Abstract
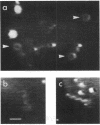
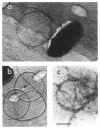
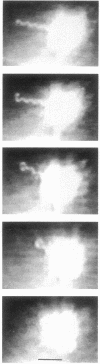
The single flagellum of the photosynthetic bacterium Rhodobacter sphaeroides was found to be medially located on the cell body. Observation of free-swimming bacteria, and bacteria tethered by their flagellar filaments, revealed that the flagellum could only rotate in the clockwise direction; switching of the direction of rotation was never observed. Flagellar rotation stopped periodically, typically several times a minute for up to several seconds each. Reorientation of swimming cells appeared to be the result of Brownian rotation during the stop periods. The flagellar filament displayed polymorphism; detached and nonrotating filaments were usually seen as large-amplitude helices of such short wavelength that they appeared as flat coils or circles, whereas the filaments on swimming cells showed a normal (small-amplitude, long-wavelength) helical form. With attached filaments, the transition from the normal to the coiled form occurred when the flagellar motor stopped rotating, proceeding from the distal end towards the cell body. It is possible that both the relaxation process and the smaller frictional resistance after relaxation may act to enhance the rate of reorientation of the cell. The transition from the coiled to the normal form occurred when the motor restarted, proceeding from the proximal end outwards, which might further contribute to the reorientation of the cell before it reaches a stable swimming geometry.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Boyd A., Mandel G., Simon M. I. Integral membrane proteins required for bacterial motility and chemotaxis. Symp Soc Exp Biol. 1982;35:123–137. [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. The induced synthesis of catalase in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1960 Jan 29;37:503–512. doi: 10.1016/0006-3002(60)90507-2. [DOI] [PubMed] [Google Scholar]
- Hotani H. Micro-video study of moving bacterial flagellar filaments. III. Cyclic transformation induced by mechanical force. J Mol Biol. 1982 Apr 25;156(4):791–806. doi: 10.1016/0022-2836(82)90142-5. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Segall J. E., Block S. M., Berg H. C. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983 Jul;155(1):228–237. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Asakura S. Helical transformations of Salmonella flagella in vitro. J Mol Biol. 1976 Sep 5;106(1):167–186. doi: 10.1016/0022-2836(76)90306-5. [DOI] [PubMed] [Google Scholar]
- Kamiya R., Hotani H., Asakura S. Polymorphic transition in bacterial flagella. Symp Soc Exp Biol. 1982;35:53–76. [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Examination of bacterial flagellation by dark-field microscopy. J Clin Microbiol. 1976 Sep;4(3):258–265. doi: 10.1128/jcm.4.3.258-265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Han D. P. Asynchronous switching of flagellar motors on a single bacterial cell. Cell. 1983 Jan;32(1):109–117. doi: 10.1016/0092-8674(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Minimal requirements for rotation of bacterial flagella. J Bacteriol. 1984 Jun;158(3):1208–1210. doi: 10.1128/jb.158.3.1208-1210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]