ 19,384 Å3) much larger than would be expected from the volume (~512 voxels ≈ 10,764 Å3) computed from the Tth RbfA structure. Possible explanations for this are that either two molecules of RbfA are binding to each 30S subunit, or binding of RbfA induces a conformational change within the 30S subunit.
Conformational Changes of the 30S Subunit due to RbfA Binding
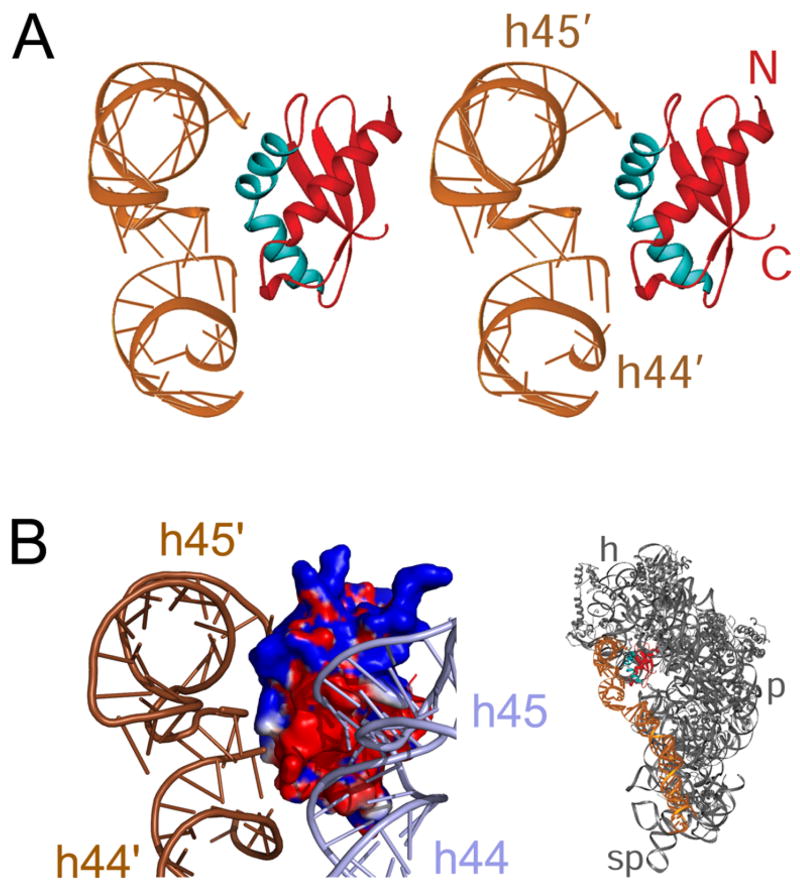
Two pieces of evidence indicate that RbfA binds as a monomer to the 30S subunit, and thus must induce a dramatic conformational change in the 16S rRNA to produce the additional density over that expected for a single RbfA molecule. Firstly, analysis of the density maps of the 30S·RbfA complex reveals much weaker density for where h45 and the top of h44 are located in the 30S control. Secondly, contouring to very high threshold values leads to a loss of all but the extra bi-lobed density (See Supplemental Figure S3). At such high thresholds only double stranded RNA helices are expected to be visible, whereas less electron dense material, such as protein, is lost. This observation indicates that the two protruding cylindrical features of the extra density must originate from a shift in rRNA helices h44 and h45, whereas the remainder of the extra density, which disappears at high threshold values, represents the bound RbfA protein. Closer inspection of the cryo-EM map clearly suggests that the upper segment of h44 (nucleotides 1400–1410 and 1490–1500) is what must shift position in the RbfA-bound 30S subunit by ~25 Å, to account for the lobe of extra density that is closer to the body of 30S (Figures 3). Since h45 is contiguous with h44, and since the loop region of h45 makes intimate contacts with the minor groove at the top of h44, it seems likely that the two regions move as a single unit. This idea is also consistent with the excellent fit of the respective RNA helices into the lobed densities, which allowed satisfactory overall docking of the h44–h45 region from the crystal structure into the cryo-EM map (Figure 3). In the crystal structure of the 30S subunit, the 3′ minor domain of the 16S rRNA, encompassing h44 and h45, establishes limited contacts with the r-proteins, and utilizes non-sequence specific backbone interactions with the rest of the 30S subunit, thus indicating the potential flexibility within this domain (Brodersen et al., 2002). Furthermore such conformational changes are not without precedent, given that shifts in h44 have been observed upon the binding of translation initiation factor IF1 (Carter et al., 2001), as well as between different elongation states of the ribosome (VanLoock et al., 2000). Note, however, that the shifts in the two cases just mentioned were not of the magnitude that is seen here.
Figure 3. Conformational changes in the 30S subunit upon RbfA binding.
A portion of the difference map (red) shown in Figure 2B corresponds to a large positional shift of the 3′-minor domain of the 16S rRNA involving the decoding site helix 44. Both positions of helix 44 (h44), as well as those of helix 45 (h45), are shown. Purple ribbons, original positions; red/orange ribbons shifted positions. B2a and B3 indicate the positions of the inter-subunit bridges. Arrow indicates direction of the shift. The landmarks of the 30S subunit are: h, head, p, platform and sp, spur.
Localization of RbfA on the 30S Subunit
We isolated the portion of the extra density that is directly attributable to RbfA by converting the fitted coordinates of h44 and h45 into electron densities and filtering them to the resolution of the cryo-EM map. The resulting mass was then subtracted from the total extra density mass (Figure 4A), leaving a density feature that matches the structure of Tth RbfA in volume and in overall shape (Figure 4B). However, the homology model of the Tth RbfA, which includes three additional amino acids at the C-terminus, shows a slightly better fit into the density (Figure 4C), as indicated by a cross-correlation coefficient (CCF) of 0.79. The conclusion is that RbfA binds in the neck region, buried deep within the cleft between the head and body of the 30S subunit. This position of RbfA is pivotal in that it is a junction point between all four domains of the 30S subunit, namely the “switch” region where h1 of the 5′ domain (body) base pairs with the single-stranded region between the central (platform) and 3′ major (head) domains, as well as the linker between the 3′ major and 3′ minor (h44–45) domains located at the base of h27 (see Supplemental Figure S1C, D). When the complex is viewed from the intersubunit side, the side of the 30S subunit that would face the 50S subunit in the 70S ribosome, RbfA is situated behind h44 and h45. The inaccessibility of the RbfA binding site in the 30S·RbfA complex suggests that binding of RbfA to the ribosome utilizes an induced fit-like mechanism, such that h44 and h45 shift concomitantly with RbfA’s interaction with the neck region of the 30S subunit. One surface of the KH domain of RbfA forms a three-stranded β-sheet, which in the 30S·RbfA complex is oriented toward the neck region, whereas the RNA-binding hkh motif, encompassing α2 and α3, faces the junction between h44 and h45 (Figure 5A). The flexible C-terminus of RbfA extends from the end of β3, deep into the body of the 30S subunit, and approaches h1 of the 16S rRNA (Figure 6A).
Figure 4. Localization of RbfA on the 30S subunit, and comparison of the atomic structure of RbfA with the cryo-EM density map.
(A) Interpretation of the extra mass in terms of 30S subunit conformational change, involving 16S rRNA helices 44 and 45 (orange), and RbfA mass (red). The mass attributable to RbfA was derived after subtraction of the density corresponding to shifted positions of helices 44 (h44′) and 45 (h45′) from the total extra mass shown in Figure 2B. (B, C) Stereo representations of the fittings of (B) the X-ray crystallographic structure (CCF = 0.78) and (C) and the homology model (CCF = 0.79) of the T. thermophilus RbfA into the corresponding cryo-EM density. The asterisk (*) in panel B points to an unoccupied region of the cryo-EM density, due to absence of three amino acid residues and a different orientation of the tail in the X-ray structure. However, the same density region is nicely accounted for by the homology model (panel C). Thumbnails to the left of the panels depict the orientations of the 30S subunit, with body (b), head (h), and platform (p) identified.
Figure 5. Interactions of RbfA with 16S rRNA helices 44 and 45.
(A) Stereo-view presentation of the interaction between the helix-kink-helix (hkh) motif of RbfA (red) and the linker region between the shifted positions of helices 44 (h44′) and 45 (h45′). The hkh motif is shown in cyan. (B) Correlation between the electrostatic distribution of RbfA (shown with regions of positive and negative potentials, in blue and red, respectively) and interaction of RbfA with h44 and h45. Both positions of h44 and h45 are shown: light purple ribbons, original positions; brown ribbons, shifted positions (marked as h44′ and h45′). Panel B was made with PyMol (http://www.pymol.org). In both panels, theRbfA-30S complex is viewed from the platform side, as depicted in the thumbnail to the lower right
Figure 6. Interactions of RbfA with other components of the 30S subunit.
(A) Proximity of the C terminus of RbfA (red) to helix 1, central pseudoknot helix 27, and helix 28 of the 16S rRNA. (B) Other neighbors of RbfA within the subunit. Positions of 16S rRNA segments and r-proteins were defined by docking of the crystallographic structure (Wimberly et al., 2000) into the cryo-EM map. Numbers prefixed by h and S identify 16S rRNA helices and 30S small subunit proteins, respectively. The C-terminus was positioned as in the Tth RbfA homology model since in the TthRbfA crystallographic structure the C-terminal end would clash with the 16S rRNA. Thumbnails to the left depict the orientations of the 30S subunit.
Contacts of Bound RbfA with Components of the 30S Subunit
The low sequence identity between different RbfA homologues suggests that RbfA interacts with the 30S subunit utilizing general electrostatic interactions, rather than specific conserved contacts (Supplemental Figure S4). RbfA interacts with three of the four domains in the 30S subunit, the head, body and 3′ minor (h44–45) domains. The most prominent interaction with the 30S subunit involves the highly basic region on one side of RbfA, formed from the N-terminus, the hkh motif (α2-α3), and the flexible loop regions located between β1-β2 and α3-β3 (Figure 5A, B, also see Supplementary Figure S5). The positively charged residues located in the hkh motif are positioned so as to interact with the negatively charged phosphate-oxygen backbone of nucleotides located in the single-stranded linker region that spans between the top of h44 and the base of h45. Similarly, the N-terminus and loop regions form a positive surface with which the backbone of h28, h29 and to a lesser extent h30 can interact. In contrast, it is interesting that a large negative area, derived from the terminal end of α1 and start of β1, is oriented towards where the top of h44 is located in the native 30S subunit (Figure 5B, see Supplemental Figure S5C). Therefore, the charge distribution supports not only our placement of RbfA and its interaction with rRNA, but also the necessity of a shift in the h44–45 region, to avoid electrostatic repulsion. In addition, the long C-terminal extensions of r-proteins S9 and S13 wind their way into the P site of the 30S subunit and interact with RbfA (Figure 6B) at the periphery of the highly basic region. Deletion of either of these extensions in E. coli shows that these extensions are not essential for viability; however, the deletion strains exhibit reduced growth rates, and the 30S subunits have a lower affinity for tRNA (Hoang et al., 2004). The other major site of contact between RbfA and the 30S subunit overlaps with the binding site of IF1 (Carter et al., 2001), encompassing h18 and r-protein S12. The loop region between β2-α2 approaches the backbone of nucleotides located at the top of h18 and is directly proximal to the long flexible loop linking β1-β2 of the OB-fold of S12 (Figure 6B). This loop contains highly conserved residues involved in mRNA-tRNA codon-anticodon discrimination at the A site; mutations within the loop affect translational fidelity (see Ogle et al., 2001).
In the 30S·RbfA complex, rigid body docking of RbfA into the cryo-EM density places the C-terminus of RbfA in a position at the neck of the 30S subunit where it clashes with the single stranded region linking h28 with the top of h44 and the upper region of h18 (Figure 6B). However, given the flexibility of this region in the known RbfA structures (Figure 1D), it is conceivable that the C-terminal amino acids relocate towards h18, such that the C-terminal end of the protein comes into close proximity with the loop region of h1 at the 5′ end of the 16S rRNA (Figure 6A).
Molecular Basis for the Overlapping Function between 30S Assembly Factors
There appears to be a complicated interplay, as well as partial overlaps in function, among a number of the 30S subunit assembly factors. It has been reported that overexpression of Era, a GTP-binding protein also involved in the 16S rRNA maturation, suppresses (at least partially), the cold-sensitive cell growth and defective ribosome assembly in a ΔrbfA strain (Inoue et al., 2003). Overexpression of RbfA, but not Era, can rescue a slow-growth phenotype and assembly defects associated with deletion of the rimM gene, which encodes another ribosome maturation factor, RimM (Bylund et al., 1998; Inoue et al., 2003). However, overexpression of RimM cannot complement the ΔrbfA strain, suggesting that some sort of hierarchy exists during 30S assembly (Bylund et al., 1998). In light of the binding position of RbfA determined here, we suggest an explanation for the partial functional redundancy of RbfA with Era and RimM.
Although the principal known function of RbfA is to facilitate processing of the 5′ ends of precursor 17S rRNA, while that of Era is to facilitate processing of 3′ ends, our study shows that RbfA binds in the immediate vicinity of Era’s binding position on the 30S subunit (Sharma et al., 2005) (Figure 7A). We suggest that Era can partially compensate for RbfA function through interaction with common structural elements of the ribosome, specifically h28 (Figure 7B), which is directly basepaired through a pseudoknot interaction with nucleotides located in the loop of h1 at the 5′ terminus of the 16S rRNA (Supplemental Figure S1A, B). Thus, the interaction of Era with h28 could stabilize h1 indirectly, in a manner that RbfA normally performs through direct interaction.
Figure 7. Comparison of the binding positions of RbfA and Era on the 30S subunit.
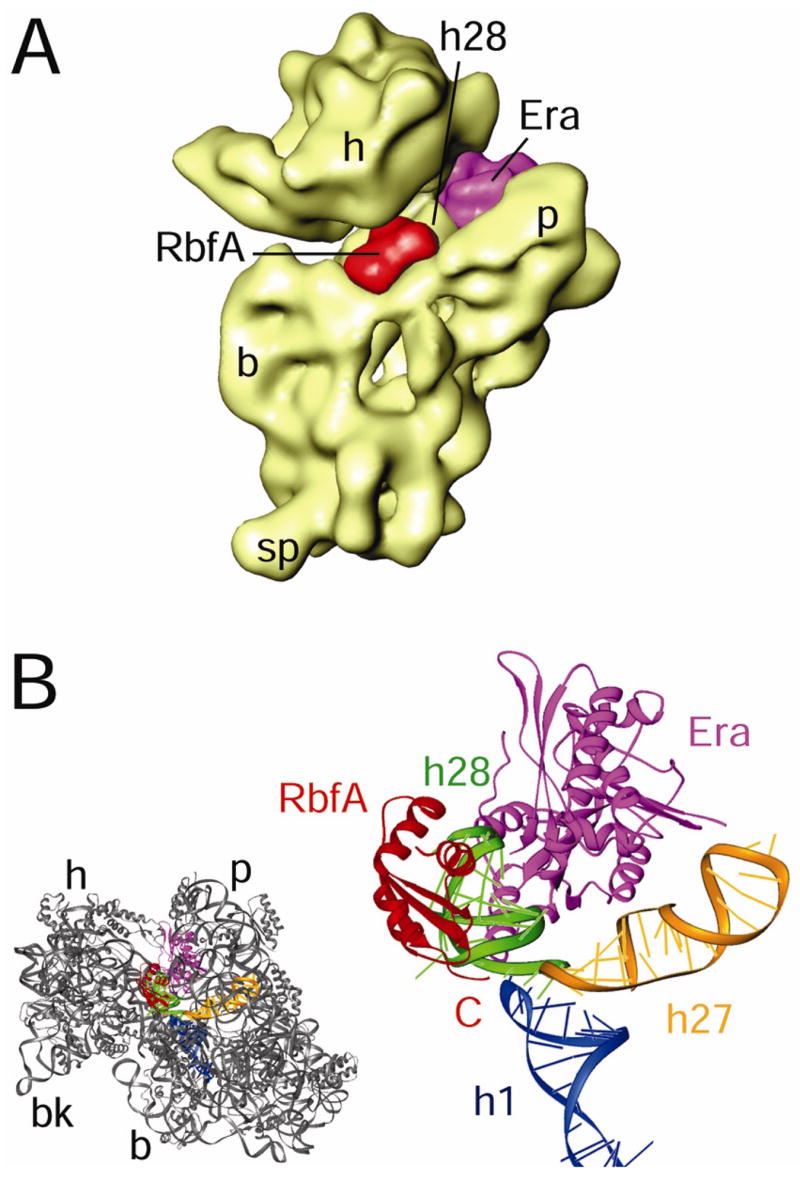
(A) Binding position of RbfA (red) and Era (magenta; Sharma et al., 2005) on the 30S subunit. (B) RbfA (red) and Era (magenta) interact with a common structural element, h28, of the 16S rRNA (cyan). The thumbnail to the left depicts the orientation of the 30S subunit.
Although the structure of RimM bound to the 30S subunit is not known, biochemical data indicate that this maturation factor interacts directly with the head region, in the vicinity of r-proteins S13, S19, and helices 31 and 33b of the 16S rRNA, leading to the suggestion that RimM plays a role in assembly of the head region (Lovgren et al., 2004). RbfA also participates in multiple interactions with components of the head, including r-protein S13 and neighbouring rRNA helices. Therefore, as compared to bound Era, bound RbfA is better positioned to bring regions of the head, body, and 3′ minor domain together at a late assembly stage; in this way the factor could compensate for the absence of RimM.
The Mechanisms of Action of RbfA during Small Subunit Assembly and Cold Shock
The binding position of RbfA on the 30S subunit supports a role for the factor during the final steps in the maturation of the 30S subunit (Jiang et al., 2007). In particular, the binding position is consistent with the proposal that RbfA facilitates correct formation of h1 at the 5′ end of the 16S rRNA through interaction between the C-terminus of RbfA and the loop region of h1 (Supplemental Figure S1A, B; Dammel and Noller, 1995). The significantly longer C-terminal extensions present in RbfA from other bacteria, such as E. coli, rather than T. thermophilus, suggests that the interaction with h1 may be even more extensive in these species. One possible explanation for the shortened tail in T. thermophilus may be related to the high temperatures at which this organism grows, by enabling efficient transition of the alternate competing conformation for h1 in the precursor 17S rRNA into the canonical h1 found in the mature 16S rRNA. It appears that, in E. coli for example, under normal growth temperatures (37 °C) RbfA is partially dispensable for this transition, although clearly the accumulation of 30S subunits containing 17S precursor observed in the ΔrbfA strain indicates that RbfA stimulates the process (Dammel and Noller, 1995; Bylund et al., 1998; Inoue et al., 2003). Indeed, the presence of a C23U mutation in the stem of h1 has been proposed to weaken formation of the helix to such an extent that under cold shock conditions, the phenotype of the mutant strain is reminiscent of the ΔrbfA strain (Dammel and Noller, 1993). The latter phenotype can be rescued by overexpression of RbfA (Dammel and Noller, 1995). The detection of other suppression mutations in the 16S rRNA, which appear to work through stabilization of h1, supports the idea that RbfA operates in an analogous manner (Dammel and Noller, 1995).
We believe that our complex most closely represents the post-processing of the 30S subunit, just prior to RbfA dissociation. However, one of the surprising findings that binding of RbfA induces dramatic conformational changes in h44 and h45 of the 30S subunit, may have important implications during maturation: The large shift of h44 significantly alters the locations of two important intersubunit bridges, namely B2a and B3 (Figure 3; Gabashvili et al., 2000; Yusupov et al., 2001); such that the association of the precursor-30S·RbfA complex with the 50S subunit would be prohibited (Dammel and Noller, 1995). Furthermore, the position of bound RbfA on the 30S subunit overlaps that of IF1 (Carter et al., 2001), as well as with the positions of the anticodon stem loops of A- and P-site bound tRNAs (Agrawal et al., 2000; Selmer et al., 2006; see Supplemental Figure S6). In addition, RbfA is expected to block the path of mRNA through the 30S subunit (Yusupova et al., 2001); and due to the shift in the 3′ end of the 16S rRNA, the anti-Shine-Dalgarno (SD) sequence is placed in an unfavourable location for base-pairing with the SD-sequence present in the 5′ UTRs of many bacterial mRNAs. Interestingly, the cold-shock protein pY binds in a location similar to that of RbfA, and has been shown to inactivate translation by preventing binding of A- and P-site tRNAs (Vila-Sanjurjo et al., 2004; Wilson and Nierhaus, 2004). Thus, we believe that RbfA prevents precursor 30S subunits, in which h1 has not formed and the 5′ end has not been processed, from entering the translation initiation cycle. However, we do not think this is the case for mature 30S subunits, since overexpression of RbfA in vivo does not inhibit growth (Xia et al., 2003), and addition of RbfA does not inhibit protein synthesis in vitro (M.K., C.T. and S.Y., unpublished). This may suggest that binding of RbfA to precursor 30S subunits is much tighter than to mature 30S subunits, and/or that RbfA may be easily released from mature, but not precursor, 30S subunits during translation initiation complex formation. Moreover, RbfA is present at very low concentrations during normal growth conditions (Xia et al., 2003), but under cold-shock conditions, when many precursor 30S subunits are trapped with an alternate h1 conformation, RbfA expression is up-regulated (Jones et al., 1996). Thus, we believe that RbfA stimulates protein synthesis indirectly, by facilitating a more rapid supply of active 30S subunits under cold-shock conditions, rather than by promoting translation of specific mRNAs.
Experimental Procedures
Purification, crystallization and structure determination of the T. thermophilus RbfA
T. thermophilus HB8 rbfA gene (TTHA0907) was cloned to expression vector pET11a (Novagen) by the RIKEN Structural Genomics Initiative (Yokoyama et al., 2000) and recombinant RbfA was expressed in E. coli strain BL21 (DE3). The cell lysate was incubated at 55°C and RbfA was precipitated by the addition of 70%-saturated ammonium sulfate (AS) to the supernatant. The precipitate was dissolved in 20mM Tris-HCl buffer (pH 8.0) containing 1.5 M NaCl and1.2 M AS, and loaded on hydrophobic column chromatography (HiPrep Buthyl). Further purification was performed by cation exchange chromatography (monoS column) and by size-exclusion chromatography (Superdex-75). The final buffer is 20 mM Tris-HCl buffer containing 250 mM KCl (pH 8.0). The protein yield was 1 mg per 1 g of cells. Crystals were obtained in drops composed of 0.5 μl protein solution (2.5 mg/ml) and 0.5 μl reservoir solution [1.2 M ammonium dihydrogen phosphate, 80 mM Tris/HCl (pH 8.5), 9.6 % glycerol, 80 mM magnesium formate, 20 mM Bis-Tris propane (pH 7.0)], by the sitting drop vapor diffusion technique at 20°C. The diffraction data was collected at BL-5A beamline in Photon Factory (Tsukuba, Japan) from a single crystal up to 1.84 Å resolution and processed using the HKL2000 suite and SCALEPACK programs (Otwinowski and Minor, 1997). General handling of the scaled data was carried out with programs in the CCP4 suite (CCP4, 1994). The phase was determined by the method of molecular replacement with program molrep, using a homology model for T. thermophilus RbfA (SWISS MODEL http://swissmodel.expasy.org/; Schwede et al., 2003) as a search model, which was generated from 1JOS (Haemophilus influenzae) with ClustalW (Higgins et al., 1994). Models were rebuilt by combining the auto-model building results of ARP-wARP, improved using O (Jones et al., 1991) and refined with CNS (Brunger et al., 1998). The structures were refined to R-factor of 19.9 % (Rfree = 24.5 %) at 1.84 Å resolution. Protein secondary structure was defined by the DSSP algorithm (Kabsch and Sander, 1983). Figures were prepared with PyMOL (DeLano; http://www.pymol.org).
Preparation of the T. thermophilus 30S subunit and the 30S·RbfA complex
The S1-depleted 30S ribosomal subunits were isolated as described earlier (Sharma et al., 2005). The 30S·RbfA complex was prepared by incubation of RbfA (a 10–40-fold excess) with 30S ribosomal subunits (360 nM) for 20 min at 65 °C in a buffer A, containing 20 mM, Hepes-KOH (pH 7.8), 10 mM Mg(OAc)2, 200 mM NH4Cl, 65 mM KCl. Reactions were stopped by incubation on ice, and pre-centrifuged for 10 min at 10000 rpm, and an aliquot was removed before the remainder was loaded onto a 10% sucrose cushion in buffer A and centrifuged in a TLN100 rotor at 78000rpm for 30 min. Binding of RbfA to the 30S was checked by running the initial reaction, and the TLN100 supernatant and pellet fractions, on 20% SDS-PAGE, with Coomassie blue staining (as seen in Figure 2A).
Cryo-EM and 3D reconstruction
Cryo-EM grids were prepared following standard procedures (Wagenknecht et al., 1988). EM data were collected under low-dose conditions on a Philips Tecnai F20 field emission gun electron microscope at 200 kV, at a magnification of 50,760X. Images were recorded between 0.7 and 3.5 μm under focus. A total of 131 micrographs was used. The micrographs were digitized on a Zeiss/Imaging scanner (Z/I Imaging Corporation, Huntsville, AL) with a step size of 14 μm, corresponding to 2.76 Å on the object scale. The three-dimensional (3D) reconstructions were calculated using a 3D projection alignment procedure (Penczek et al., 1994). Each data set was subdivided into defocus groups and then analyzed with SPIDER software (Frank et al., 1996) to generate CTF-corrected 3D cryo-EM maps (Penczek et al., 1997). Initially, 154,476 particles were manually selected. The first 3D map obtained from all of the manually selected particle images showed a fragmented mass of extra density in the neck region of the 30S subunit, suggesting a sub-stoichiometric binding of RbfA to the 30S subunit. Therefore, the data set was subjected supervised classification (Valle et al., 2002), by use of the 3D map from the total image set as one of the references. The other reference was the map of the T. thermophilus control 30S subunit (Sharma et al., 2005). Finally, 61,207 particles that classified with the new reference map were used to calculate the 3D reconstructions of the 30S·RbfA complex. The classification helped to enrich the population of RbfA-bound 30S images. However, the possibility that the classified population was still contaminated with images of free (uncomplexed) 30S subunits could not be ruled out. The resolution of the final 3D cryo-EM map of the 30S·RbfA complex was 12.5 Å, according to the FSC criterion with a 0.5 cutoff (Bottcher et al., 1997; Malhotra et al., 1998), or 8.9 Å, according to the 3σ cutoff criterion (Orlova et al., 1998).
Fitting of X-ray crystallographic structures
To attain an optimum fitting of the X-ray crystallographic structure of the T. thermophilus 30S subunit (Wimberly et al., 2000; PDB ID 1J5E), we divided the X-ray coordinates into four structural domains (head, body, platform, and 16S rRNA 3′-minor domain), and then fitted each domain individually into the cryo-EM map of the 30S·RbfA complex. Subsequently, portions of the h44 and h45 rRNA helices from the 3′-minor domain of the X-ray crystallographic structure were separately fitted into the protruding cylindrical features of the extra mass visible in the cryo-EM map of the 30S·RbfA complex. Fitted coordinates were converted into electron density maps with a pixel size of 2.76 Å, and the resulting maps were filtered to match the resolution of the cryo-EM map. The density assigned to RbfA was isolated through comparison of the cryo-EM map of the 30S·RbfA complex and the density map obtained from the fitting of the 30S X-ray coordinates into the cryo-EM map of the 30S·RbfA complex. The cross correlation coefficient (CCF) values between the fitted coordinates for T. thermophilus RbfA and the corresponding cryo-EM density maps were determined after conversion of the fitted coordinates into the density map, through computation of averaged densities within volume elements scale-matched to those of the cryo-EM map (i.e., with a pixel size of 2.76 Å, and after filtration of the X-ray map to the resolution of the cryo-EM density map). Visualization of the fitted atomic structures and the cryo EM density maps was done with Ribbons (Carson, 1991) and IRIS EXPLORER (Numerical Algorithms group, Inc., Downers Grove, IL), respectively.
Supplementary Material
Acknowledgments
We thank S. Wakatsuki for the use of BL-5A at the Photon Factory and R. Nakayama-Ushikoshi, T. Terada and M. Shirouzu for sample preparation. This work was supported by NIH R01 grant GM61576 (to R.K.A.), and also by the RIKEN Structural Genomics/Proteomics Initiative (RSGI), the National Project on Protein Structural and Functional Analysis, Ministry of Education, Culture, Sports, Science and Technology of Japan (to S.Y.), and Deutsche Forschungsgemeinschaft grant FU579/1–2 (to P.F.). The authors acknowledge use of the Wadsworth Center’s EM infrastructure.
Footnotes
Accession Numbers:
The cryo-EM map of the 30S·RbfA complex has been deposited in the EM database (http://www.ebi.ac.uk) under ID code EMD -1413. The coordinates of the Tth RbfA crystal structure, the homology model of RbfA as fitted into the cryo-EM map, and components of 30S ribosomal subunit located in RbfA neighborhood, have been deposited in the Protein Data Bank (www.rcsb.org) under the accession code 2DYJ, 2R1C, and 2R1G, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal RK, Spahn CM, Penczek P, Grassucci RA, Nierhaus KH, Frank J. Visualization of tRNA movements on the Escherichia coli 70S ribosome during the elongation cycle. J Cell Biol. 2000;150:447–460. doi: 10.1083/jcb.150.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix JH, Nierhaus KH. DnaK-facilitated ribosome assembly in Escherichia coli revisited. RNA. 2003;9:787–793. doi: 10.1261/rna.5360203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. doi: 10.1038/386088a0. [DOI] [PubMed] [Google Scholar]
- Brodersen DE, Clemons WM, Jr, Carter AP, Wimberly BT, Ramakrishnan V. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16 S RNA. J Mol Biol. 2002;316:725–768. doi: 10.1006/jmbi.2001.5359. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Bylund GO, Wipemo LC, Lundberg LA, Wikstrom PM. RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli. J Bacteriol. 1998;180:73–82. doi: 10.1128/jb.180.1.73-82.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M. Ribbons 2.0. J Appl Crystallogr. 1991;24:103–106. [Google Scholar]
- Carter AP, Clemons WM, Jr, Brodersen DE, Morgan-Warren RJ, Hartsch T, Wimberly BT, Ramakrishnan V. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science. 2001;291:498–501. doi: 10.1126/science.1057766. [DOI] [PubMed] [Google Scholar]
- COLLABORATIVE COMPUTATIONAL PROJECT NUMBER 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Culver GM. Assembly of the 30S ribosomal subunit. Biopolymers. 2003;68:234–249. doi: 10.1002/bip.10221. [DOI] [PubMed] [Google Scholar]
- Culver GM, Noller HF. In vitro reconstitution of 30S ribosomal subunits using complete set of recombinant proteins. Methods Enzymol. 2000;318:446–460. doi: 10.1016/s0076-6879(00)18069-3. [DOI] [PubMed] [Google Scholar]
- Daigle DM, Brown ED. Studies of the interaction of Escherichia coli YjeQ with the ribosome in vitro. J Bacteriol. 2004;186:1381–1387. doi: 10.1128/JB.186.5.1381-1387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammel CS, Noller HF. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 1993;7:660–670. doi: 10.1101/gad.7.4.660. [DOI] [PubMed] [Google Scholar]
- Dammel CS, Noller HF. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 1995;9:626–637. doi: 10.1101/gad.9.5.626. [DOI] [PubMed] [Google Scholar]
- Datta PP, Bhadra RK. Cold shock response and major cold shock proteins of Vibrio cholerae. Appl Environ Microbiol. 2003;69:6361–6369. doi: 10.1128/AEM.69.11.6361-6369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatur W, Fournier M. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Gabashvili IS, Agrawal RK, Spahn CM, Grassucci RA, Svergun DI, Frank J, Penczek P. Solution structure of the E. coli 70S ribosome at 11.5 Å resolution. Cell. 2000;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- Graumann P, Schroder K, Schmid R, Marahiel MA. Cold shock stress-induced proteins in Bacillus subtilis. J Bacteriol. 1996;178:4611–4619. doi: 10.1128/jb.178.15.4611-4619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno H, Hanawa-Suetsugu K, Kimura T, Takagi K, Sugiyama W, Shirata S, Mikami T, Odagiri F, Osanai Y, Watanabe D, et al. A novel GTPase activated by the small subunit of ribosome. Nucleic Acids Res. 2004;32:5303–5309. doi: 10.1093/nar/gkh861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang L, Fredrick K, Noller HF. Creating ribosomes with an all-RNA 30S subunit P site. Proc Natl Acad Sci U S A. 2004;101:12439–12443. doi: 10.1073/pnas.0405227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Swapna GV, Rajan PK, Ke H, Xia B, Shukla K, Inouye M, Montelione GT. Solution NMR structure of ribosome-binding factor A (RbfA), a cold-shock adaptation protein from Escherichia coli. J Mol Biol. 2003;327:521–536. doi: 10.1016/s0022-2836(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Inoue K, Alsina J, Chen J, Inouye M. Suppression of defective ribosome assembly in a rbfA deletion mutant by overexpression of Era, an essential GTPase in Escherichia coli. Mol Microbiol. 2003;48:1005–1016. doi: 10.1046/j.1365-2958.2003.03475.x. [DOI] [PubMed] [Google Scholar]
- Iost I, Dreyfus M. DEAD-box RNA helicases in Escherichia coli. Nucleic Acids Res. 2006;34:4189–4197. doi: 10.1093/nar/gkl500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Sullivan SM, Walker AK, Strahler JR, Andrews PC, Maddock JR. Identification of novel Escherichia coli ribosome-associated proteins using isobaric tags and multidimensional protein identification techniques. J Bacteriol. 2007;189:3434–3444. doi: 10.1128/JB.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PG, Inouye M. The cold-shock response--a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Jones PG, Inouye M. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol Microbiol. 1996;21:1207–1218. doi: 10.1111/j.1365-2958.1996.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Jones PG, Mitta M, Kim Y, Jiang W, Inouye M. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:76–80. doi: 10.1073/pnas.93.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zhou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogra A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Lovgren JM, Bylund GO, Srivastava MK, Lundberg LA, Persson OP, Wingsle G, Wikstrom PM. The PRC-barrel domain of the ribosome maturation protein RimM mediates binding to ribosomal protein S19 in the 30S ribosomal subunits. RNA. 2004;10:1798–1812. doi: 10.1261/rna.7720204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JA, Schnobrich DJ, Culver GM. The DnaK chaperone system facilitates 30S ribosomal subunit assembly. Mol Cell. 2002;10:129–138. doi: 10.1016/s1097-2765(02)00562-2. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Penczek P, Agrawal RK, Gabashvili IS, Grassucci RA, Junemann R, Burkhardt N, Nierhaus KH, Frank J. Escherichia coli 70 S ribosome at 15 Å resolution by cryo-electron microscopy: localization of fMet-tRNAfMet and fitting of L1 protein. J Mol Biol. 1998;280:103–116. doi: 10.1006/jmbi.1998.1859. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM, Jr, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Carter AP, Ramakrishnan V. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem Sci. 2003;28:259–266. doi: 10.1016/S0968-0004(03)00066-5. [DOI] [PubMed] [Google Scholar]
- Orlova EV, Dube P, Harris JR, Beckman E, Zemlin F, Markl J, van Heel M. Structure of keyhole limpet hemocyanin type 1 (KLH1) at 15 Å resolution by electron cryomicroscopy and angular reconstitution. J Mol Biol. 1997;271:417–437. doi: 10.1006/jmbi.1997.1182. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Penczek PA, Grassucci RA, Frank J. The ribosome at improved resolution: new techniques for merging and orientation refinement in 3D cryo-electron microscopy of biological particles. Ultramicrosc. 1994;53:251–270. doi: 10.1016/0304-3991(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Penczek PA, Zhu J, Schroder R, Frank J. Three-dimensional reconstruction with contrast transfer function compensation from defocus series. Scanning Microsc. 1997;11:147–154. [Google Scholar]
- Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell. 2002;108:557–572. doi: 10.1016/s0092-8674(02)00619-0. [DOI] [PubMed] [Google Scholar]
- Rubin SM, Pelton JG, Yokota H, Kim R, Wemmer DE. Solution structure of a putative ribosome binding protein from Mycoplasma pneumoniae and comparison to a distant homolog. J. Struct. Funct. Genomics. 2003;4:235–243. doi: 10.1023/b:jsfg.0000016127.57320.82. [DOI] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Sharma MR, Barat C, Wilson DN, Booth TM, Kawazoe M, Hori-Takemoto C, Shirouzu M, Yokoyama S, Fucini P, Agrawal RK. Interaction of Era with the 30S ribosomal subunit implications for 30S subunit assembly. Mol Cell. 2005;18:319–329. doi: 10.1016/j.molcel.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Traub P, Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968;59:777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Sengupta J, Swami NK, Grassucci RA, Burkhardt N, Nierhaus KH, Agrawal RK, Frank J. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J. 2002;21:3557–3567. doi: 10.1093/emboj/cdf326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLoock MS, Agrawal RK, Gabashvili IS, Qi L, Frank J, Harvey SC. Movement of the decoding region of the 16 S ribosomal RNA accompanies tRNA translocation. J Mol Biol. 2000;304:507–515. doi: 10.1006/jmbi.2000.4213. [DOI] [PubMed] [Google Scholar]
- Vila-Sanjurjo A, Schuwirth BS, Hau CW, Cate JHD. Structural basis for the control of translational initiation during stress. Nat Struct Mol Biol. 2004;11:1054–1059. doi: 10.1038/nsmb850. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T, Grassucci R, Frank J. Electron microscopy and computer image averaging of ice-embedded large ribosomal subunits from Escherichia coli. J Mol Biol. 1988;199:137–147. doi: 10.1016/0022-2836(88)90384-1. [DOI] [PubMed] [Google Scholar]
- Williamson JR. After the ribosome structures: how are the subunits assembled? RNA. 2003;9:165–167. doi: 10.1261/rna.2164903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DN, Nierhaus KH. The how and Y of cold shock. Nat Struct Mol Biol. 2004;11:1026–1028. doi: 10.1038/nsmb1104-1026. [DOI] [PubMed] [Google Scholar]
- Wilson DN, Nierhaus KH. The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol. 2007;42:187–219. doi: 10.1080/10409230701360843. [DOI] [PubMed] [Google Scholar]
- Wimberly BT, Brodersen DE, Clemons WM, Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B. Ribosomal proteins: their structure and evolution. In: Hardesty B, Kramer G, editors. Structure, function and genetics of ribosomes. Springer; NY: 1986. pp. 326–361. [Google Scholar]
- Xia B, Ke H, Shinde U, Inouye M. The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J Mol Biol. 2003;332:575–584. doi: 10.1016/s0022-2836(03)00953-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Hirota H, Kigawa T, Yabuki T, Shirouzu M, Terada T, Ito Y, Matsuo Y, Kuroda Y, Nishimura Y, Kyogoku Y, Miki K, Masui R, Kuramitsu S. Structural genomics projects in Japan. Nat Struct Biol. 2000;7:943–945. doi: 10.1038/80712. [DOI] [PubMed] [Google Scholar]
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 Å resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- Yusupova GZ, Yusupov MM, Cate JH, Noller HF. The path of messenger RNA through the ribosome. Cell. 2001;106:233–241. doi: 10.1016/s0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.