Abstract
Chromatin is increasingly recognized as a highly dynamic entity. Chromosome sites in lower and higher eukaryotes undergo frequent, rapid and constrained local motion and occasional slow, long-range movements. While the dynamic properties of chromatin have been described by visualization in vivo, recent observations have revealed some of the functional relevance of chromatin mobility. Paradoxically, both the mobility and immobility of chromatin appears to have functional consequences: Local diffusional motion of chromatin appears important in gene regulation, but global chromatin immobility playa a key role in maintenance of genomic stability.
Introduction
Chromatin is defined as the complex between DNA and its associated proteins. The fundamental unit of chromatin is the nucleosome which consists of ~ 200bp of DNA wrapped around an octamer of core histones. Interactions between individual nucleosomes lead to the folding of nucleosomes into the 30 nm fiber and into large-scale configurations to eventually form a chromosome. This arrangement is thought to compact the enormous amount of linear genome information into the limited volume of the nucleus in such a way that all necessary regulatory sequences are still accessible. The chromatin of each chromosome occupies a spatially limited nuclear subvolume and appears physically anchored in a distinct and limited region of the cell nucleus, suggesting a relatively static overall organization. At the same time, advanced live microscopy methods have recently been developed to track chromatin in vivo. The findings from these experiments have revealed a high-degree of local dynamics and they have challenged the static view of chromatin. Remarkably, it now appears that both the highly dynamic properties of chromatin as well as the static aspects of chromosome organization are functionally important. We discuss here how chromatin moves in vivo over long and short distances and how these regimes of motion are important for gene expression and genome stability.
Short-range chromatin motion
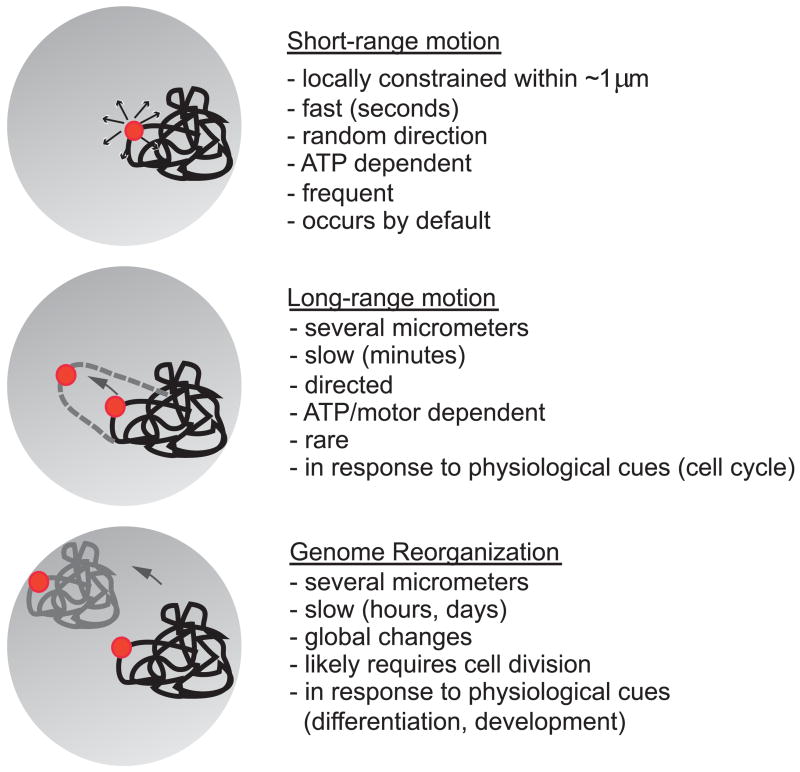
The motion of chromatin in vivo can be tracked by imaging of fluorescent proteins bound to unique chromosome sites engineered into the chromatin fiber [1]. Experiments using such systems have revealed universal characteristics of chromatin dynamics. In S. cerevisiae, D. melanogaster and mammalian cells, chromatin undergoes rapid, locally constrained motion by oscillating within a volume of 0.5–0.7um in radius [2–5]. Careful analysis uncovers rapid motion of less than 0.2 um in distance and, superimposed onto it, less frequent movements over ~0.5um (Fig. 1). Although this local chromatin motion is sensitive to ATP-levels, it is generally non-directional and occurs in a random walk. The apparently paradoxical observation of energy-dependence but random motion is likely due to the fact that chromatin motion is largely the result of continuous, default opening and closing events of the chromatin fiber by remodeling machines, most of which depend on ATP. This idea is further supported by the finding that the constraints in chromatin motion are imposed by the fibrous nature of chromatin, since elegant experiments in S. cerevisiae demonstrate that an excised gene locus is able to diffuse rapidly and in an unconstrained manner through the yeast nucleus [6]**.
Figure 1. Comparative overview of the three types of chromatin motion.
Short range motion: A particular chromosome site (red) undergoes locally constrained motion within a range of ~ 1um. Long-range motion: Chromosome sites may undergo long-range, possibly directed and motor driven, movement over long distances. These appear to be rare. Reorganization: Global reorganization of the genome via repositioning of entire chromosomes and chromosome regions occurs during differentiation and development.
A remarkable outcome of these experiments has been the realization that the local motion of chromatin is virtually identical regardless of the organism or the size of its nucleus [7,8]. It is, however, important to point out that this same range of motion has vastly different relevance, and potential functional implications, in the various organisms. While a typical ~1um exploration of a locus in a mammalian nucleus means that the locus is essentially immobile in the context of the cell nucleus, the same motion in a much smaller yeast nucleus means that a locus can essentially explore the entire nuclear volume and can thus gain access to various, potentially functionally distinct, nuclear compartments [8]. This behavior is clearly exemplified by the direct comparison of the motion of heterochromatin regions in mammalian cells and S.pombe visualized using HP1/Swi6 as a marker [9,10]. While heterochromatin domains are essentially immobile in the mammalian nucleus, they rapidly crisscross the nucleus of S.pombe within tens of seconds. The differences in the extent of motion of a chromatin locus in the various organisms must be taken into account when considering the contribution of chromatin motion in genome function.
Long-range chromatin motion
Although locally highly dynamic, chromatin in higher organisms is positionally relatively stable over long periods of time. Photobleaching experiments reveal very little movement of chromatin over distances larger than 1–2 um during the cell cycle [11,12] (Fig. 1). While this suggests that long-range motions are not a default property of chromatin, movements over several micrometers have occasionally been observed. In Drosophila, movements of a locus of ~ 3 um over 10 min occurs in early G2 but diminishes as cells progress and enter meiosis [2]. In mammalian cells, irreversible tethering of the transcriptional activator VP16 to chromatin leads to the dynamic internalization of a marker locus [13]**. Remarkably, this migration is dependent on the actin/myosin system, suggesting that in contrast to local, short-range motion, more global movements of chromatin may occur in a directed and active fashion. The functional relevance of these directed long range motions is currently unknown.
There are several reasons why long-range chromatin movements may have been difficult to detect and their functional relevance underestimated. First, the rapid, short-term motions appear to be far more frequent with the long-range movements being the rare exception. Second, long-range motions seem to be exquisitely sensitive to light damage and their observation requires low light techniques, which are not routinely used [13]**. Third, long-range motions may be very tightly controlled and may only occur as part of physiological responses. They are less likely to be captured by observation of generic cultured cells in vitro and require analysis of chromatin movements in physiological situation such as that observed during cell cycle progression in D. melanogaster spermatogenesis [2]. It may not be surprising that long-range, directed chromatin movements are not the norm but possibly the exception and only occur in response to specific physiological cues. It is not entirely trivial to envision how directionality of movement would be established and how, if the actin/myosin system mediates this movement, the starting and endpoint of the motion are determined. Intriguingly, the nucleus contains several molecular motor proteins of unknown function and it will be key to determine whether they are involved in physiological long range chromatin displacements.
Despite the scarcity of direct visualization of long-range motion, we know that global movements of chromatin occur in vivo. Centromere and telomeres are known to undergo changes in position during differentiation and development and numerous genes have been shown to undergo changes in their intranuclear location or their position relative to other genome regions [14]. These changes, however, likely occur over much longer time periods and involve passage of cells through mitosis; they should thus be characterized as chromatin reorganizations rather than motion (Fig. 1). Considering the overall positional stability of chromatin over long distances during interphase, such global genome reorganization is likely driven by rearrangement of chromatin regions as cells go through mitosis. In support, the position of chromosomes is only partially conserved in daughter cells and the arrangement of chromatin regions is not inherited through mitosis but newly established in early G1 [11,12,15]. A fascinating and entirely unanswered question is what the mechanisms are that determine the resulting semi-conserved patterns of genome organization of daughter cells and whether physiological cues can influence repositioning events during cell division.
Chromatin motion in gene expression
The dynamic motion of chromatin is increasingly becoming an integral component of models of genome function. In particular, chromatin movement has been linked to transcriptional regulation. An attractive hypothesis is that a gene locus can move into a favorable place within the nucleus for regulatory purposes, either activation or repression. This model is supported by the behavior of genes such as IgH and Igκ which change their intranuclear position by moving from the nuclear periphery in hematopoetic precursors toward the interior as they are activated in B-cells [16]. The β-globin locus undergoes a similar change during its activation in erythroid differentiation and α- and β-globin loci frequently associate with each other at intranuclear splicing factor compartments at the time of their peak expression during differentiation [17**,18**]. In addition, co-regulated genes from the same or different chromosomes associate with a single nuclear transcription center [19]. Another modality of chromatin motion is the dissociation of a gene locus from the main body of the chromosome on which it localizes. Such expulsion of a locus from the chromosome territory has been linked to high expression of larger chromatin domains such as the MHC class II locus, the epidermal differentiation or the Hox gene cluster [20–23].
These and many other observations clearly show that genes move within the nucleus and that the movement is often related to their activity. But it is less clear whether the observed movement of gene loci is essential for gene expression. In the case of the internalization of the β-globin locus during erythroid maturation, the gene becomes activated prior to its change in localization and activity does not depend on its internal nuclear position [17]**. Similarly, association with splicing factor compartments as observed for human α-globin was not seen for β-globin or for mouse globins [18]**. With respect to expulsion from chromosome territories, many highly expressed genes do not dissociate from chromosomes and while the Hoxd locus loops out from chromosomes in cells along the anterior-posterior axis, it does not do so in the limb bud [22]**. Rather than these positioning events being required for proper gene expression, it seems more plausible that the repositioning of a gene is a consequence of its activity status. This does not mean that positioning is not important. Observations in S. cerevisiae suggest that the association of an activated locus with the nuclear periphery is neither sufficient nor necessary for activity, but serves to optimally express the gene [24]**. Thus the movement of a locus is a fine-tuning step in the hierarchy of gene regulation.
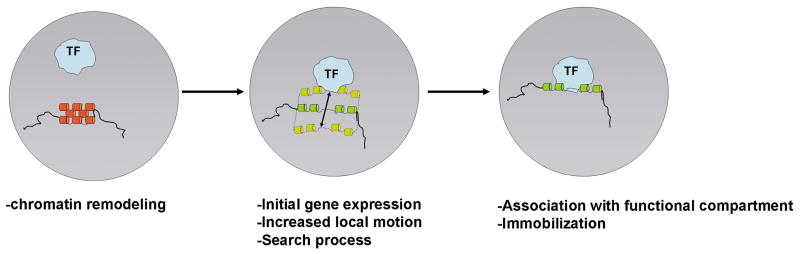
Taking into consideration these various observations on chromatin motion and positioning, a plausible scenario that emerges is the following [14,25] (Fig. 2): As a gene becomes activated, its chromatin structure is altered locally by the action of chromatin remodeling machines. This leads to changes in the higher order chromatin structure and likely modulates the local accessibility of transcriptional regulators to the locus. It is possible, and likely, that transcription commences at this point, albeit possibly at a low level. Since the intranuclear mobility of the locus is probably strongly affected by its higher order chromatin structure, the locus is now increasingly mobile. Its motion is most likely still locally constrained, although its potential for long range motion may be increased. This altered mobility of the partially activated, or merely potentiated, locus now allows it to explore its nuclear environment in a stochastic and random manner by local, constrained diffusion and to find a functional compartment that corresponds to and supports its functional status, such as a transcription factory or a splicing factor compartment. While this association most likely perpetuates the gene’s activity status, it does not necessarily confer stability, since at least some genes appear to be expressed in transcriptional bursts [26**,27]. A similar sequence of events may occur during silencing, but in that case a gene locus primed for repression would undergo locally constrained motion before it stochastically associates with a heterochromatin domain for stronger and more permanent silencing (Fig. 2).
Figure 2. Chromatin motion in gene activation.
The local motion of chromatin is critical for proper gene expression. Upon chromatin remodeling from an inactive state (red), a locus undergoes increased local motion. The locus may be transcriptionally silent, potentiated or active at low level at this point. The increased range of motion permits the locus to explore its environment and eventually associate with a transcription center (TF). This association potentially stabilizes the transcriptional status of the locus (green).
In this view, the local diffusional motion of chromatin is a critical property in gene expression as it allows the locus to find its ideal environment and be optimally expressed. This model is consistent with the widespread observation that the position of a locus relative to any nuclear landmark is not essential for its expression. Genes which do not associate with a transcription factory might still be expressed, albeit at a lower level; similarly, transcripts from genes which do not find a splicing factor compartment can still be spliced, presumably by freely diffusing splicing factors, albeit possibly at a lower level. In addition, this model is in line with the notion that long-range motion is a rare occurrence that might be reserved for events that are particularly sensitive to optimal regulation. A key prediction from this model is that the motion of remodeled chromatin is distinct from that of basal chromatin. While the observations using tethering of VP16 to an artificial chromosome site support this idea [3,13]**, it will be essential to develop technology to probe the motion of endogenous chromatin under physiological conditions. An ultimate test for the role of chromatin mobility in gene expression will be to uncouple the local motion of a locus from its activity for example by immobilizing a gene and testing its ability to be properly regulated. The technology to carry out these experiments is in place and we will undoubtedly know the answer to this question soon.
Chromatin immobility in the formation of chromosome translocations and maintenance of genome stability
The dynamic properties of chromatin are also key to the mechanisms of formation of cancerous chromosomal translocations and maintenance of genome stability. Chromosome translocations occur by illegitimate joining of two unrepaired double strand breaks (DSB) present in an individual cell nucleus. The mechanisms by which translocations form in vivo have been controversial [28]. One possibility is that DSBs occur on spatially distant chromosomes and that the broken chromosome ends roam the nuclear space in search of potential translocation partners. In contrast, it has been suggested that translocations can only occur between breaks located on neighboring or proximal chromosomes. These two models make divergent predictions with regard to the importance of the mobility of the broken chromosome ends: The former model requires that DSBs can move over large distances within the nucleus to find other broken chromosome ends, while the latter does not require that DSBs are mobile and, in fact, implies that they are not since otherwise the DSBs might move away from each other.
Several recent studies have addressed this issue and a consensus is emerging demonstrating that DSBs are largely immobile in mammalian cells. Two early analyses of damaged DNA in chemically fixed cells came to divergent conclusions. Induction of DNA damage using ultrasoft X-rays showed that damaged regions were held in a relatively fixed position within the nucleus [29]. In contrast, irradiation with α-particles led to the movement of damaged sites over several micrometers [30]. The discrepancy between these observations has recently been resolved by analysis of DSB dynamics in living cells. Using the core histone H2B as an in vivo marker, no significant motion of damaged DNA regions after damage by UV or γ-irradiation was detected [31]. This observation on bulk damaged DNA was extended by the development of an experimental system to visualize the dynamic behavior of broken chromosome ends in living cells after controlled induction of a single DSB [32]**. Direct tracking of the movement of the broken chromosome ends by specific fluorescent tags on either side of the break demonstrated that cut ends were unable to roam the nuclear volume over large distances and breakage of the chromosome only resulted in slightly increased local motion of the DNA break. Surprisingly, the positional stability of the broken ends was not dependent on the structural proteins H2AX and SMC1 nor on the MDC1/RAD51/NBS1 repair complex which acts as primary sensor of breaks and had been implicated in anchoring DNA ends. Increased local mobility followed by long range mobilization of broken DNA ends was, however, observed in the absence of the heterodimer Ku80/Ku70, pointing to its importance in aligning and holding broken chromosomes in place. These observations in living cells strongly suggest that broken chromosome ends are positionally stable in the mammalian cell nucleus. As a consequence, translocations must preferentially occur between already proximally positioned simultaneous DSBs rather than spatially distant chromosome lesions.
Things might be very different in yeast. S.cerevisiae cells have been shown to contain a limited number of nuclear sites which act as DNA damage repair centers [33]. These centers seem to serve repair of multiple DNA breaks since breaks on different chromosomes associate with a single repair site, suggesting that DSBs migrate to repair centers. This is in stark contrast to mammalian cells where multiple DSBs do not cluster around a common repair site, but rather each break creates a new repair focus [32]**. It is probably not a coincidence that the differential dynamic behavior of DSBs in yeast and mammalian cells directly mirrors the ability of chromatin in these systems to explore the nuclear volume. While the default short-range motion of chromatin over ~1um allows a yeast DSB to explore a large fraction of the yeast nucleus creating a high probability of encountering a DNA repair center, the same extent of motion limits the DSBs to a small fraction of the mammalian nucleus, thereby limiting their ability to encounter another DSB or a repair site. The differential ability of a DSB to explore varying fractions of the nucleus in yeast and mammals thus seems to have contributed to the development of very different strategies of organizing DNA repair in the nucleus of yeast and mammals.
The observed immobility of broken chromosome ends in mammalian cells has significant implications for the mechanism by which chromosomal translocations form [28] (Fig. 3). Since DSBs on different chromosomes are unable to diffuse through the nucleus, the illegitimate joining of unrepaired breaks must occur predominantly amongst neighboring chromosomes. The emerging view then is that translocations happen when two DSBs on distinct chromosomes are positioned such that they can interact with each other as part of their locally constrained motion. If DSBs are further apart than their intrinsic reach of local motion, they will not translocate. It is important to point out that the formation of translocations does not require long-range motion and the ability of a broken chromosome end to move over long distances sufficient for formation of translocations. We know this because in the absence of Ku80, broken chromosome ends separate, at times over several micrometers, but the majority of them remain unrepaired and do not undergo translocations [32]**. It thus seems that translocations occur preferentially amongst proximally positioned chromosomes.
Figure 3. Chromosome immobility in the formation of translocations.
DNA double strand breaks are positionally immobile over long distances and only undergo local constrained motion with a defined range of motion (circles). As a consequence, distant DSBs (green chromosome) are unable to interact and only proximal breaks (red, blue chromosomes) are candidates for illegitimate joining and translocation formation.
The observations on chromatin mobility of DSBs pointing to translocations amongst proximal chromosomes are complemented by an independent body of evidence based on mapping of the 3D spatial position of frequent translocation partners. A series of studies have demonstrated that the frequently translocated chromosome regions and specific break points are on average found in preferential spatial proximity and are often direct neighbors prior to undergoing translocations [14,28]. This concept has recently been extended by the finding that translocation frequencies amongst chromosomes in human lymphocytes not only correlate with physical association of the involved chromosomes, but with the degree of intermingling of the chromatin fibers at the interface between the two chromosomes [14, 34**].
The positional stability of DSBs has direct physiological consequences. For one, it may explain the well-established observation that tumors from different tissues exhibit distinct recurrent chromosomal translocations [35]. It is well known that the 3D spatial organization of genomes is tissue specific [36,37]; thus, chromosomes and gene loci physically associate with different partners in different tissues. Since the immobility of DSBs restricts their choice of interaction partners to their immediate physical neighbors, the set of translocation partners is limited and directly determined by the tissue-specific arrangement of chromosomes.
Possibly the most important physiological consequence of the positional immobility of DSBs is its protective role against genomic instability. If broken chromosome ends were highly mobile within the nucleus, the probability of illegitimate joining would be very high every time two DSBs occur within the same cell nucleus. The positional immobility prevents illegitimate joining of spontaneously occurring distant DSBs since they cannot be brought into spatial proximity. This might afford the cell the opportunity to repair at least one of the breaks before they have an opportunity to interact with each other and illegitimately join to form a translocation. Interestingly, in yeast where any given locus has access to a higher proportion of the nucleus, and thus can interact with a larger fraction of the genome, recombination events between sites on different chromosomes are significantly more frequent than in mammalian cells [38].
A key question that remains is what the mechanisms are that keep broken chromosomes in their spatial position. One possibility is that the inability to move over large distances is an intrinsic property of chromosomes and is simply caused by the complex higher order folding of the chromatin fiber which restricts the ability of a locus to move. On the other hand, it is possible that specific mechanisms are at play which constrain broken ends from diffusing away from each other. This idea is supported by the observation that loss of the non-homologues end joining factor Ku80 leads to loss of positional immobility in mammalian cells [32]**. An obvious goal is to identify additional factors that contribute to maintaining broken chromosome ends in their position. This seems particularly important since some of these factors might act as tumor suppressors by preventing broken chromosome ends from finding each other and thus protecting the genome from illegitimate joining of chromosomes.
Conclusions
Chromatin undergoes frequent, locally constrained dynamic motions in vivo, but only limited long-range movements. Chromatin mobility is often simply considered an intrinsic property of chromatin without much functional relevance. However, analysis of cellular processes using in vivo imaging methods has begun to reveal that both the local mobility, as well as global chromatin immobility, are functionally important. Interestingly, it appears that the locally constrained motion of chromatin is important for transcription by allowing a locus to find an optimal environment corresponding to its activity status, and that local motion is also key in formation of translocations by allowing proximal DSBs to find each other. At the same time the limited global motion reduces the frequency of chromosome translocations. These roles of chromatin mobility are likely just the tip of the iceberg. It will be particularly important to probe whether directed long-range chromatin motions are more prevalent than currently thought and, if so, what the mechanisms of motion are, and whether they involve motor proteins. As we are increasingly able to visualize and manipulate genome processes in living cells, the full importance of chromatin mobility and dynamics in nuclear events will become evident. Incorporating the dynamic aspect of chromatin will be an important factor in understanding the complexity to genome regulation.
Acknowledgments
We thank Rebecca Chiffer for help with the manuscript. Work in the Misteli laboratory is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belmont A. Dynamics of chromatin, proteins, and bodies within the cell nucleus. Curr Opin Cell Biol. 2003;15:304–310. doi: 10.1016/s0955-0674(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez J, Belmont AS, Sedat JW. Multiple regimes of constrained chromosome motion are regulated in the interphase Drosophila nucleus. Curr Biol. 2001;11:227–1239. doi: 10.1016/s0960-9822(01)00390-6. [DOI] [PubMed] [Google Scholar]
- 3.Tumbar T, Belmont AS. Interphase movements of a DNA chromosome region modulated by VP16 transcriptional activator. Nat Cell Biol. 2001;3:134–139. doi: 10.1038/35055033. [DOI] [PubMed] [Google Scholar]
- 4.Heun P, Laroche T, Shimada K, Furrer P, Gasser SM. Chromosome dynamics in the yeast interphase nucleus. Science. 2001;294:2181–2186. doi: 10.1126/science.1065366. [DOI] [PubMed] [Google Scholar]
- 5.Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–445. doi: 10.1016/s0960-9822(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 6.Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell. 2004;119:955–967. doi: 10.1016/j.cell.2004.11.008. ** This study demonstrates that the locally limited motion of chromatin is due to its constraint in the fiber since an excised locus can rapidly diffuse though the nuclear volume. [DOI] [PubMed] [Google Scholar]
- 7.Gasser SM. Visualizing chromatin dynamics in interphase nuclei. Science. 2002;296:1412–1416. doi: 10.1126/science.1067703. [DOI] [PubMed] [Google Scholar]
- 8.Chubb JR, Bickmore WA. Considering nuclear compartmentalization in the light of nuclear dynamics. Cell. 2003;112:403–406. doi: 10.1016/s0092-8674(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 9.Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 10.Cheutin T, Gorski SA, May KM, Singh PB, Misteli T. In vivo dynamics of Swi6 in yeast: Evidence for a stochastic model of heterochromatin. Mol Cell Biol. 2004;24:3157–3167. doi: 10.1128/MCB.24.8.3157-3167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter J, Schermelleh L, Cremer M, Tashiro S, Cremer T. Chromosome order in HeLa cells changes during mitosis and early G1, but is stably maintained during subsequent interphase stages. J Cell Biol. 2003;160:685–697. doi: 10.1083/jcb.200211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlich D, Beaudouin J, Kalbfuss B, Daigle N, Eils R, Ellenberg J. Global Chromosome Positions Are Transmitted through Mitosis in Mammalian Cells. Cell. 2003;112:751–764. doi: 10.1016/s0092-8674(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 13.Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. **This study describes long-range directed motion of a chromosome site in an actin/myosin-dependent manner. [DOI] [PubMed] [Google Scholar]
- 14.Misteli T. Beyond the sequence: Cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Thomson I, Gilchrist S, Bickmore WA, Chubb JR. The radial positioning of chromatin is not inherited through mitosis, but is established de novo in early G1. Curr Biol. 2004;14:166–172. doi: 10.1016/j.cub.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Sub-nuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 17.Ragoczy T, Bender MA, Telling A, Byron R, Groudine M. The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation. Genes Dev. 2006 doi: 10.1101/gad.1419506. **This is a detailed analysis of the relationship between gene activity and localization in a physiologically relevant context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JM, Leach J, Reittie JE, Atzberger A, Lee-Prudhoe J, Wood WG, Higgs DR, Iborra FJ, Buckle VJ. Coregulated human globin genes are frequently in spatial proximity when active. J Cell Biol. 2006;172:177–187. doi: 10.1083/jcb.200507073. **This study demonstrates the spatial association of co-regulated genes at splicing factor compartments and suggests that this association is the result of a self-organization process rather than a directed form of higher-order gene regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, et al. Active genes dynamcially colocalize to shared sites of ongoing transcription. Nature Genetics. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 20.Williams RR, Broad S, Sheer D, Ragoussis J. Subchromosomal positioning of the epidermal differentiation complex (EDC) in keratinocyte and lymphoblast interphase nuclei. Exp Cell Res. 2002;272:163–175. doi: 10.1006/excr.2001.5400. [DOI] [PubMed] [Google Scholar]
- 21.Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, Campbell RD, Goldsworthy M, Powis SH, Ragoussis J, et al. Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci. 2000;113(Pt 9):1565–1576. doi: 10.1242/jcs.113.9.1565. [DOI] [PubMed] [Google Scholar]
- 22.Morey C, Da Silva N, Perry P, Bickmore W. Nuclear reorganisation and chromatin decondensation are conserved, but distinct, mechanisms linked to Hox gene activation. Development. 2007;134:909–919. doi: 10.1242/dev.02779. **This study provides evidence that looping out of a locus from a chromosome is not merely a consequence of chromatin decondensation. [DOI] [PubMed] [Google Scholar]
- 23.Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–778. doi: 10.1038/nature04845. **This study demonstrates that association of a locus with the nuclear periphery acts to optimize expression levels. [DOI] [PubMed] [Google Scholar]
- 25.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 26.Chubb JR, Bloomfield G, Xu Q, Kaller M, Ivens A, Skelton J, Turner BM, Nellen W, Shaulsky G, Kay RR, et al. Developmental timing in Dictyostelium is regulated by the Set1 histone methyltransferase. Dev Biol. 2006;292:519–532. doi: 10.1016/j.ydbio.2005.12.054. ** This study elegantly demonstrates the pulsatile nature of “constitutively” active genes. [DOI] [PubMed] [Google Scholar]
- 27.Wijgerde M, Grosveld F, Fraser P. Transcription complex stability and chromatin dynamics in vivo. Nature. 1995;377:209–213. doi: 10.1038/377209a0. [DOI] [PubMed] [Google Scholar]
- 28.Meaburn KJ, Misteli T, Soutoglou E. Spatial genome organization in the formation of chromosomal translocations. Semin Cancer Biol. 2007;17:80–90. doi: 10.1016/j.semcancer.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelms BE, Maser RS, MacKay JF, Lagally MG, Petrini JH. In situ visualization of DNA double-strand break repair in human fibroblasts. Science. 1998;280:590–592. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- 30.Aten JA, Stap J, Krawczyk PM, van Oven CH, Hoebe RA, Essers J, Kanaar R. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science. 2004;303:92–95. doi: 10.1126/science.1088845. [DOI] [PubMed] [Google Scholar]
- 31.Kruhlak MJ, Celeste A, Nussenzweig A. Spatio-temporal dynamics of chromatin containing DNA breaks. Cell Cycle. 2006;5:1910–1912. doi: 10.4161/cc.5.17.3169. [DOI] [PubMed] [Google Scholar]
- 32.Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, Danuser G, Misteli T. Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol. 2007;9:675–682. doi: 10.1038/ncb1591. ** This study describes the first experimental system for the analysis of a single double strand break in living mammalian cells. It demonstrates that DSBs are positionally immobile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisby M, Mortensen UH, Rothstein R. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol. 2003;5:572–577. doi: 10.1038/ncb997. [DOI] [PubMed] [Google Scholar]
- 34.Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006;4:e138. doi: 10.1371/journal.pbio.0040138. ** This study demonstrates physical intermingling of neighboring chromosomes in the interphase nucleus and reports a correlation between the degree of intermingling and the probability of formation of chromosome translocations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitelman F, Johansson B, Mertens F. Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nat Genet. 2004;36:331–334. doi: 10.1038/ng1335. [DOI] [PubMed] [Google Scholar]
- 36.Parada L, McQueen P, Misteli T. Tissue-specific spatial organization of genomes. Genome Biology. 2004;7:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cremer M, Kupper K, Wagler B, Wizelman L, Hase Jv J, Weiland Y, Kreja L, Die-bold J, Speicher MR, Cremer T. Inheritance of gene density-related higher order chromatin arrangements in normal and tumor cell nuclei. J Cell Biol. 2003;162:809–820. doi: 10.1083/jcb.200304096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haber JE, Leung WY. Lack of chromosome territoriality in yeast: promiscuous rejoining of broken chromosome ends. Proc Natl Acad Sci U S A. 1996;93:13949–13954. doi: 10.1073/pnas.93.24.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]