Summary
Activity-dependent rapid structural and functional modifications of central excitatory synapses contribute to synapse maturation, experience-dependent plasticity, learning and memory, and are associated with neurodevelopmental and psychiatric disorders. However the signal transduction mechanisms that link glutamate receptor activation to intracellular effectors that accomplish structural and functional plasticity are not well understood. Here we report that NMDA receptor activation in pyramidal neurons causes CaMKII-dependent phosphorylation of the guanine-nucleotide exchange factor (GEF) kalirin-7 at residue threonine 95, regulating its GEF activity, leading to activation of small GTPase Rac1 and rapid enlargement of existing spines. Kalirin-7 also interacts with AMPA receptors and controls their synaptic expression. By demonstrating that kalirin expression and spine localization are required for activity-dependent spine enlargement and enhancement of AMPA-mediated synaptic transmission, our study identifies a novel signaling pathway that controls structural and functional spine plasticity.
Keywords: Rac1, GluR1, CaMKII, synaptic plasticity, postsynaptic density, cytoskeleton, actin, synaptic transmission
Introduction
How central excitatory synapses change during plasticity is a central question in neuroscience because knowledge of the mechanisms of this process will help us understand normal brain functions such as cognition, learning, memory, as well as pathological conditions such as mental retardation, mental illness and addiction. In the last decade, enormous progress has been made in understanding the phenomenology and mechanisms of functional plasticity of central synapses. However, much less is known about the mechanisms that control synaptic structural plasticity of existing synapses. Such structural changes of spines complement functional changes, and it is becoming clear that synapse structure and function are tightly correlated (Kasai et al., 2003; Kopek et al., 2006; Luscher et al., 2000). Activity-dependent rapid structural modifications of central excitatory synapses, together with synaptic functional modifications, underlie synaptic plasticity (Engert & Bonhoeffer, 1999; Maletic-Savatic et al, 1999; Toni et al, 1999), and are crucial for experience-dependent brain plasticity (Trachtenberg et al., 2002), as well as learning and memory (Leuner et al., 2003; Kozorovitskiy et al., 2005). Dendritic spine morphogenesis is central to synaptic structural plasticity (Luscher et al., 2000; Yuste & Bonhoeffer, 2001): most excitatory synapses on pyramidal neurons in brain are located on dendritic spines (Fiala et al., 2002), and spine structure is a determinant of synapse function (Kasai et al, 2003). Altered synaptic structural and functional plasticity also contribute to neurodevelopmental and psychiatric disorders that affect cognition (Fiala et al., 2002): aberrant spine morphogenesis and plasticity occurs in mental retardation (Kaufman & Moser, 2000), Fragile-X disorder (Irwin et al., 2000), autism spectrum disorders (Pickett & London, 2005), schizophrenia and depression (Glantz & Lewis, 2001), and addiction to drugs (Robinson & Kolb, 1999). However, the molecular mechanisms that regulate activity-dependent synaptic structural plasticity are not well understood.
In forebrain pyramidal neurons, induction of long-term potentiation (LTP) in hippocampal slices using different protocols (Engert & Bonhoeffer, 1999; Maletic-Savatic et al., 1999; Toni et al., 1999; Kopec et al., 2006), or glutamate uncaging on spine heads (Matsuzaki et al., 2004), causes rapid enlargement of spine heads and simultaneous delivery of α-amino-5-hydroxy-3-methyl-4-isoxazole propionic acid (AMPA) receptors (AMPAR) containing GluR1 into spines. Similar modifications have been observed in the cortex in response to LTP (Connor et al., 2006) or experience (Takahashi et al., 2003). N-methyl-D-aspartate (NMDA) receptor-dependent activation of calcium/calmodulin-dependent protein kinase II (CaMKII) is necessary for induction of both structural and functional plasticity (Matsuzaki et al., 2004; Toni et al., 1999). CaMKII is a serine/threonine kinase regulated by Ca2+/calmodulin, which is highly abundant in spines. CaMKII is activated and autophosphorylated during LTP, and its activation is necessary and sufficient for the induction of LTP (Lisman et al., 2002) and of activity-dependent spine plasticity (Maletic-Savatic et al., 1999; Jourdain et al., 2003; Matsuzaki et al., 2004). CaMKII is required for experience-dependent cortical plasticity (Galzewski et al., 1996) and learning and memory in vivo (Gieze et al., 1998; Lisman et al., 2002). Nevertheless, the substrates of CaMKII necessary for induction of activity-dependent structural plasticity of mature synapses are not known. At the effector end of the postsynaptic signaling cascades, increased synaptic expression of AMPA receptors (AMPAR) (Kopec et al., 2006; Shi et al. 1999; Song & Huganir, 2002) and actin remodeling (Fischer et al., 1998; Fukazawa et al., 2003; Matsuzaki et al., 2004) underlie the maintenance of synaptic potentiation and the associated spine enlargement. On the other hand, the intracellular signaling mechanisms that functionally link NMDA receptor (NMDAR) activation to actin dynamics and receptor trafficking to the synaptic surface are not well understood (Tada and Sheng, 2006).
The brain-specific neuronal guanine-nucleotide exchange factor (GEF) kalirin-7, an activator of the small GTPase Rac1 and regulator of actin cytoskeletal dynamics, is enriched in dendritic spines through interactions of its C-tail with postsynaptic scaffolding proteins including PSD-95, SAP-102 and SAP97, where it controls spine morphogenesis (Penzes et al., 2000; 2001). For these reasons kalirin-7 is in an optimal position to relay signals initiated by NMDAR activation to the intracellular machinery that drives cytoskeletal rearrangements and receptor trafficking, to control activity-dependent structural and functional spine plasticity in mature spines. Importantly, Rho GTPases including the kalirin-7 target Rac1 are key regulators of spine morphogenesis, Rac1 specifically inducing spine formation and enlargement (Tashiro et al., 2000). Mutations in genes encoding several components of the Rho GTPase signaling pathway cause mental retardation (Ramakers, 2002), demonstrating a key role for proteins in these pathways in human cognitive development.
To understand the mechanisms that control activity-dependent plasticity of existing spines, and coordinate it with synaptic functional plasticity, in this study we investigated the signal transduction mechanisms that functionally link activation of glutamate postsynaptic receptors with the machinery that controls rapid spine structural and functional modifications that maintain plasticity. We report that in developed pyramidal neurons, activation of NMDARs causes rapid spine enlargement, associated with activation of Rac1 and CaMKII. In turn, CaMKII interacts with kalirin-7 in spines, and phosphorylates it. Activity-dependent phosphorylation of kalirin-7 and Rac1 activation require the association of kalirin-7 with the postsynaptic density, as well as active CaMKII. We show that kalirin-7 also interacts with the GluR1 subunit of AMPARs, controlling their synaptic expression and maintenance, and AMPAR-mediated synaptic transmission. We demonstrate that CaMKII activity, kalirin expression, and spine localization of kalirin are required for activity-dependent insertion of GluR1 into spines, spine enlargement, and enhancement of AMPA-mediated synaptic transmission. Taken together, our study identifies a novel signaling pathway that controls structural and functional spine plasticity.
Results
NMDA receptor-dependent rapid spine enlargement and Rac1 activation
Rapid enlargement of dendritic spines, induced by specific patterns of synaptic stimulation and occurring in parallel with enhancement of synaptic transmission, is thought to contribute to the establishment of long-lasting synapse potentiation (Maletic-Savatic et al., 1999; Toni et al., 1999; Matsuzaki et al., 2004; Kopec et al., 2006). However the intracellular mechanisms that control activity-dependent rapid spine enlargement are not known. To induce activity-dependent spine remodeling we activated synaptic NMDARs on cultured cortical pyramidal neurons by acutely unmasking receptors previously inhibited with APV (Xie et al., 2005). We examined neurons at div 28, when the majority of synapses are mature and the formation and elimination of synapses reach equilibrium (Holtmaat et al, 2005). This treatment induced rapid spine enlargement: average spine areas were 30–70% larger (basal, 0.62±0.04; 5 min activated, 0.78±0.054; 30 min activated: 0.95±0.055 μm2, p<0.001; Figure 1A, S6 A–B), while inducing significant spine elongation at later time points (Xie et al., 2005). We confirmed this activity-dependent rapid spine enlargement in live neurons using time-lapse imaging (Figure 1B). Activation of synaptic NMDARs using an independent protocol in neurons cultured in absence of APV by a short treatment with the coagonist glycine (Lu et al., 2001) induced similar spine enlargement (Figure S1A). These changes in spine morphology have a comparable time course to those associated with induction of LTP or caused by glutamate uncaging in other studies (Kopec et al. 2006; Matsuzaki et al., 2004). This enlargement of spines was also associated with functional enhancement of synaptic transmission, indicated by increased average AMPA-mediated mEPSC frequency (basal 3.11±0.17/sec, activated 5.05±0.75/sec, p<0.05; Figure 1C).
Figure 1. NMDA receptor-dependent spine enlargement is associated with Rac1 and CaMKII activation.
(A) Quantification of spine areas of cultured cortical pyramidal neurons in basal and activated conditions: 5-min and 30-min activated are significantly different from basal, p<0.005. (B) Time-lapse imaging of single spine before (basal) and after (activated) NMDAR activation. (C) Traces show AMPA-mediated mEPSC recordings from neurons in basal and activated (30 min) conditions, *p<0.05. (D) Quantification of integrated intensities of Rac1-GTP bands in control (basal) and activated neurons, normalized for protein loading, **p<0.005. (E) Western blot of extracts of control and activated neurons with an anti-phospho-Thr286-CaMKII antibody. (F) Time-dependent phosphorylation of CaMKIIα/β in dendrites of cortical neurons upon NMDAR activation; immunostaining with anti phospho-CaMKIIα/β antibody, ***p<0.001. (G) Effects of KN-62 (10 μM) on NMDAR activation-dependent CaMKII phosphorylation and spine enlargement, **p<0.005. (H) Quantification of (G): activated is significantly different from basal, p<0.001. Scale bars: 10 μm.
To investigate the molecules that link NMDAR activation with structural remodeling of spines, we examined the activation of the small GTPase Rac1. This G-protein is a central regulator of the actin cytoskeleton, spine enlargement, activity-dependent dendrite growth (Sin et al., 2002; Tashiro et al., 2000; Wiens et al., 2005), and synaptic clustering of AMPAR during synapse maturation (Wiens et al., 2005). We found that in pyramidal neurons, activation of NMDARs also resulted in activation of Rac1 with the same temporal dynamics as spine morphogenesis, (5 min, 1.92±0.23; 30 min, 4.42±0.74; p<0.005; Figure 1D) suggesting that activation of the Rac1 may underlie activity-dependent spine enlargement, as well as AMPAR synaptic expression.
CaMKII is required for activity-dependent spine enlargement
CaMKII is required for the induction of LTP, spine enlargement associated with potentiation, and multiple forms of plasticity and learning and memory (Galzewski et al., 1996; Gieze et al., 1998; Maletic-Savatic et al., 1999; Lisman et al., 2002; Jourdain et al., 2003; Matsuzaki et al., 2004); hence, we reasoned that it may also be crucial for mediating downstream signaling from NMDARs to the actin cytoskeleton. To determine whether NMDAR activation induced activation of CaMKII, we examined the phosphorylation of CaMKII in neurons in which we activated NMDARs, as compared to untreated control neurons (p<0.001; Figure 1E–F). As expected, activation of NMDARs resulted in rapid phosphorylation of CaMKII (within 30 min), which was blocked by the CaMKII inhibitor KN-62 (Matsuzaki et al., 2004) (p<0.005; Figure 1G). Importantly, preincubation with KN-62 also blocked activity-dependent spine enlargement and increase in linear spine density (area: basal, 0.65±0.04; activated, 0.98±0.03; activated+KN-62, 0.62±0.05 μm2, p<0.001; Figure 1G–H, S6C), demonstrating that NMDAR activity-dependent spine enlargement requires CaMKII activation.
CaMKII interacts with kalirin-7
The downstream targets of CaMKII that may regulate spine enlargement are not known. We reasoned that this function may be fulfilled by GEFs for Rac1, which could catalyze CaMKII-dependent activation of Rac1 leading to actin remodeling. We have previously reported that the brain-specific Rac1-GEF kalirin-7 is enriched in dendritic spines and regulates spine morphogenesis (Penzes et al., 2000; 2001). Bioinformatics algorithms indicate that kalirin-7 contains several putative CaMKII phosphorylation sites (Figure S1B). Hence kalirin-7 may relay signals from NMDARs, via CaMKII, to control activity-dependent spine remodeling. To test whether CaMKII and kalirin-7 interacted functionally, we examined their physical association in rat forebrain homogenates and cultured pyramidal neurons. CaMKII coimmunoprecipitated with kalirin-7 and PSD-95 from rat forebrain homogenates (Figure 2A), suggesting that these proteins participate in the same multiprotein complex. Indeed, in dissociated cultures of cortical neurons, kalirin-7 and CaMKII colocalized in spines (Figure 2B). These results place kalirin-7 in proximity of CaMKII in spines, potentially enabling it to phosphorylate kalirin-7. To test whether CaMKII can phosphorylate kalirin-7, we coexpressed them in hEK293 cells and examined the phosphorylation of kalirin-7 by Western blotting with a phospho-threonine (Thr) antibody (Lisman et al., 2002). While kalirin-7 alone was not phosphorylated, its coexpression with CaMKIIα (constitutively active, -CA, or wild type) resulted in its phosphorylation (Figure 2C). This was dependent on the presence of phosphatase inhibitors and was blocked by KN-62 (Figure 2C, D). These results suggest that activation of NMDARs may induce Thr-phosphorylation of kalirin-7. Indeed, activation of NMDARs in cortical neuronal cultures resulted in Thr phosphorylation of kalirin-7 (Figure 2E), suggesting that kalirin-7 phosphorylation by CaMKII may occur in synapses upon NMDAR activation.
Figure 2. CaMKII interacts with and phosphorylates kalirin-7.
(A) Coimmunoprecipitation of kalirin-7 with CaMKII and PSD-95 from rat forebrain homogenate; negative controls were IgG and myc antibodies. (B) Colocalization of CaMKII and kalirin-7 in spines of pyramidal neurons. (C) Western blot for phospho-Thr (p-Thr): constitutively active CaMKIIα (CaMKII-CA) phopshorylates kalirin-7, dependent on the presence of phosphatase inhibitors. Kalirin-7 was immunoprecipitated from hEK293 cells overexpressing myc-kalirin-7 with or without CaMKII-CA. D) Phosphorylation of kalirin-7 by wild-type CaMKII is blocked by KN-62. Cells were preincubated with or without 10 μM KN-62 before immunoprecipitation; Western blot with p-Thr and myc antibodies; quantification of integrated intensities of p-Thr bands, normalized for protein loading, *p<0.05. (E) NMDAR activation-dependent phosphorylation of kalirin-7 in neurons; kalirin was immunoprecipitated and analyzed by Western blotting for p-Thr. (F) Epitope-tagged kalirin-7 or truncated forms were overexpressed in hEK293 cells with CaMKII-CA; kalirin was immunoprecipitated and phosphoproteins were analyzed by Western blotting with a p-Thr antibody. (G) Purified CaMKII/calmodulin directly phosphorylates immunopurified kalirin-7 in vitro, in presence of purified CaMKII/calmodulin and Y-[32P]-ATP; arrowhead: phospho-kalirin-7 band, asterisk: non-specific band. Scale bar: 10 μm.
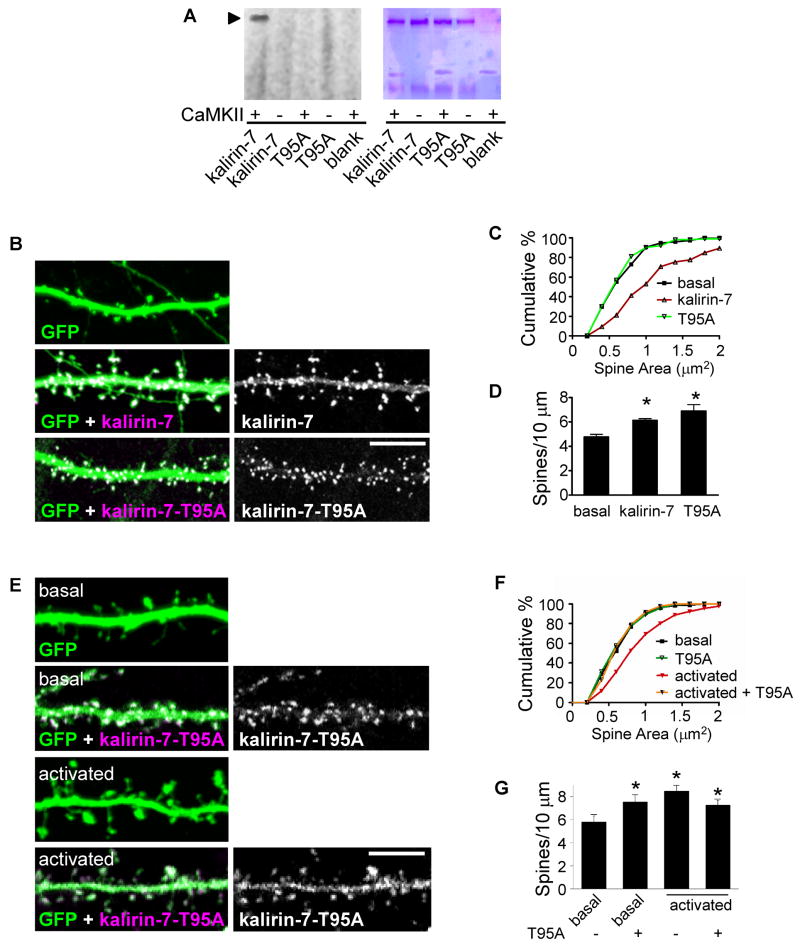
To identify the region of kalirin sequence that contained the CaMKII phosphorylation sites, we coexpressed truncated forms of kalirin containing or lacking specific domains with CaMKIIα and examined the phosphorylation of kalirin-7 (Figure 2F). While full-length kalirin-7 was phosphorylated on Thr residues, a mutant lacking the N-terminus (kalirin-5), or mutants containing only the DH-PH or spectrin domains were not. This indicates that the CaMKII phosphorylation site is within the N-terminal 300 amino acids of kalirin-7, in agreement with the predicted CaMKII phosphorylation site at Thr 95 (T95) (Figure S1B). Remarkably, purified CaMKII/calmodulin phosphorylates purified kalirin-7 in vitro (Figure 2G), and the presence of inhibitors of PKA, PKC or Cdk5 did not reduce CaMKII-dependent phosphorylation (Figure S1C), indicating that kalirin is directly phosphorylated by CaMKII.
To determine whether T95 was the CaMKII phosphorylation site, we generated a point mutation in kalirin-7 changing the threonine to alanine (T95A) (Figure S1D). In an in vitro phosphorylation assay, purified CaMKII/calmodulin did not phosphorylate the immunopurified T95A, demonstrating that CaMKII phosophorylates kalirin-7 at T95 (Figure 3A). This was further confirmed by Western blotting with a p-Thr antibody of kalirin-7 or T95A coexpression in the presence or absence of CaMKII-CA (Figure S1E). While coexpression of CaMKII-CA with wild-type kalirin-7 resulted in an enhanced activation of Rac1, coexpression with kalirin-7-T95A did not, suggesting that phosphorylation of kalirin-7 at T95 by CaMKII modulates its Rac1-GEF activity (Figure S1F). To test whether availability for phosphorylation of this site affected the ability of kalirin-7 to regulate spine morphology, we transfected neurons (div 28) with plasmids expressing GFP alone, or together with wild-type kalirin-7 or kalirin-7-T95A (Figure 3B). Both wild type and mutant kalirin-7 were expressed and targeted to spines with similar efficiencies. While expression of kalirin-7 caused an increase in spine areas compared to controls, as expected, expression of kalirin-7-T95A had no effect on spine sizes (basal, 0.61±0.04; kalirin-7, 1.1±0.07; T95A, 0.6±0.03 μm2, p<0.001; Figure 3C) suggesting that the presence of a phosphorylatable Thr at this site is necessary for the ability of kalirin-7 to cause spine enlargement. Meanwhile, expression of kalirin-7 or kalirin-7-T95A increased the linear spine density (basal, 4.76±0.23; kalirin-7, 6.13±0.14; T95A, 6.89±0.54 spines/10 μm, p<0.001; Figure 3D), indicating that spine density is not affected by phosphorylation at this site. Importantly, overexpression of kalirin-7-T95A in neurons prevented their ability to undergo NMDAR-dependent spine enlargement (basal, 0.61±0.02; basal+T95A, 0.6±0.07; activated, 0.93±0.03; activated+T95A, 0.6±0.01 μm2, p<0.001; Figure 3E–F, S6E) but not spine density increases (Figure 3G). These experiments demonstrate that CaMKII interacts with kalirin-7 and enhances its GEF activity by phosphorylating it on T95, modulating the ability of kalirin-7 to induce activity-dependent spine enlargement. NMDAR activation caused a rapid translocation of kalirin-7 from dendritic shafts to nearby spines (Figure S2A) without affecting the relative distribution of kalirin-7 in dendrites vs. soma (Figure S2B). This was paralleled by an increase in phosphorylated PAK (P-PAK), a Rac1/Cdc42 substrate, in dendrites but not in soma (Figure S2C) demonstrating that the components of this signaling pathway are present in spines and are further enriched there upon induction of plasticity (Figure S2D). However, as a fraction of these proteins are present in the cell body as well (Figure S2B–C), there may also be a somatic contribution to the observed effects.
Figure 3. Identification of the phosphorylated site of kalirin-7.
(A) Kalirin-7 is directly phosphorylated by CaMKII, but kalirin mutant T95A is not; arrowhead: phospho-kalirin-7 band. Kalirin-T95A (T95A) was immunopurified and analyzed as in Figure 2G. (B) Thr-95 is a key regulatory site for ability of kalirin-7 to induce spine enlargement. Spine morphology was analyzed on neurons (div 28) transfected with plasmids expressing GFP alone, or together with wild-type kalirin-7 or kalirin-7-T95A. (C). Quantification of spine areas in (B), p<0.001. (D). Quantification of linear spine densities in (B): *p<0.05. (E) Thr-95 is key regulatory site for activity-dependent spine enlargement. Spine morphology was analyzed as in (B), in activated or untreated control neurons. (F). Quantification of spine areas in (E), p<0.001. (G) Quantification of spine densities in (E), p<0.05. Scale bars: 10 μm.
NMDA receptor activation-dependent Rac1 activation is mediated by CaMKII and kalirin-7
To examine the requirement for the association of kalirin-7 with the postsynaptic density (PSD) and for its targeting to spines, we developed a cell-permeant interfering peptide that mimics the C-terminus of kalirin-7 (Penzes et al., 2000). This peptide competes with endogenous kalirin-7 for interactions with PSD-95 and other PDZ proteins, interfering with its synaptic targeting and association with NMDAR complexes. Incubation of cultured neurons with fluorescein-tagged interfering peptide (K7 int), followed by washout of excess peptide, resulted in labeling of neurons, indicating that the peptide effectively entered the cells (Figure 4A). Immunostaining using a kalirin antibody revealed that in neurons treated with interfering peptide, the punctate staining of kalirin in synapses was eliminated, as compared to untreated control neurons (Figure 4A). Importantly, while the interfering peptide (but not a mutated control peptide, K7 mut) significantly depleted kalirin-7 from spines and dendrites, it did not affect localization of PSD-95, the protein mediating kalirin-7 targeting to spines (Figure 4B), or of NMDAR subunit 2A (NR2A), which binds to PSD-95 (Figure S3), confirming the effectiveness and specificity of interference with kalirin-7 spine targeting.
Figure 4. CaMKII and spine targeting are required for activity-dependent kalirin-7 phosphorylation and Rac1 activation.
(A) A kalirin-7 interfering peptide is able to penetrate neurons: neurons were incubated with a fluorescein-tagged kalirin-7 interfering peptide (K7 int), a control peptide (K7 mut), or left untreated. Retained peptide was detected based on its tag (fluo) or using a kalirin-7 antibody. (B) K7 int (arrowheads) but not K7 mut displaces kalirin-7 from spines. PSD-95 is not displaced (arrows). (C) Activity-dependent phosphorylation of kalirin-7 in neurons requires its association with the postsynaptic density (PSD) and active CaMKII. Neurons were left untreated or preincubated with K7 int, K7 mut, or KN-62, and subjected to APV withdrawal; Western blot with p-Thr and kalirin antibodies: quantification of normalized p-Thr integrated intensities, ***p<0.001. (D) and (E) Neurons were left untreated or were preincubated with K7 int, K7 mut (D), or KN-62 (E), and subjected to APV withdrawal; quantification of normalized integrated intensities of Rac1-GTP bands,***p<0.001. Scale bars: 10 μm.
We next used this interfering peptide to test whether activity-dependent phosphorylation of kalirin-7 required its association with the PSD and targeting to spines. Preincubation of neurons with the K7 int, but not with K7 mut, prevented NMDAR activity-dependent Thr-phosphorylation of kalirin-7. Similarly, KN-62 blocked NMDAR activity-dependent phosphorylation of kalirin-7 (p<0.001; Figure 4C). These results indicate that association of kalirin-7 with the PSD, as well as CaMKII activity, are necessary for activity-dependent Thr-phosphorylation of kalirin-7.
Activity-dependent and CaMKII-dependent phosphorylation of kalirin-7 may regulate its GEF activity, and activation of Rac1. To test this, we blocked NMDAR-induced activation of endogenous Rac1 in cultured neurons, using the interfering peptide and KN-62 (Figure 4D-E); NMDAR activity resulted in activation of Rac1, which was blocked by interfering peptide or KN-62, but not by the control peptide. Taken together, our data demonstrate that in cortical pyramidal neurons, CaMKII colocalizes and interacts with kalirin-7 in spines, phosphorylating it on a Thr residue in an activity-dependent manner, and that this interaction mediates NMDA receptor-dependent activation of Rac1.
Kalirin-7 expression and spine localization are required for maintenance of spine morphology
Kalirin-7 stimulates spine formation and maturation (Penzes et al. 2001, 2003). However, when we examined the expression of kalirin-7 in rat cerebral cortex forebrain during postnatal development, we found that while kalirin-7 is absent at postnatal day 2 (P2), or is expressed at very low levels at P8–P15, it was very abundant at P28, when most synapses have undergone maturation (Figure 5A). This suggests that kalirin-7 may play an important role in mature synapses, perhaps in their activity-dependent structural plasticity. To examine the dependence of mature spine morphology and maintenance on kalirin expression, we employed RNA interference (RNAi) to knock down the expression of endogenous kalirin in neurons. We tested the efficiency and specificity of pGsuper-RNAi (referred to as RNAi) in knocking down the expression of kalirin-7 in hEK293 cells (Figure S4A) and in neurons (Figure S4B–E). We then examined the effect of knocking down kalirin expression on the morphology of dendrites and spines on mature cortical neurons (div 28). Neurons subjected to RNAi for 5 days had significantly fewer and smaller spines compared to neurons expressing pGsuper plasmid alone (Figure 5B). To further confirm knockdown specificity, we transfected neurons with RNAi together with a rescue plasmid, in which 2 silent point mutations have been introduced in the kalirin sequence targeted by siRNA, as well as with a mutated RNAi plasmid. While RNAi caused a significant loss of spines, coexpression of RNAi with rescue plasmid, or expression of mutated RNAi had no effect (Figure 5B, C). To determine whether a partial knockdown of kalirin, achieved by a shorter treatment with RNAi, resulted in a less dramatic effect on spines, we treated neurons with RNAi for 3 or 5 days (Figure 5D). While kalirin knockdown for 5 days resulted in significant loss of spines (p<0.001), knockdown for 3 days had a much more moderate effect, consisting of unaffected spine densities and moderately reduced spine areas, likely caused by partial reduction of kalirin levels (Figure 5E–F, S6F). Importantly, kalirin knockdown did not affect NMDAR function and localization to spines, as indicated by the persistent NMDAR activity-dependent phosphorylation of CaMKII (Figure S5B) and of NR1 clusters (Figure S5C) in spines in the presence of RNAi.
Figure 5. Kalirin-7 expression and spine localization are required for maintenance of mature spine morphology.

(A) Expression of kalirin-7 in rat cerebral cortex during postnatal development. (B) Effects of kalirin knockdown on spine number in cortical pyramidal neurons. Neurons (div 24) were transfected with empty vector pGsuper (basal), pGsuper-RNAi (RNAi), pGsuper-RNAi together with a rescue plasmid (RNAi+rescue K7), and a mutated RNAi with 2 point mutations, for 5 days. (C) Quantification of spine linear densities in (B), ***p<0.00. (D) Time-dependent (3–5 days) effect of kalirin knockdown on spine areas and linear density. (E) Quantification of spine linear densities in (D), ***p<0.001. (F) Quantification of spine areas in (D), p<0.05. Quantification of K7 int and K7 mut on the areas of dendritic spines after incubation for 2 hours (G) and 2 days (H). p<0.05. Scale bar: 10 μm.
To determine whether displacing kalirin-7 from spines affected spine morphology, we incubated neurons expressing GFP with K7 int and K7 mut for 2 hours or 2 days. Peptide incubation for 2 hrs did not significantly affect spine areas (basal, 0.78±0.04; K7 mut, 0.81±0.04; K7 int, 0.82±0.05 μm2) but had a modest effect on linear spine densities (basal, 6.12±0.38; K7 mut, 6.11±0.35; K7 int, 4.88±0.19 spines/10 μm, p<0.05; Figure. 5G, S6G), suggesting that it did not interfere with short-term spine maintenance. However, incubation with interfering peptide for 2 days resulted in a reduction in spine sizes and number (area: basal, 0.78±0.04; K7 mut, 0.77±0.03; K7 int, 0.63±0.04 μm2, p<0.05; spine density: basal, 6.12±0.38; K7 mut, 6.38±0.42; K7 int, 3.51±0.45 spines/10 μm; p<0.05; Figure 5H, S6H). These experiments demonstrate that kalirin expression is required for spine maintenance in a dosage-dependent manner, and that its localization to spines is required for the long-term maintenance of spines.
Kalirin-7 interacts with AMPA receptors, controls their maintenance in spines, and modulates AMPA receptor-mediated synaptic transmission
The structure, stability, and function of excitatory synapses are thought to be coordinately regulated in plasticity, in that activity-dependent spine enlargement parallels synapse stabilization and functional strengthening of synapses caused by increased AMPAR levels at the postsynaptic membrane (Kasai et al., 2003; Luscher et al., 2000; Matsuzaki et al., 2004). This suggest that mechanisms that control the remodeling of actin in spines not only underlie spine enlargement and stabilization, but may also contribute to the synaptic trafficking and stabilization of glutamate receptors (Allison et al., 1998). Consistently, actin polymerization is required for the maintenance of LTP and memory (Fukazawa et al., 2003). Moreover, Rac1, a central regulator of actin dynamics, also regulates AMPAR clustering during synapse maturation (Wiens et al., 2005). Hence kalirin-7, as an upstream activator of Rac1 and actin remodeling, may control spine structure and synaptic stability of receptors. Because GluR1-containing AMPARs are required for potentiation (Kopec et al., 2006; Shi et al., 1999; Song and Huganir, 2002), we examined their interaction with kalirin-7. We found that in cultured neurons kalirin-7 and GluR1 immunoreactivities colocalized in spine heads (Figure 6A). In addition, kalirin-7 coimmunoprecipitated with GluR1 from rat forebrain homogenates (Figure 6B). These results place kalirin-7 in proximity of GluR1, supporting a role for kalirin-7 in the regulation of GluR1-containing AMPARs in spines.
Figure 6. Kalirin-7 controls AMPA receptor maintenance in spines.
(A) Kalirin-7 colocalizes with GluR1 in spines of pyramidal neurons (div 28). (B) Kalirin-7 coimmunoprecipitates with GluR1 from rat forebrain homogenate, while control IgG does not. (C) Effect of kalirin knockdown (3–5 days) on the maintenance of GluR1 clusters, quantified in (D), *p<0.001. (E) Partial knockdown of kalirin (3 days) causes altered localization of GluR1 clusters; in RNAi-treated neurons GluR1 clusters are often in spine neck/base (arrowheads). (F) Line scans of GluR1 immunofluorescence across representative spines and the adjacent dendritic shaft in basal (top) and RNAi-treated (bottom) neurons. (G) Effect of kalirin knockdown (5 days) on AMPAR-mediated basal synaptic transmission. Traces show representative recordings of AMPAR-mediated mEPSCs. (H) Quantification of mEPSC frequencies and amplitudes in (G), *p<0.05, **p<0.005. (I) Elevated GluR1 spine expression induced by kalirin-7 overexpression (2 days) is blocked by actin polymerization inhibitor latrunculin A (LAT-A, 5 μM, 24 hr). (J) Integrated intensities of GluR1 immunofluorescence in the dendritic spine and dendritic shaft were quantified and the spine/shaft ratios were plotted. Dashed lines: dendritic shafts; arrows: GluR1 cluster in the shaft, arrowheads: GluR1 cluster in spines; ***p<0.001. Scale bars: 10 μm (A, C, I); 1 μm (E, F).
To test whether kalirin-7 was required for the maintenance of GluR1 clusters at synapses, we examined the effect of knocking down kalirin expression on GluR1 content in spines (Figure 6C–F). Knockdown for 5 days resulted in dramatically reduced GluR1 cluster intensities (p<0.001). On the other hand, a shorter knockdown for 3 days resulted in formation of thinner spines, with only slightly lower GluR1 content (p>0.05; Figure 6C, D). Interestingly, while in control neurons GluR1 clusters were mainly in the spine head, in neurons subjected to RNAi for 3 days GluR1 clusters were in the spine neck or at the base of the spines (Figure 6C, E). These experiments provide evidence that kalirin-7 is in proximity of GluR1-containg AMPARs and regulates their content in spines. Indeed, while in control neurons GluR1 clusters are enriched in the spine head, in neurons where expression of kalirin has been knocked down GluR1 is partially removed from spine heads, as shown by line scans of GluR1 immunostaining intensities across the spine head and adjacent to the shafts (Figure 6F).
By regulating AMPAR content in spines, kalirin may control basal synaptic transmission. To test this possibility, we measured the effect of knocking down kalirin expression on AMPAR-mediated synaptic transmission. In cultured neurons, knockdown of kalirin (for 5 days) resulted in significantly diminished AMPAR-mediated basal synaptic transmission, as indicated by reduced frequency (basal, 4.63±1.07/sec; 5 days RNAi 1.74±0.43/sec; p<0.05) and amplitudes (basal, 18.21±1.52; 5 days RNAi, 11.39±0.61 pA; p<0.01) of AMPAR-mediated mEPSCs (Figure 6G, H). Altogether, these data provide evidence that kalirin controls maintenance of AMPARs at the synapse and AMPAR-mediated synaptic transmission.
To investigate the downstream mechanisms linking kalirin-7 activity with AMPAR surface expression, we examined the role of actin polymerization. Actin cytoskeleton in spines is crucial for the maintenance and dynamics of both spine structure (Fischer et al., 1998) and synaptic glutamate receptors (Allison et al., 1998). A likely hypothesis is that kalirin-7 functions in spines by activating Rac and thereby controlling actin cytoskeletal dynamics in spines. To test this, we transfected neurons with kalirin-7 for one day, then incubated trasfected or untransfected neurons with 5 μM latrunculin A (LATA-A; an inhibitor of actin polymerization) or vehicle further for one day. We found that actin polymerization was required for kalirin-dependent spine enlargement and enhanced GluR1 synaptic expression. As expected, actin polymerization was also necessary for basal maintenance of spine structure and synaptic GluR1 clustering (Ratios of spine/shaft GluR1: basal, 1.58±0.34; LAT-A, 0.56±0.10; kalirin-7, 3.61±0.45; kalirin-7+ LAT-A,1.83±0.26; p<0.001; Figure 6I–J). These experiments strongly suggest that kalirin-7 promotes spine enlargement and GluR1 enrichment in spines by enhancing actin polymerization.
CaMKII activity and kalirin spine targeting are required for activity-dependent synaptic delivery of AMPA receptors
Activity-dependent strengthening of excitatory synapses entails the addition of GluR1-containing AMPARs to synapses, resulting in a net increase in AMPAR content in spines and enhanced AMPA-mediated synaptic transmission, which is associated with spine enlargement (Kopec et al., 2006; Matsuzaki et al., 2004; Shi et al., 1999). These coordinated changes in spine structure and function have been observed in a diverse range of experimental systems and are thought to underlie activity-dependent synapse maturation, experience-dependent plasticity, and information storage. Activity-dependent changes in synapse structure and function rely in part on rearrangements of the actin cytoskeleton, hence actin and its regulators are likely to play key roles in activity-dependent plasticity. Indeed, LTP-induced accumulation of actin in spines is required for LTP in vivo (Fukazawa et al., 2003), and mutations in several genes encoding components of the Rho-like GTPase signaling pathways are associated with mental retardation and defects in spine morphology in humans (Ramakers, 2002). Regulation of Rac1 and actin by the NMDAR-CaMKII-kalirin-7 pathway may therefore play a key role in activity-dependent spine enlargement and AMPAR delivery.
To test this model, we first determined whether NMDAR activity-dependent delivery of GluR1-containing AMPARs to spines was dependent on CaMKII activity. We therefore activated NMDARs in neurons expressing GFP in the absence or presence of the CaMKII inhibitor KN-62 (Figure 7A). Quantification of the changes of GluR1 content in spines revealed that preincubation with KN-62 blocked the NMDAR activation-induced enhancement of GluR1 content in spines (activated, 2.4±0.58; activated+KN-62, 0.74±0.04; p<0.001; Figure 7B). This indicates that CaMKII activity is required for GluR1 delivery to spines in cultured neurons, similarly with hippocampal preparations (Hayashi et al., 2000).
Figure 7. NMDAR-dependent spine delivery of AMPA receptors depends on CaMKII and kalirin spine targeting.
(A) Preincubation of neurons with KN-62 (activated+KN-62) blocks activity-induced (activated) delivery of GluR1 to spines. (B) Quantification of average integrated GluR1 cluster immunofluorescence intensities, normalized to basal, ***p<0.001. (C) Interfering peptide K7 int blocks the increase in GluR1 content in spine heads following activation of NMDARs, while control K7 mut peptide does not. (D) Quantification of GluR1 cluster integrated intensities (C), **p<0.005. (E) NMDAR activation increases cell-surface GluR1; incubation with K7 int interferes with the activity-dependent increase of surface GluR1 expression, K7 mut does not. Total amount of GluR1 expressed on cell surface was determined by cross-linking surface receptors. (F) Quantification of normalized GluR1 surface expression in cortical neurons, *p<0.05. (G) Quantification of spine areas in (F), p<0.001. Scale bars, 10 μm.
To determine whether displacement of kalirin-7 from spines affected spine GluR1 content, we preincubated neurons expressing GFP with or without K7 int or K7 mut peptides, and activated NMDARs for 30 min (Figure 7C). Quantification of GluR1 immunofluorescence in spines revealed that K7 int blocked the increase in spine GluR1 following activation of NMDARs, while K7 mut did not (activated+K7 mut, 2.6±0.28; activated+K7 int, 1.1±0.36; p<0.005; Figure 7D). Quantification of the effect of interfering and control peptides on activity-dependent spine enlargement, as visualized by GFP, revealed that in the presence of K7 mut peptide, spine areas increased as in the absence of peptide, whereas K7 int peptide inhibited this increase (basal, 0.72±0.04; activated, 0.98±0.4; activated+K7 mut, 0.97±0.06; activated+K7 int, 0.68±0.04 μm2; p<0.001; Figure 7G, S6I).
We used an additional, biochemical method to crosslink surface-only GluR1 (Boudreau & Wolf, 2005) to test whether interference with kalirin-7 localization to PSDs affected activity-dependent enhancement of GluR1 expression on the neuronal cell surface. Western blotting with a GluR1 antibody revealed that as expected, NMDAR activation increased cell-surface GluR1; this activity-dependent increase of surface GluR1 expression was abolished by preincubation with K7 int but not with K7 mut (p<0.05; Figure 7E, F).
Kalirin is required for activity-dependent enhancement of AMPA receptor-mediated synaptic transmission
To determine whether kalirin is required for the activity-dependent increase in GluR1 content in spines, we knocked down kalirin expression in neurons. Because dramatic alterations of dendritic morphology caused by full knockdown of kalirin may indirectly affect AMPAR content in spines, we took advantage of the dosage-dependent effect of kalirin knockdown on spine morphology and GluR1 content. Knockdown of kalirin-7 for 3 days, while having small non-significant effects on spine size and basal GluR1 content in spines (Figures 5, 6), blocked APV withdrawal-induced spine enlargement (pGsuper basal, 0.77±0.05; pGsuper activated, 1.28±0.09; RNAi basal, 0.70±0.06; RNAi activated 0.75±0.05 μm2. p<0.001) and GluR1 delivery to spines (pGsuper basal, 1.00±0.12; pGsuper activated, 1.92±0.18; RNAi basal, 0.94±0.17; RNAi activated 1.03±0.13. p<0.001; Figure 8A–B, S6J).
Figure 8. NMDAR-dependent spine enlargement and enhancement of AMPA receptor-mediated synaptic transmission depend on kalirin expression.
(A) Partial kalirin knockdown (3 days) prevents NMDAR activation-induced spine enlargement and GluR1 spine delivery (RNAi activated), compared to neurons expressing control plasmid and subjected to APV withdrawal (pGsuper activated); basal spine morphologies and GluR1 cluster intensities were similar in unstimulated neurons expressing control plasmid (pGsuper basal) or RNAi (RNAi basal). (B) Quantification of spine areas and intensities in (A), ***p<0.001. (C) Effect of kalirin knockdown (3 days) on NMDAR activation-dependent enhancement of AMPAR-mediated synaptic transmission. Traces show representative recordings of AMPAR-mediated mEPSCs. (D) Quantification of AMPA mEPSC frequencies and amplitudes in (C). (E) Model of the regulation of activity-dependent synapse structural and functional plasticity by the NMDAR/CaMKII/kalirin-7/Rac1 pathway. Scale bars, 10 μm.
Activity-dependent synaptic delivery of AMPARs contributes to the enhancement of synaptic transmission during potentiation. To determine whether kalirin was required for activity-dependent enhancement of synaptic transmission, we examined the effect of knocking down kalirin expression on APV-induced changes in AMPA-mediated mEPSCs in cortical pyramidal neurons. While NMDAR activation caused an increase in average AMPAR-mediated mEPSC frequency (Figure 1C), partial knockdown of kalirin expression prevented this increase (RNAi basal, 4.09±0.60/sec; RNAi activated 4.01±1.01/sec; p=0.95; Figure 8C–D). This result demonstrates that normal levels of kalirin expression are required for NMDAR activity-dependent enhancement of AMPA-mediated synaptic transmission. Together with kalirin’s role in the maintenance of spines and AMPAR clusters at synapses, these data provide evidence that kalirin controls activity-induced rapid enhancement of spine size and AMPAR content, mechanisms that underlie the coordinated structural and functional strengthening of synapses.
Discussion
Here we provide evidence that in mature pyramidal neurons, a signal transduction pathway initiated by plasticity-inducing activation of NMDARs, causes activation and autophosphorylation of CaMKII, resulting in the phosphorylation of kalirin-7 anchored to the PSD in spines (Figure 8E). CaMKII interacts with kalirin-7, phosphorylates it on Thr 95, and modulates the ability of kalirin-7 to induce spine enlargement. This indicates that the presence of a phosphorylatable Thr at this site is necessary for the ability of kalirin-7 to regulate spine morphogenesis, also revealing an important regulatory mechanism of kalirin. This interaction of kalirin-7 with CaMKII mediates NMDAR-dependent activation of Rac1, which in turn causes the rapid enlargement of spines heads that underlies activity-dependent synaptic structural plasticity.
Unexpectedly, kalirin-7 also associates with GluR1-containing AMPRs, controls their synaptic expression and maintenance, and thereby modulates AMPAR-mediated synaptic transmission. Kalirin interacts with several PDZ domain-containing proteins, such as PSD-95, SAP97, and SAP-102 (Penzes et al., 2001), that also interact directly or indirectly with GluR1 and may mediate this association. Importantly, in mature synapses, CaMKII and kalirin-7 are required for activity-dependent spine enlargement and enhancement of AMPA-mediated synaptic transmission. This study therefore identifies an essential link between the mechanisms necessary for induction of activity-dependent rapid structural and functional plasticity of existing synapses, and the molecular machinery that regulates postsynaptic actin dynamics that underlie spine morphogenesis, receptor trafficking, and receptor anchoring at the synapse.
Because all components of this signaling pathway are enriched in spines, our methods of stimulating NMDARs act mainly at synapses (Liao et al., 2001; Lu et al., 2001), and because NMDAR activation causes the translocation of CaMKII (Liao et al., 2001), and kalirin from dendrite shafts into nearby spines, leading to predominant phosphorylation of the Rac-target PAK in spines, it is very likely that a large fraction of these effects take place in dendrites and spines. However, because a fraction of CaMKII and kalirin-7 are present in the soma, they may also contribute to the observed effects.
Kalirin-7 may fulfill several unique functions in the propagation of the signal from NMDARs to actin. First, it may provide an amplification step in the activity-dependent control of actin dynamics: since kalirin-7 is an enzyme, only a few kalirin molecules may be required to activate multiple Rac1 molecules, which would then lead to more extensive actin rearrangements than those caused by direct stoichiometric interactions of actin filaments with glutamate receptors. Second, a multiprotein complex containing NMDARs, CaMKII, and kalirin-7 would provide signal channeling, achieved here through spatial localization of the signaling cascade, resulting in an increased signaling specificity and temporal efficiency. Third, since kalirin-7 is a multidomain protein, which is also regulated by ephrinB/EphB signaling (Penzes et al., 2003), cadherins, and fyn (unpublished data), kalirin-7 may allow for the integration of multiple signaling inputs to regulate spine morphology, providing a higher degree of control than separate parallel pathways would. Fourth, because kalirin-7 controls both spine structure and synaptic function, it may provide a key link in the coordinated activity-dependent regulation of spine structure and function. Fifth, because postsynaptic morphology may also affect presynaptic function through trans-synaptic signaling molecules, including the ephrinB/EphB system, kalirin-7 may indirectly modulate presynaptic function, as suggested by the effects of kalirin knockdown on AMPA-mediated mEPSC frequencies.
The expression of kalirin-7 is highest in neurons with mature synapses, and low (although detectable) in immature developing neurons, suggesting that it plays an important functional role in mature synapses. This further supports a crucial role for kalirin-7 in the activity-dependent structural and functional plasticity of mature synapses. In developing neurons kalirin, together with other GEFs such as Tiam1 (Tolias et al., 2005), may control activity-dependent dendrite development and synapse maturation. Tiam1 appears to mediate the activity-dependent increase in spine density during synapse development and maturation (Tolias et al., 2005), while kalirin-7 affects primarily activity-dependent increases in spine sizes. In addition, the fact that Tiam1 knockdown affects the soma size and the complexity of the dendritic tree, suggests a more general function for Tiam1 in neuronal morphology. Our results, together with the established important roles of the upstream regulators of kalirin-7 (NMDARs and CaMKII) and its targets (Rac1 and actin) in plasticity, strongly suggest that kalirin-7 may be an important regulator of the experience-dependent modifications of forebrain circuits during postnatal development, and may play an important role in learning and memory. Future studies using gene targeted animals will establish the role of kalirin in these processes in vivo.
Our RNAi experiments suggest a selective dependence of spine and GluR1 plasticity vs. maintenance processes on the duration of reduced kalirin levels. This is likely due to the time required for the disassembly of structural components of spines, the need for somatic supply of kalirin for spine maintenance but not plasticity (3 and 5-day RNAi affect kalirin levels comparably in dendrites, but not soma, Figure S4C–D), and to potential parallel mechanisms that may maintain spines for short but not long periods of reduced kalirin.
Although structural changes in spiny synapses, associated with various physiological conditions such as various forms of training, stress, social contexts, or diseases like mental retardation or schizophrenia, have been described for decades, it has only recently become clear that spine structural plasticity is intimately associated with synaptic functional plasticity (Kasai et al, 2003; Kopec et al, 2006). Indeed, stimuli that induce synaptic plasticity in mature synapses, such as high-frequency stimulation (Engert & Bonhoeffer, 1999; Maletic-Savatic et al., 1999; Toni et al., 1999), glutamate uncaging (Matsuzaki et al, 2004), or chemical LTP (Kopec et al., 2006), also cause rapid enlargement of existing spines. In these studies, activity-dependent spine modifications occurred on a range of time scales, but in general spines become enlarged rapidly, within 5 min, and remained larger than the initial sizes for extended periods (40–80 min) (Kopec et al., 2006; Maletic-Savatic et al., 1999; Matsuzaki et al. 2004; Park et al., 2006) or reverted to the initial sizes (Lang et al., 2004). It is most likely that these discrepancies are due to the different experimental conditions. However, the time course and mechanisms of spine plasticity in cortical neurons are not well understood. In this study we find that in dissociated cortical pyramidal neurons, NMDAR activation-dependent enlargement of dendritic spines occurs within 5–30 min, and requires the activation of CaMKII. Spine plasticity in cortical neurons occurs with a similar time course as spine structural changes in slice preparations, indicating similar phenomenology and mechanisms. Moreover, spine structural plasticity in cortical neurons occurs with similar time courses as the synaptic functional changes in cortical and in hippocampal neurons (Kasai et al., 2003; Kopec et al., 2006), indicating that structural and functional plasticity may be mechanistically linked.
Structural and functional plasticity plays an important role in many neuronal types, including hippocampal, cortical, striatal, nucleus accumbens, and cerebellar neurons. In this study we focus on cortical pyramidal neurons, which share many physiological and morphological properties with hippocampal pyramidal neurons, including LTP and experience-dependent spine structural and functional plasticity (Takahashi et al., 2003; Connor et al., 2006). Spine plasticity has been extensively examined in the rodent cortex in vivo, and in various neurodevelopmental and psychiatric diseases and disease models, and is highly relevant for the pathophysiology of these diseases. We expect that mechanisms similar to those described here govern plasticity in subpopulations of pyramidal neurons in the forebrain.
Activity-dependent rapid spine enlargement parallels the enhancement of AMPAR synaptic content and AMPAR-dependent synaptic transmission, suggesting that the structure and function of excitatory synapses are coordinately regulated during plasticity (Kasai et al., 2003; Luscher et al., 2000; Matsuzaki et al., 2004). However, the molecular mechanisms that accomplish the coordinated regulation of synaptic functional and structural modifications during plasticity have not been uncovered. Because actin polymerization is required for the maintenance of LTP and memory (Fukazawa et al., 2003), one likely possibility is that mechanisms that control actin remodeling in spines, such as those involving kalirin-7, may also contribute to the synaptic trafficking and stabilization of glutamate receptors (Allison et al., 1998). Indeed, here we show that kalirin also controls the maintenance of AMPARs at the synapse, is crucial for the maintenance of basal AMPAR-mediated synaptic transmission, and is required in spines for activity-dependent enhancement of AMPAR-mediated synaptic transmission.
Interestingly, we found that kalirin participates in a protein complex with GluR1. The association of kalirin with GluR1 is most likely mediated by scaffolding proteins such as SAP97 or PSD-95, which interact with both proteins, but may also be direct. In this manner, kalirin-7 is in proximity of GluR1-containing cellular membranes. These effects of kalirin on spine morphology and AMPARs depend on actin polymerization, since they are prevented by the actin polymerization inhibitor latrunculin A. Kalirin-7 may hence regulate AMPAR maintenance in spines by regulating the actin cytoskeleton in spines, to which AMPARs are anchored (Allison et al., 1998).
Altered morphologies of spiny synapses have been associated with mental retardation, neuropsychiatric disorders, and drug addiction (Fiala et al, 2002). Specifically, aberrant spine morphology in forebrain occurs in many types of mental retardation (Kaufman & Moser, 2000), including fragile-X (Irwin et al, 2000) and autism spectrum disorders (Pickett & London, 2005). Rho-like small GTPases (including Rac1) are central regulators of the development and morphology of spines (Tashiro et al, 2000), and their essential role in regulating spine morphological plasticity is supported by the fact that many types of non-syndromic mental retardation, associated with altered spine morphogenesis, are caused by mutations in genes encoding proteins in the Rho GTPases signaling pathways (Ramakers, 2002). These disorders affect human cognitive abilities, suggesting a link between dendritic spine structural plasticity, Rho GTPase signaling, and cognition. Defects in postsynaptic signaling mediated by kalirin may also contribute to psychiatric disorders. Postmortem studies on schizophrenic subjects reported morphological alterations in dendritic spines in the cerebral cortex (Glantz & Lewis, 2001). Kalirin may play an important role in this spine pathology, because it interacts with the schizophrenia candidate protein DISC1 (Disrupted-In-Schizophrenia-1) (Millar et al., 2003), and the expression of kalirin (also called Duo) is specifically reduced in prefrontal cortex in postmortem subjects with schizophrenia (Hill et al., 2006). Therefore our results may suggest potential strategies for treatments of these neurodevelopmental and psychiatric diseases.
Experimental Procedures
Reagents and plasmid constructs
The plasmids encoding myc-kalirin-7 and myc-kalirin-5 were described previously (Penzes et al., 2001). The myc-kalirin-7-T95A mutant was generated by site-directed mutagenesis using the QuickChange II XL mutagenesis kit (Stratagene). The sequence of the forward mutagenesis primer was: 5′-GTTTGCAAACGTGGCTTCGCCGTCATCATCGACATGCGGGGC-3′. The following plasmids were also used: CaMKIIα-T286D (CaMKII-CA) and CaMKIIα, were kind gifts from Dr. Tobias Meyer (Stanford University); pEGFP-N2; myc-DH-PH; FLAG-spectrin. Polyclonal antibodies recognizing kalirin were described previously (Penzes et al., 2001); antibodies to GluR1 and NR2A were generous gifts from Dr. Richard L. Huganir (Johns Hopkins University). The following antibodies were purchased: CaMKIIα monoclonal, PSD-95 monoclonal, GluR1 monoclonal, GFP monoclonal (Chemicon); myc monoclonal (University of Iowa Hybridoma Bank, Santa Cruz); p-Thr polyclonal (Invitrogen); Tiam-1 monoclonal (Calbiochem); Rac1 monoclonal and p-CaMKIIα/β polyclonal (Upstate); IgG polyclonal (Sigma). Latrunculin-A was a gift from Dr. Anis Contractor (Northwestern University).
Neuronal Treatments
For APV withdraw, neuron cultures were maintained in presence of 200 μM D,L-APV. Cells were pre-incubated in ACSF (in mM: 125 NaCl, 2.5 KCl, 26.2 NaHCO3, 1 NaH2PO4, 11 glucose, 5 Hepes, 2.5 CaCl2 and 1.25 MgCl2) with 200 μM APV for 30 min at 37°C. Coverslips were then washed in ACSF and transferred into treatment medium (ACSF without MgCl2, plus 10 μM glycine, 100 μm picrotoxin and 1 μM strychnine) (Liao et al, 1999). For neurons cultured in dishes, ACSF+APV medium was removed, washed with ACSF, and then incubated in treatment medium. For peptide treatments, cells were incubated with 10 μm of either K7 int or K7 mut for 2 hr, followed by a wash, and incubated in culture medium overnight before treatment or being fixed. Although the examined synaptic PDZ proteins and ligands are not affected by the peptide (Figure S3), it can not be completely ruled out that other PDZ-mediated interactions may have also been affected. Experiments with KN-62; cells were transferred to ACSF+APV, KN-62 (10 μM) was added for 90 min. Cells were then treated as above in the presence of KN-62. All treatments were allowed to proceed for the indicated times; cells were then immediately fixed, or used for biochemistry.
Quantitative Analysis of Spine Morphologies
Confocal images of single- and double-stained neurons were obtained with a Ziess LSM5 Pascal confocal microscope. To determine the lateral resolution of our confocal microscope, we imaged fluorescent microspheres (Duke Scientific) of known diameters, mounted in ProLong antifade solution, using the 63x oil-immersion objective (NA = 1.4). Quantification indicated that the lateral point spread function was smaller than 0.5 μm, and that areas equal to or larger than 0.21 μm2 were accurately measured. Therefore we did not include structures in our analysis that had an area of less than 0.25 μm2, as these structures are approaching the limit of our microscope’s resolution. It should also be noted that measurements of small spine features, such as thin spine necks, could be overestimated in our measurements. An anti-GFP antibody was used to circumvent potential unevenness of GFP diffusion in spines. Images of neurons were taken using the 63x oil-immersion objective as z-series of 3–8 images, averaged 4 times, taken at 0.37 μm intervals, 1024×1024 pixel resolution at a scan speed of 8 s per section. The acquisition parameters were kept the same for all scans. Two-dimensional maximum projection reconstructions of images, morphometric analysis and quantification were done using MetaMorph software (Universal Imaging). Synapses formed onto healthy neurons with pyramidal morphologies were imaged as described above. Cultures that were directly compared were stained simultaneously and imaged with the same acquisition parameters. For each condition, 6–12 neurons each from at least two separate experiments were used, and two dendrites from each neuron were analyzed. Experiments were done blind to conditions and on sister cultures. To examine the morphologies of dendritic spines, individual spines on dendrites were manually traced, and the area, maximum length and head width of each spine was measured by MetaMorph. Student’s unpaired t tests were used to determine the statistical significance of differences between two groups; one-way ANOVA were used to compare three or more groups, followed by Tukey-b post hoc for multiple comparisons. Statistical analysis were performed in Excel and SPSS. Cumulative plots were analyzed using Kolmogorov-Smirnov test (K-S test).
RNAi and mutagenesis
To deliver siRNA (small interfering RNA) molecules, we used the plasmid pGsuper, derived from “pSuper” which expresses siRNA and EGFP simultaneously (Elbashir et al., 2001; Kojima et al., 2004). Several gene-specific inserts were designed using the BLOCK-iT software (Invitrogen) to encode 21-nucleotide sequences derived from the target transcript, separated by spacer loops of 9 nucleotides, and followed by the reverse complement sequence of the target sequence. These siRNAs were unique to the kalirin gene and were not targeting any other sequences in the database.
Electrophysiology
Transfected neurons expressing GFP were identified by fluorescence before recording. The external solution contained ACSF with 1.25 MgCl2 and 200 μM APV, or with 10 μM glycine, pH 7.3, 320–330 mOsm/L. Miniature AMPA-mediated excitatory postsynaptic currents (mEPSCs) were measured from whole-cell voltage patch-clamp recordings with a gap-free model using Pulse 8.4 software data acquire system (HEKA, Germany). Signals were low-pass filtered at 1 kHz, sampled at 10 kHz, and amplified with an axopatch 200B amplifier (Axon Instruments). EPSCs were recorded at a holding potential of −60 mV in the presence of tetrodotoxin (1 μM), picrotoxin (100 μM) and strychnine (1 μM) at room temperature. Patch pipettes were pulled from borosilicate glass and had a resistance of approximately 3–5 M. Internal pipette skolution contained the following (in mM): CsMeSO3 120; NaCl 5; TEA-Cl 10; HEPES 10; QX314 5; EGTA 1.1; ATP-Mg2 4; GTP-Na2 0.3; pH 7.2 adjusted with CsOH; 270–280 mOsm/L. Electrophysiological signals were analyzed using Clampfit 9.2 (Axon Instruments) and Mini Analysis Program 6.0.3 (Synaptosoft).
Supplementary Material
See more in “supplemental methods”.
Acknowledgments
We thank Dr. Richard L. Huganir (Johns Hopkins Univ.) for the GluR1 and NR2A polyclonal antibodies, Dr. Dane Chetkovich and Ms. Emma Hayes (Northwestern Univ.) for assistance with phosphorylation assays, Dr. Tobias Meyer (Stanford Univ.) for CaMKII expression plasmids; Dr. Gary G. Borisy and Dr. Shin-ichiro Kojima (Northwestern Univ.) for the pGsuper plasmid and assistance with RNAi; Dr. Gordon Shepherd for reading the manuscript (Northwestern Univ.); Dr. Anis Contractor (Northwestern Univ.) and Dr. Suzanne Zukin (Albert Einstein College of Medicine) for useful discussions, Dr. David Wokosin, and Dr. Jaime Grutzendler (Northwestern Univ.) for advice on image quantification; Dr. Jack Waters (Northwestern Univ.) for help with confocal measurements; and Ms. Kelly Jones for careful editing. This work was supported by the NIH-NIMH (MH071316), NAAR, NARSAD (to P.P.), and NIH-NINDS (to D.J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor S, Williams PT, Armstrong B, Petit TL, Ivanco TL, Weeks AC. Long-term potentiation is associated with changes in synaptic ultrastructure in the rat neocortex. Synapse. 2006;59:378–382. doi: 10.1002/syn.20248. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the -calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001;58:203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. Requirement for α-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer J, Malinow R. Driving AMPA Receptors into Synapses by LTP and CaMKII: Requirement for GluR1 and PDZ Domain Interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kojima S, Vignjevic D, Borisy GG. Improved silencing vector co-expressing GFP and small hairpin RNA. Biotechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc Natl Acad Sci U S A. 2004;101:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Zhang X, O’Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Christie S, Porteous DJ. Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochem Biophys Res Commun. 2003;311:1019–1025. doi: 10.1016/j.bbrc.2003.10.101. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acid Research. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Pickett J, London E. The neuropathology of autism: a review. J Neuropathol Exp Neurol. 2005;64:925–935. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci. 2005;25:10627–10636. doi: 10.1523/JNEUROSCI.1947-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–661. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See more in “supplemental methods”.