Abstract
Objective
To evaluate uterine development of women with Turner syndrome (TS) receiving conventional medical care.
Study design
In a cross sectional study we used ultrasound for uterine evaluation in 86 women with TS aged 18–45 years participating in an intramural NIH study, and who had abnormal karyotypes in >70% of white blood cells. Outcomes were uterine dimensions and shape. Information on hormone treatment was obtained by personal interview.
Results
Twenty five percent had a mature in size and shape uterus, and 31% had an immature uterus, with the remainder in a transitional category. Twenty percent of all participants were not taking hormone replacement (HRT) in the preceding year. The majority on treatment were taking conjugated estrogens or oral contraceptives. Factors associated with uterine maturity were history of spontaneous puberty, and duration and type of HRT, with estradiol based treatment being the most effective. The age at starting HRT was not a critical factor.
Conclusions
Women with TS may develop a normal uterus even at a late start of HRT given adequate duration of treatment and regardless of karyotype.
Keywords: Turner syndrome, uterine development, estrogen replacement, ultrasound
Turner syndrome (TS) is defined as deficiency of all or part of the second sex chromosome in phenotypic females and is relatively common, affecting ~1/2500 female births (1). Premature ovarian failure affects nearly 95% of girls and women with this disorder, who usually require exogenous estrogen treatment to induce puberty and maintain feminization and bone health throughout the adult years (2). Concerns about the timing of pubertal induction have related mostly to optimization of statural growth in response to pharmacological growth hormone treatment, because a delay in the age of estrogenization allows a longer period of longitudinal bone growth (3, 4). However, several European studies have suggested that conventional pubertal induction does not produce optimal development of the uterus in TS(5–7).
It is not clear from these studies whether impaired uterine development was due to delayed estrogen treatment, too low dosage, or perhaps the use of progestins with androgenic properties. There does not seem to be any inherent defect in uterine capacity in TS, because development is normal in girls with TS with spontaneous puberty (5, 8). At this time we do not have complete information on the requirements for normal uterine growth. It is not known whether there is a ‘window’ of developmental time during childhood or adolescence, when uterine growth and maturation must occur to achieve sufficient maturity for pregnancy, or whether uterine development can be induced at a later age with sufficiently high, or sufficiently long estrogen treatment. In the present study, we addressed the pattern and type of ovarian hormone replacement and karyotype as factors involved in uterine development in adult women ages 18–40 years of age with TS.
Methods
Study subjects
In a cross-sectional study conducted between January, 2002 and June, 2006, we evaluated the uterine development of 86 women with TS, age 18 to 45, who were participants in an ongoing comprehensive intramural natural history study on TS. They were recruited through NIH web site ads. The study was NICHD IRB approved and all participants signed informed consents. In order to qualify for the study the participants had to have a karyotype by G-banding consistent with TS in over 70% of 50 white blood cells.
Methods
Evaluation included imaging of the uterus and gonads by transabdominal ultrasound in 68, and by transvaginal ultrasound in 20 of the participants. High-resolution gray scale sonography was performed on an Accuson Sequoia scanner (Accuson, Mountain View, CA) or on an ATL scanner (Advanced Technology Laboratories, Bothwell, WA) using a multifrequency transducer (5–8 MHz) for the transvaginal scans and a multifrequency transducer (3–5 MHz) for the transabdominal scans. We measured the longitudinal uterine diameter, the anterior-posterior fundal segment (upper) and anterior-posterior cervical uterine segment (lower), all in a sagittal plane, and the maximal transverse uterine diameter in a transverse plane.
Uterine development was evaluated in terms of a size by the uterine length and uterine volume and in terms of shape by calculation of the upper to lower uterine ratio. We used size and shape normative data (6) to characterize uterine maturity in the following way: Mature uterus: length ≥6.5 cm and upper/lower segment ratio ≥1.10; Transitional uterus: length 5.0–6.4 cm and ratio ≥1.10 or length ≥6.5 cm but ratio <1.10; and Immature uterus: length< 5.0 cm or length 5.0–6.4 cm but ratio <1.10. The uterine volume was calculated by the formula: Vol (mL) = Length (cm) ×Transverse diameter (cm) × AP fundal diameter (cm) × 0.5233 (9).
Each study participant filled out a questionnaire and had a detailed interview regarding pubertal development, age at initiation of hormone replacement therapy (HRT), type of estrogen used, years of estrogen use, and history of growth hormone therapy (GH Rx). We calculated the years of estrogen use by adding the time intervals during which the subject was taking various estrogen containing medications. In case of spontaneous menarche, we added the time interval from the menarche to development of amenorrhea or starting estrogen containing medication.
Results are presented as mean values with standard deviation for continuous variables and as numbers and percent for nominal variables. We explored the contribution of the following independent variables to uterine size and maturity: age, height, body surface area (BSA), age at estrogen exposure, years of estrogen use, GH Rx, current HRT use, type of estrogen, and history of spontaneous menarche. To study the individual effect of the above variables on the uterine volume we used either simple liner regression (when the independent variables were continuous) or ANOVA post-hoc Bonferroni test (when the independent variables were nominal). We used multiple ordinal logistic regression model to study the effect of a combination of continuous and nominal independent variables on uterine maturity (ordinal variable). A SPSS statistical program (StatView and JMP) was used.
Results
Study subjects
The mean age of the study population was 31.8 ±7.3 years, range 18–45. The majority participants were white (93.1%). Other racial/ethnic groups included Asian, Hispanic and Black, each 2.3%.
Most study subjects (93%) had karyotypes consistent with TS in 100% of the peripheral white blood cells (Table I). Only 6/86 subjects (7%) had mosaicism for cells with normal female karyotype (46,XX). However, these constituted only 2–14% of the karyotyped cells. None of the subjects had cells containing a Y chromosome (intact or abnormal). Thus our study includes only women with TS who have either none or only very minimal mosaicism for cells with normal karyotypes.
Table 1.
Karyotype distribution
| Karyotype | N | % |
|---|---|---|
| 45,X in 100% of WBC | 49 | 57.0% |
| 45,X/46,XX or 45,X/46,XX/47,XXX* | 6 | 7.0% |
| 46,Xi(Xq) or 45,X/46,Xi(Xq) | 17 | 19.8% |
| 46,Xdel(Xp) or 45,X/46,Xdel(Xp) | 4 | 4.6% |
| 46,Xdel(Xq) or 45,X/46,Xdel(Xq) | 3 | 3.5% |
| 45,X/46,Xr(X) | 4 | 4.6% |
| Other: 45,X/46,X & another type of abnormal X-chromosome | 3 | 3.5% |
Karyotype 46,XX was found in only 2 to14% of the peripheral WBC
Ovarian Hormone Replacement Therapy
Fifteen percent (13/86) had experienced spontaneous menarche at an average age of 12.2 ± 1.7 years and by their late teens they had developed amenorrhea and had started estrogen replacement. All other subjects (73/86) had not had spontaneous puberty and had started taking estrogen at an average age of 15.7 ± 4.1 years. Thirty percent (26/86) had been treated with growth hormone.
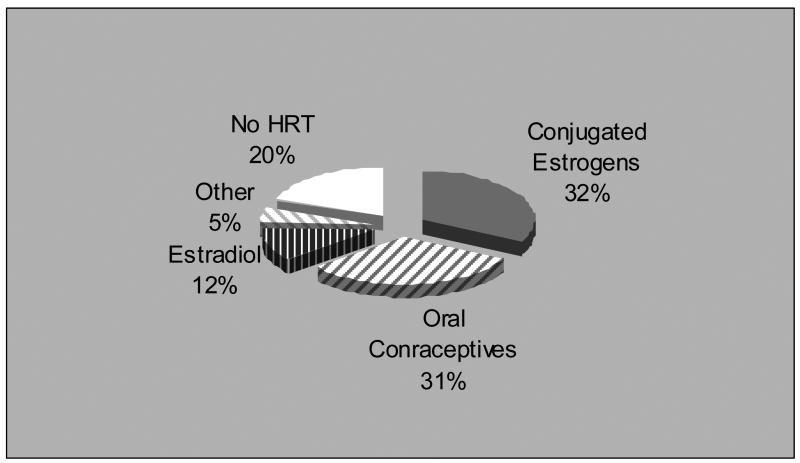
Eighty percent of the participants had been prescribed HRT at the time of study (Table II; available at www.jpeds.com; Figure). The majority were taking either oral conjugated estrogens (CE) or oral contraceptives (OC). Relatively few were using transdermal or oral estradiol (E2). In addition to estrogen, most were taking a progestin, the preferred progestin being either medroxyprogesterone or one of the three most common components of the oral contraceptives (norethindrone, levonor/desogestrel or norgestimate). One fifth of the participants were not taking HRT, the reasons being concern about side effects, experience of discomfort or financial constraints.
Figure 1.
Distribution of various forms of HRT in TS
Uterine size and maturity
Approximately a quarter of the women with TS (21/86; 24.4%) had fully developed in size and shape uterus (Table III). Most (38/86; 44.2%) had somewhat smaller in size uterus (transitional) and almost a third had an immature “cylindrical shaped” uterus (27/86; 31.4%). Women who had developed mature uteri were as likely to have “pure” 45.X karyotype as were women with immature uteri (Table III).
Table 3.
Variables influencing uterine volume
| Continuous independent variables | |||
|---|---|---|---|
| R2 | F-value | p | |
| Age | 0.24 | 5.08 | 0.027 |
| Height | 0.008 | 0.68 | 0.411 |
| Weight | 0.017 | 1.46 | 0.230 |
| Body Surface Area | 0.016 | 1.40 | 0.240 |
| Age at Estrogen Exposure | 0.022 | 1.85 | 0.177 |
| Years of Estrogen Exposure | 0.161 | 15.94 | 0.0001 |
| Nominal Independent Variables | |||
| Level | Volume (mL) | ||
| Mean ± SD | |||
| History of GH Use | Yes (n=26) | 18.3 ± 12.0 | P=0.066 |
| No (n=60) | 22.8 ± 12.3 | ||
| Current HRT | Yes (n=69) | 23.0 ± 12.6 | P=0.0019 |
| No (n=17) | 15.0 ± 8.6 | ||
| Spontaneous Menarche | Yes (n=13) | 30.0 ± 16.9 | P=0.014 |
| No (n=73) | 19.9 ± 10.7 | ||
| Type of Estrogen | E2 (n=10) | 28.0 ± 10.0 | E2 vs None, P=0.0015;
E2 vs OC, P=0.045; CE vs None, P=0.0032 |
| CE (n=28) | 25.0 ± 14.6 | ||
| OC (n=27) | 19.7 ± 11.0 | ||
| None (n=20) | 15.4 ± 8.6 | ||
E2 denotes estradiol, CE - conjugated estrogens, OC - oral contraceptives
Factors influencing uterine maturity
In separate regression analyses uterine size (volume) was influenced significantly by age, years of estrogen use, current use of HRT, history of spontaneous menarche and type of estrogen medication (Table IV). Notably, women who were taking oral contraceptives had uterine size similar to those who were not currently taking HRT, although those who were taking estradiol based hormone replacement had significantly larger uteri (Table IV). There was no correlation between the age at first exposure to estrogens and the size of the uterus. In addition, none of the measures of body size (height, weight and BSA) were correlated to the size of the uterus.
We explored the combined influence of several independent variables (age at first exposure to estrogens, years of estrogen use, type of HRT and history of spontaneous menarche) on the degree of uterine maturity in an ordinal multiple logistic regression analysis. The uterine maturity was expressed as an ordinal dependent variable in the following way: immature uterus = 0, transitional uterus = 1, mature uterus = 2. We did not include age in the model because it was strongly correlated to years of estrogen use (r = 0.59, P < 0.00001). The overall χ2 of the model was 17.5 with a p-value of 0.0077. The degree of uterine maturity was positively associated with years of estrogen use (χ2 = 4.0, P=0.045), estradiol based HRT (χ2 = 4.1, P=0.044) and with history of spontaneous menarche (χ2 = 5.3, P=0.021), and negatively with the lack of current HRT (χ2 = 4.3, P=0.038). The age at first exposure to estrogen, again, had no influence over the uterine maturity (χ2 = 0.15, P=0.701).
Discussion
This study shows that women with TS treated with oral or transdermal estradiol or oral conjugated estrogen, in combination with oral medroxyprogesterone or micronized progesterone for several years, may attain a normal, mature uterine size and configuration. The age of pubertal induction was not critical. Stature and history of GH treatment did not impact the degree of uterine development. Karyotype was not a contributing factor, and 45,X women did have normal uterine development given adequate treatment (Table IV). A recent study from Germany found that only 45,X/46,XX mosaic females developed normal uterine proportions although none with 45,X did (7). In fact, karyotype was the only significant predictor of normal uterine development in this study, with age at estrogen initiation, age at start of cyclic progestin or duration of estrogen showing no correlation to the uterine development (7). The different findings in our study (12/21 or 57% of the subjects with mature uteri had 45,X karyotype) may be explained by the longer average duration of estrogen treatment in our subjects. The mere currency of HRT is not enough in itself to guarantee a normal uterine size if it has not been administered long enough – as illustrated in Table III, the majority of the women with TS who had immature uterus (70%) had been taking HRT at the time of the study.
The optimal age for pubertal initiation and the safest and most effective protocol for pubertal development and maintenance HRT in girls and young women with premature ovarian failure are important issues that lack strong, evidence based support at present. According to some studies age at pubertal induction was an important factor in achieving a normal uterine size (5,6,8). In previous years, expert opinion recommended pubertal induction with low dose estrogen treatment beginning between age 12 – 15 yrs, with gradual increases in dose until feminization is adequate, and the addition of a cyclic progestin on a regular basis after 12–24 months (10). The average age of initiation in our group of community-treated patients was rather late at almost 16 years of age. This may be due to a trend in recent years to delay the start of estrogen treatment to promote additional statural growth under GH treatment (11). Our study suggests that age at estrogen initiation is not critical to adequate uterine development.
Previous studies have found that the dose of estrogen is an important contributor to uterine size and maturity (8.9). Our study indicates that in addition, the type of hormone replacement therapy may play an important role. The real test of adequate uterine development is successful pregnancy, and although initial reports on assisted reproduction outcomes in TS did suggest a potential uterine problem (12), a recent review of women with TS participating in oocyte donation programs in the U.S. found that of 146 women treated, 101 (69%) became pregnant; 94 of these pregnancies resulted in the birth of a live baby, for a miscarriage rate of only 6.4% (13). This important observation indicates that given adequate hormonal treatment, women with TS may develop a uterus able to sustain a term pregnancy. Although the uterus may sustain a pregnancy, the cardiovascular system of the mother with TS may not (13), so the paramount concern in considering pregnancy must be the mother’s risk factors for pregnancy complications (14).
Our study has certain limitations. As with every cross sectional study, it may have unsuspected bias. Unexplained remained the fact that when compared to historic reference data, women with TS in our study had smaller uterine volumes even when they fulfilled the criteria for uterine maturity(6, 9, 15). In addition, the small number of women taking transdermal estrogen therapy (n=5, Table II) did not allow valid conclusions regarding the effect of different modes of estrogen administration, i.e. oral versus transdermal. Future studies are needed to compare uterine size and maturity of girls with TS who have had the currently recommended “optimal” pubertal induction and hormone replacement therapy to a group of age matched girls with normal karyotypes and normal pubertal development.
Table 2.
Immature versus mature uterus in Turner syndrome
| Measure | Immature Uterus N=27 | Mature Uterus N=21 |
|---|---|---|
| Length (cm) ± SD (range) | 5.0 ± 0.9 (2.8–6.4) | 7.2 ± 0.7 (6.5–8.1) |
| AP (cm) ± SD (range) | 1.7 ± 0.7 (0.7–4.8) | 2.5 ± 0.5 (1.5–3.8) |
| Transverse (cm) ± SD (range) | 2.8 ± 0.7 (1.8–4.0) | 3.7 ± 0.9 (1.5–5.0) |
| Volume (mL) ± SD (range) | 12.8 ± 7.2 (3.1–35.2) | 35.2 ± 12.3 (17.2 – 69.6) |
| Uterine Ratio ±SD | 1.08 ± 0.16 (0.93–1.78) | 1.44 ± 0.21 (1.12–1.85) |
| Age (years) ± SD
45,X karyotype in 100% of the cells (n,%) Spontaneous puberty (n,%) Currently taking HRT (n,%) |
28.9 ± 6.6
14/27 (52%) 1/27 (3.7%) 19/27 (70%) |
34.8 ± 6.8
12/21 (57%) 5/21 (24%) 19/21 (90%) |
Supplementary Material
Acknowledgments
This work was supported by the intramural research program of NICHD, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87:81–3. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 2.Hanton L, Axelrod L, Bakalov V, Bondy CA. The importance of estrogen replacement in young women with Turner syndrome. J Womens Health (Larchmt) 2003;12:971–7. doi: 10.1089/154099903322643893. [DOI] [PubMed] [Google Scholar]
- 3.Kiess W, Conway G, Ritzen M, Rosenfield R, Bernasconi S, Juul A, et al. Induction of puberty in the hypogonadal girl--practices and attitudes of pediatric endocrinologists in Europe. Hormone Research. 2002;57:66–71. doi: 10.1159/000057952. [DOI] [PubMed] [Google Scholar]
- 4.Attie KM, Chernausek CS, Frane J, Rosenfeld RG. Growth hormone use in Turner syndrome: a preliminary report on the effect of early vs delayed estrogen. In: Albertsson-Wikland KRM, editor. Turner syndrome in a life-span perspective. New York, NY: 1995. pp. 175–181. [Google Scholar]
- 5.Paterson WF, Hollman AS, Donaldson MD. Poor uterine development in Turner syndrome with oral oestrogen therapy. Clin Endocrinol (Oxf) 2002;56:359–65. doi: 10.1046/j.1365-2265.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 6.Snajderova M, Mardesic T, Lebl J, Gerzova H, Teslik L, Zapletalova J. The uterine length in women with Turner syndrome reflects the postmenarcheal daily estrogen dose. Horm Res. 2003;60:198–204. doi: 10.1159/000073233. [DOI] [PubMed] [Google Scholar]
- 7.Doerr HG, Bettendorf M, Hauffa BP, Mehls O, Partsch C-J, Said E, et al. Uterine size in women with Turner syndrome after induction of puberty with estrogens and long-term growth hormone therapy: results of the German IGLU Follow-up Study 2001. Hum Reprod. 2005;20:1418–1421. doi: 10.1093/humrep/deh764. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell CM, Coleman L, Zacharin MR. A 3-year prospective study to assess uterine growth in girls with Turner's syndrome by pelvic ultrasound. Clinical Endocrinology. 2003;58:446–450. doi: 10.1046/j.1365-2265.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 9.Porcu E, Venturoli S, Fabbri R, Orsini LF, Sganga E, Brondelli L, et al. Uterine development and endocrine relationships after menarche. Am J Obstet Gynecol. 1989;161:174–7. doi: 10.1016/0002-9378(89)90259-7. [DOI] [PubMed] [Google Scholar]
- 10.Saenger P, Wikland KA, Conway GS, Davenport M, Gravholt CH, Hintz R, et al. Recommendations for the Diagnosis and Management of Turner Syndrome. J Clin Endocrinol Metab. 2001;86:3061–3069. doi: 10.1210/jcem.86.7.7683. [DOI] [PubMed] [Google Scholar]
- 11.Chernausek SD, Attie KM, Cara JF, Rosenfeld RG, Frane J. Growth hormone therapy of Turner syndrome: the impact of age of estrogen replacement on final height. Genentech, Inc., Collaborative Study Group. J Clin Endocrinol Metab. 2000;85:2439–2445. doi: 10.1210/jcem.85.7.6684. [DOI] [PubMed] [Google Scholar]
- 12.Foudila T, Soderstrom-Anttila V, Hovatta O. Turner's syndrome and pregnancies after oocyte donation. Hum Reprod. 1999;14:532–535. doi: 10.1093/humrep/14.2.532. [DOI] [PubMed] [Google Scholar]
- 13.Karnis MF, Zimon AE, Lalwani SI, Timmreck LS, Klipstein S, Reindollar RH. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: a national survey. Fertility and Sterility. 2003;80:498–501. doi: 10.1016/s0015-0282(03)00974-9. [DOI] [PubMed] [Google Scholar]
- 14.Bondy CA for the The Turner Syndrome Consensus Study G. Care of Girls and Women with Turner Syndrome: A Guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1374. jc.2006–1374. [DOI] [PubMed] [Google Scholar]
- 15.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–86. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.