Abstract
Septic shock is a leading cause of morbidity and mortality. However, genetic factors predisposing to septic shock are not fully understood. Excessive production of proinflammatory cytokines, particularly tumor necrosis factor (TNF)-α, and the resultant severe hypotension play a central role in the pathophysiological process. Mitogen-activated protein (MAP) kinase cascades are crucial in the biosynthesis of proinflammatory cytokines. MAP kinase phosphatase (MKP)-1 is an archetypal member of the dual specificity protein phosphatase family that dephosphorylates MAP kinase. Thus, we hypothesize that knockout of the Mkp-1 gene results in prolonged MAP kinase activation, augmented cytokine production, and increased susceptibility to endotoxic shock. Here, we show that knockout of Mkp-1 substantially sensitizes mice to endotoxic shock induced by lipopolysaccharide (LPS) challenge. We demonstrate that upon LPS challenge, Mkp-1−/− cells exhibit prolonged p38 and c-Jun NH2-terminal kinase activation as well as enhanced TNF-α and interleukin (IL)-6 production compared with wild-type cells. After LPS challenge, Mkp-1 knockout mice produce dramatically more TNF-α, IL-6, and IL-10 than do wild-type mice. Consequently, Mkp-1 knockout mice develop severe hypotension and multiple organ failure, and exhibit a remarkable increase in mortality. Our studies demonstrate that MKP-1 is a pivotal feedback control regulator of the innate immune responses and plays a critical role in suppressing endotoxin shock.
Sepsis represents a serious challenge to public health, accounting for ∼750,000 hospitalizations and 215,000 deaths annually in the United States alone (1, 2). The overall mortality is ∼30%, rising to 40% in the elderly, and is >50% in patients with septic shock (1). Septic shock is associated with abnormal coagulation, profound and unresponsive hypotension, vasodilatory shock, and multiple organ failure. Sepsis describes a complex clinical syndrome that results from a harmful or damaging host response to microbial infection due to a dysregulated innate immune reaction, which is characterized by an excessive production of proinflammatory cytokines, such as TNF-α and IL-1β (3, 4). Mononuclear cells play a key role in the synthesis of proinflammatory cytokines, particularly TNF-α, IL-1β, and IL-6, as well as an array of other inflammatory mediators. These proinflammatory cytokines can in turn trigger secondary inflammatory cascades, including the production of cytokines, lipid mediators, and reactive oxygen species, as well as the up-regulation of cell adhesion molecules that facilitate the migration of inflammatory cells into tissues. By inducing the expression of inducible nitric oxide synthase and augmenting the production of nitric oxide, proinflammatory cytokines can decrease systemic vascular resistance, resulting in profound hypotension. Moreover, these cytokines also stimulate the procoagulation pathway, leading to thrombosis of microvasculature and impaired tissue perfusion. The combination of hypotension and microvascular occlusion results in tissue ischemia and ultimately leads to multiple organ failure.
The inflammatory responses to microbial infection are initiated by innate immunity via Toll-like receptors (TLRs) that recognize the pathogen-associated molecular patterns characteristic of microbial products (5). The inflammatory response to LPS is mediated mainly by a receptor complex composed of LPS-binding protein CD14 and TLR4, which is critically important in the innate immune recognition of Gram-negative pathogens (6). Upon activation of TLR4, the adaptor protein MyD88 is recruited to the receptor, which in turn triggers a cascade of signaling events leading to the activation of transcription factor NF-κB and mitogen-activated protein (MAP) kinases. NF-κB plays a pivotal role in the transcription of a variety of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6. MAP kinases, including extracellular signal–regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38, also modulate cytokine expression at multiple levels. ERK is required for the transport of TNF-α mRNA from the nucleus to the cytoplasm. Knockout mice deficient in the proto-oncogene Tpl-2 demonstrate defects in ERK activation in response to LPS as well as exhibit a substantial attenuation in circulatory TNF-α levels after LPS challenge and marked resistance to LPS-induced shock (7). p38 is also a crucial mediator for the production of proinflammatory cytokines (8) and has been identified as the target of a class of small molecule inhibitors capable of inhibiting the production of inflammatory cytokines (9). Furthermore, knockout of MAP kinase–activated protein kinase 2 (MK2), a downstream target of p38, abolishes the production of proinflammatory cytokines and protects mice from LPS-induced mortality (10). It has been shown that p38 may mediate TNF-α production by enhancing the stability of TNF-α mRNA and accelerating its translation (11, 12).
Because a group of dual specificity MAP kinase phosphatases (MKPs) play a pivotal role in the feedback control of MAP kinases, we hypothesize that MKPs act to dephosphorylate and inactivate MAP kinase, thus restraining the production of proinflammatory cytokines and preventing septic shock. Previously, we have shown that MKP-1 is induced by bacterial products and plays an important role in the regulation of TNF-α production in isolated macrophages (13, 14). In this report, we have studied the physiological function of MKP-1 in vivo during the inflammatory responses to LPS using Mkp-1 knockout mice. Our studies indicate that MKP-1 is a pivotal feedback control regulator of innate immune responses and plays a critical role in suppressing endotoxic shock.
RESULTS
MKP-1 is responsible for the attenuation of both JNK and p38 after LPS stimulation
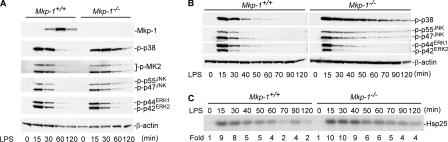
To examine the physiological function of MKP-1 in the regulation of the innate immune responses during bacterial infection, thioglycollate-elicited peritoneal macrophages from both Mkp-1 +/+ and Mkp-1 −/− mice were stimulated with LPS. In Mkp-1 +/+ macrophages, LPS stimulation resulted in an increase in MKP-1 protein levels within 30 min, with levels peaking at ∼60 min. The increase of the MKP-1 level temporally coincided with the inactivation of p38, JNK, and ERK (Fig. 1 A). No MKP-1 protein was detected in the Mkp-1 −/− macrophages, even with LPS stimulation (Fig. 1 A, top). Mkp-1 −/− cells exhibited substantially prolonged p38 and JNK activation compared with Mkp-1 +/+ macrophages (Fig. 1 A). However, the activation kinetics of ERK were similar in both cell groups, indicating that MKP-1 does not play a significant role in ERK inactivation in this system. MK2, often referred to as MAP kinase–activated protein kinase 2, is a physiological target of p38 (15). MK2 plays a critical role in the innate immune response to bacterial infection and is required for the production of many inflammatory cytokines in vivo after LPS challenge (10). Consistent with the sustained p38 activation in Mkp-1 −/− macrophages, LPS stimulation also resulted in a prolonged MK2 activation in Mkp-1 −/− macrophages (Fig. 1 A, third panel). The findings that Mkp-1–deficient cells had sustained p38 and JNK activities after LPS stimulation were reinforced by detailed analysis of the activation kinetics of p38, JNK, and ERK (Fig. 1 B). The activity of MK2 was also examined by immune complex kinase assays using Hsp25 as a substrate. MK2 activity in Mkp-1 knockout macrophages was sustained after LPS stimulation relative to wild-type cells (Fig. 1 C). These results clearly indicate that MKP-1 plays a predominant role in the termination of the JNK and p38 pathways in vivo.
Figure 1.
Mkp-1 deficiency results in prolonged p38 and JNK activation. (A) The kinetics of MAP kinase activation in Mkp-1 +/+ and Mkp-1 −/− macrophages after LPS stimulation. Elicited peritoneal macrophages from both Mkp-1 +/+ and Mkp-1 −/− mice were stimulated with 100 ng/ml LPS for the indicated times, and cell lysates were analyzed for phospho-p38, phospho-JNK, phospho-ERK, phospho-MK2, and MKP-1 using Western blotting. (B) Detailed kinetics of p38, JNK, and ERK inactivation after LPS challenge. Mkp-1 +/+ and Mkp-1 −/− samples were processed together, with the exception of the initial electrophoresis in which Mkp-1 +/+ and Mkp-1 −/− samples were run on different gels. (C) Comparison of MK2 activities between Mkp-1 +/+ and Mkp-1 −/− macrophages using immune complex kinase assays. Peritoneal macrophages were treated with 100 ng/ml LPS for the indicated times, and MK-2 kinase activity was examined by immune complex kinase assays using [γ-32P]ATP and a mouse Hsp25 as substrate. Kinase activities were quantitated using a phosphorImager and expressed as fold-activation relative to controls.
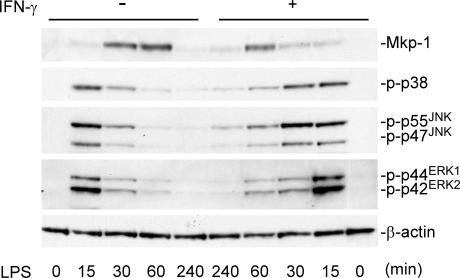
IFN-γ has been shown to activate macrophages, resulting in more robust production of inflammatory cytokines (16). We found that the pretreatment of resident peritoneal macrophages with IFN-γ inhibited MKP-1 induction by LPS and resulted in sustained p38 and JNK activation in LPS-stimulated resident peritoneal macrophages (Fig. 2). This observation suggests that IFN-γ may increase the activity of macrophages in part through attenuation of MKP-1 induction. Collectively, our results indicate that MKP-1 is a primary regulator of MAP kinases, especially p38 and JNK, in peritoneal macrophages activated by the Gram-negative bacterial cell wall component, LPS.
Figure 2.
IFN-γ enhances activation of p38 and JNK by inhibiting MKP-1 expression. Resident peritoneal macrophages were treated with or without 50 U/ml IFN-γ overnight before LPS stimulation (100 ng/ml) at the indicated times. The levels of MKP-1 protein and phosphorylated MAP kinases were examined using Western blotting. Please note that the order for the + IFN-γ samples is from right to left.
Mkp-1 deficiency alters the pattern of cytokine production in LPS-stimulated innate immune cells
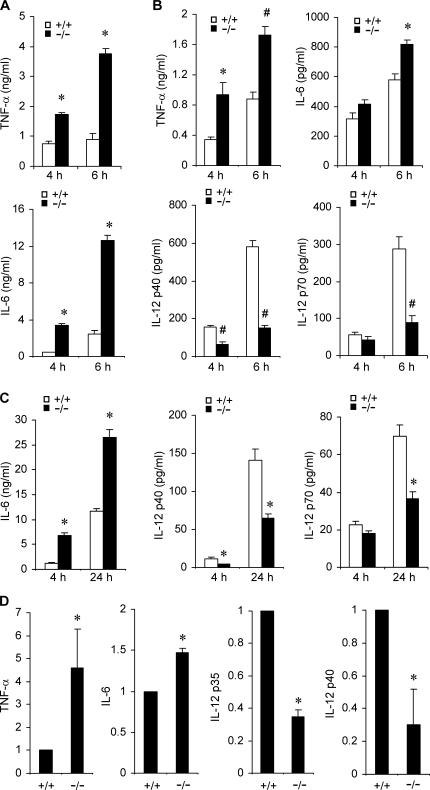
Because MAP kinases are pivotal in modulating innate immune responses (17), we investigated the effect of Mkp-1 deficiency on cytokine production by several different types of innate immune cells. First, resident peritoneal macrophages were isolated from Mkp-1 +/+ and Mkp-1 −/− mice, primed with IFN-γ, and stimulated with LPS. The concentrations of cytokines secreted into the media were determined by ELISA. In the absence of LPS stimulation, TNF-α and IL-6 production was undetectable (not depicted). In response to LPS stimulation, IFN-γ–primed resident peritoneal macrophages from Mkp-1 −/− mice produced significantly more TNF-α and IL-6 than did their wild-type counterparts (Fig. 3 A). To assess the role of MKP-1 in cytokine production in inflammatory cells, we elicited both wild-type and Mkp-1 −/− mice with thioglycollate and isolated peritoneal macrophages from these mice. Similar to what was observed for IFN-γ–primed resident macrophages, thioglycollate-elicited peritoneal macrophages from Mkp-1 −/− mice also mounted a more robust TNF-α production than did wild-type cells upon LPS stimulation (Fig. 3 B, top left). Likewise, IL-6 production by thioglycollate-elicited Mkp-1 −/− peritoneal macrophages was also significantly elevated compared with wild-type cells at 6 h, but not at 4 h, likely reflecting the delayed expression of IL-6 relative to TNF-α (Fig. 3 B, top right). IL-12 is a classic Th1 cytokine produced by macrophages that can promote the differentiation of naive Th cells into Th1 cells (18). Surprisingly, production of IL-12 by thioglycollate-elicited Mkp-1 −/− peritoneal macrophages in response to LPS was significantly decreased relative to Mkp-1 +/+ peritoneal macrophages (Fig. 3 B, bottom). Enhanced IL-6 production and attenuated IL-12 biosynthesis upon LPS stimulation were also observed in unprimed Mkp-1 −/− resident peritoneal macrophages compared with their Mkp-1 +/+ counterparts (Fig. 3 C).
Figure 3.
Knockout of Mkp-1 alters cytokine expression in macrophages. (A) Cytokine production by IFN-γ–primed resident peritoneal macrophages. Resident peritoneal macrophages primed with IFN-γ overnight were stimulated with LPS for 4 and 6 h. Cytokine concentrations in the medium were analyzed by ELISA. Data are presented as the mean ± SEM (n = 3 in each group). *, Mkp-1 −/− different from Mkp-1 +/+, P < 0.001. (B) Cytokine production by thioglycollate-elicited peritoneal macrophages after stimulation with 100 ng/ml LPS for 4 and 6 h. Data are presented as mean ± SEM (n = 4 in each group). *, Mkp-1 −/− different from Mkp-1 +/+, P < 0.05; #, Mkp-1 −/− different from Mkp-1 +/+, P < 0.005. (C) Cytokine production by unprimed resident peritoneal macrophages from both Mkp-1 +/+ and Mkp-1 −/− mice. Resident peritoneal macrophages were stimulated with 100 ng/ml LPS for the indicated times, and cytokine concentrations in the medium were assayed by ELISA. Data are presented as the mean ± SEM (n = 6 in each group). *, Mkp-1 −/− different from Mkp-1 +/+, P < 0.001. (D) Expression of TNF-α, IL-6, IL-12p35, and IL-12p40 mRNA in LPS-stimulated wild-type and Mkp-1 −/− resident peritoneal macrophages. Resident peritoneal macrophages were primed overnight with 50 U/ml IFN-γ and then stimulated with 100 ng/ml LPS for 4 h. qRT-PCR was performed to assess the levels of mRNA for TNF-α, IL-6, IL-12p35, and IL-12p40. The relative mRNA levels were normalized to the GAPDH mRNA. Values represent expression levels relative to those in wild-type cells. Data are means ± SEM of three independent experiments. *, different from Mkp-1 −/− cells, P < 0.05.
To study the effect of Mkp-1 deficiency on the expression of inflammatory cytokines, resident peritoneal macrophages were isolated from Mkp-1 +/+ and Mkp-1 −/− mice, primed with IFN-γ, and stimulated with LPS for 4 h. The expression levels of TNF-α, IL-6, IL-12p35, and IL-12p40 mRNAs were assessed by quantitative real-time RT-PCR (qRT-PCR; Fig. 3 D). TNF-α mRNA levels in the LPS-stimulated Mkp-1 −/− macrophages were 3.5-fold higher than those in the LPS-stimulated Mkp-1 +/+ macrophages. IL-6 mRNA levels were ∼50% higher in the LPS-stimulated Mkp-1 −/− macrophages than those in their wild-type counterparts. In contrast, the expression levels of both IL-12p35 and IL-12p40 were significantly decreased in the LPS-stimulated Mkp-1 −/− macrophages relative to their wild-type counterparts. In fact, compared with the mRNA levels in the LPS-stimulated wild-type macrophages, IL-12p35 and IL-12p40 mRNA levels in the Mkp-1 −/− macrophages were decreased by 65 and 70%, respectively. Therefore, the difference observed in cytokine levels in Mkp-1 −/− cells is likely due to the modulation of cytokine gene expression.
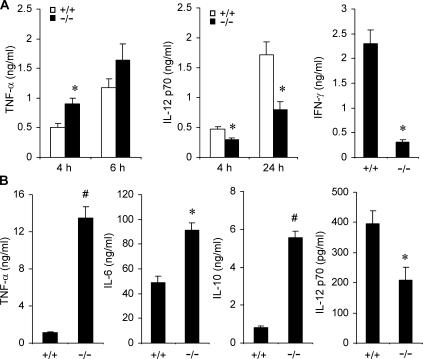
To understand the role of Mkp-1 in the production of inflammatory cytokines in other cell populations, splenocytes were isolated from both Mkp-1 +/+ and Mkp-1 −/− mice and stimulated with LPS to assess the effect of Mkp-1 deletion on cytokine production. In response to LPS, splenocytes from Mkp-1 +/+ mice produced considerable amount of TNF-α (Fig. 4 A, left). The amount of TNF-α secreted by the Mkp-1–deficient splenocytes was appreciably greater than that produced by the Mkp-1 +/+ splenocytes (Fig. 4 A, left). However, compared with the more robust TNF-α production by the Mkp-1–deficient splenocytes, the production of two classic Th1 cytokines, IFN-γ and IL-12, was actually attenuated in these cells relative to that in the wild-type splenocytes (Fig. 4 A, middle and right).
Figure 4.
Knockout of Mkp-1 shifts the pattern of cytokine expression in splenocytes and dendritic cells. (A) Cytokine expression in LPS-stimulated splenocytes. Splenocytes isolated from Mkp-1 +/+ and Mkp-1 −/− mice were stimulated with 100 ng/ml LPS for the indicated times, and cytokine concentrations in the medium were assayed by ELISA. IFN-γ levels were measured 24 h after LPS treatment. Data are presented as the mean ± SEM (n = 3–9 for each group). *, Mkp-1 −/− different from Mkp-1 +/+, P < 0.05. (B) Cytokine production by bone marrow–derived dendritic cells from both Mkp-1 +/+ and Mkp-1 −/− mice. Cells were stimulated with 1 μg/ml LPS for 24 h. Data are presented as mean ± SEM (n = 3 in each group). *, Mkp-1 −/− different from Mkp-1 +/+ , P < 0.05; #, Mkp-1 −/− different from Mkp-1 +/+, P < 0.005.
Dendritic cells are professional antigen-presenting cells that play a vital role in the development of adaptive immunity in response to pathogens by facilitating T lymphocyte activation and differentiation (19). The effect of Mkp-1 knockout on cytokine production in dendritic cells was examined. Dendritic cells were derived from bone marrow isolated from Mkp-1 +/+ and Mkp-1 −/− mice by culturing in the presence of GM-CSF. LPS stimulation of dendritic cells resulted in significant production of TNF-α (Fig. 4 B). Remarkably, Mkp-1 −/− cells produced ∼10-fold more TNF-α and 75% more IL-6 when compared with wild-type dendritic cells (Fig. 4 B, first and second panels). Interestingly, the production of IL-10, a classic antiinflammatory cytokine (20), in dendritic cells was also dramatically increased in Mkp-1 −/− cells relative to the wild-type cells (Fig. 4 B, third panel). In contrast, IL-12 production in dendritic cells was significantly attenuated in Mkp-1–deficient cells relative to wild-type cells (Fig. 4 B, fourth panel). Collectively, our studies indicate that loss of Mkp-1 results in profound alteration in the cytokine expression profiles in a variety of immune cells.
Knockout of Mkp-1 dramatically augments the biosynthesis of both the proinflammatory cytokine TNF-α and the antiinflammatory cytokine IL-10 in vivo
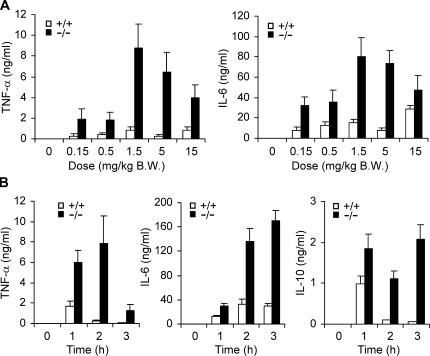
To study the role of MKP-1 in the regulation of cytokine production in vivo, Mkp-1 +/+ and Mkp-1 −/− mice were injected i.p. either with vehicle (PBS) or with different doses of LPS. Neither Mkp-1 +/+ nor Mkp-1 −/− mice injected with PBS produced a detectable amount of TNF-α or IL-6 in their plasma (Fig. 5 A). Compared with wild-type animals, Mkp-1 −/− mice were more sensitive to LPS and produced significantly higher levels of both TNF-α (Fig. 5 A, left) and IL-6 (Fig. 5 A, right) over a broad range of LPS doses. A time course of cytokine production in response to LPS was also examined in Mkp-1 +/+ and Mkp-1 −/− mice. In the absence of LPS challenge, serum levels of TNF-α, IL-6, and IL-10 were undetectable (Fig. 5 B). In response to LPS challenge, serum TNF-α levels in Mkp-1 +/+ mice were substantially elevated within 1 h followed by a rapid decline thereafter (Fig. 5 B, left). In Mkp-1 −/− mice challenged with LPS, serum TNF-α levels were 3.5-fold higher than those of Mkp-1 +/+ mice at 1 h, and the levels continued to increase at 2 h. By 2 h, the serum TNF-α levels in Mkp-1 −/− mice were ∼29-fold higher than those in Mkp-1 +/+ mice. Although TNF-α levels in Mkp-1 −/− mice declined substantially by 3 h, the levels of TNF-α remained significantly higher than those in wild-type mice. In fact, TNF-α levels in Mkp-1 −/− mice at 3 h were not significantly different from the maximal TNF-α levels observed in Mkp-1 +/+ mice at 1 h (Fig. 5 B, left). In Mkp-1 +/+ mice, serum IL-6 was elevated within 1 h and plateaued by 2 h (Fig. 5 B, middle). Compared with Mkp-1 +/+ mice, Mkp-1 −/− mice produced substantially more IL-6. Furthermore, serum IL-6 levels in Mkp-1 −/− mice continued to increase through the observed 3-h period. Similar to TNF-α, IL-10 was increased transiently in Mkp-1 +/+ mice (Fig. 5 B, right). On the contrary, LPS-induced serum IL-10 in Mkp-1 −/− mice remained elevated throughout the 3-h experimental period (Fig. 5 B, right). Collectively, our results indicate that Mkp-1 knockout has profound effects on the production of TNF-α, IL-6, and IL-10 in vivo.
Figure 5.
Deficiency in Mkp-1 enhances LPS-triggered production of TNF-α, IL-6, and IL-10 in vivo. (A) Both Mkp-1+/+ and Mkp-1 −/− mice were injected i.p. with either vehicle (PBS) or LPS at indicated doses. Mice were killed 90 min later and plasma cytokine concentrations were measured by ELISA. Data are presented as mean ± SEM (n = 6 in each group). Two-way ANOVA demonstrates a significant effect of both genotype (P < 0.001) and LPS dose (P = 0.005) on TNF-α and IL-6 concentrations. (B) Mice were injected i.p. with LPS (1.5 mg/kg body weight) and killed at the indicated times. Serum cytokines were measured using ELISA. Data are presented as mean ± SEM (n = 10). Two-way ANOVA demonstrates a significant effect of both genotype and time after LPS challenge on TNF-α, IL-6, and IL-10 concentrations (P < 0.001).
Deficiency in Mkp-1 renders mice susceptible to endotoxin shock and multiple organ failure
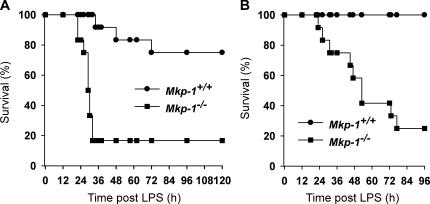
TNF-α is a pivotal inflammatory cytokine that plays an important role in the pathogenesis of septic shock (21, 22). Because Mkp-1 −/− mice exhibited a marked elevation in TNF-α upon LPS challenge, we examined the effect of Mkp-1 deficiency on survival. Both Mkp-1 +/+ and Mkp-1 −/− mice were injected i.p. with LPS, and survival of these animals was monitored over 4–5 d. An LPS dose of 5 mg/kg body weight resulted in severe distress in both Mkp-1 +/+ and Mkp-1 −/− mice. For Mkp-1 knockout mice, mortality was observed within 22 h after LPS challenge (Fig. 6 A). By 32 h, 83% of the Mkp-1 −/− mice had died, and no further death was noted through 120 h. In contrast, for the Mkp-1 +/+ mice, the first mortality occurred at 34 h. By the end of the experiment (120 h), only 25% of the Mkp-1 +/+ mice had died (Fig. 6 A). An LPS dose of 1.5 mg/kg body weight caused few signs of distress in the Mkp-1 +/+ mice, and all Mkp-1 +/+ mice survived through the 96-h period (Fig. 6 B). However, the Mkp-1 −/− mice given 1.5 mg/kg LPS were severely distressed, and 75% of them died over the 96-h experimental period (Fig. 6 B).
Figure 6.
Increased mortality in Mkp-1 knockout mice in response to LPS challenge. (A) Survival curves of Mkp-1 +/+ and Mkp-1 −/− mice after challenge i.p. with 5 mg LPS per kg body weight. Kaplan-Meier analysis demonstrates a significant difference in survival between Mkp-1 +/+ and Mkp-1 −/− mice (P < 0.001; n = 12 in each group). (B) Survival curves of Mkp-1 −/− mice and their wild-type littermates after challenge i.p. with 1.5 mg LPS per kg body weight (P < 0.0005; n = 12 in each group).
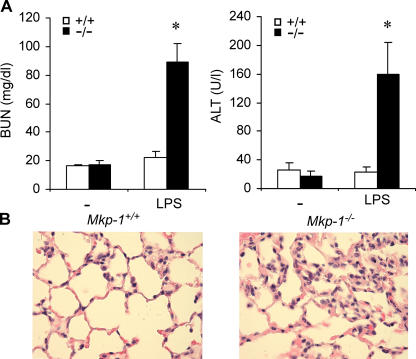
To examine the effects of Mkp-1 deficiency on the function of key organs, wild-type and Mkp-1−/− mice were challenged with either vehicle (PBS) or LPS at a dose of 1.5 mg/kg body weight and killed 24 h after LPS injection. Blood samples were collected, and blood urea nitrogen (BUN) levels, a measurement of renal function, were measured (Fig. 7 A, left). BUN levels in vehicle-treated mice were similar in both Mkp-1+/+ and Mkp-1−/− mice (16.3 ± 1.3 vs. 17.0 ± 3.4 mg/dl, respectively). LPS challenge did not result in a significant change in BUN for Mkp-1+/+ mice. However, a substantial increase in BUN levels was observed in Mkp-1−/− mice after LPS challenge (89.1 ± 12.6 mg/dl). Blood alanine aminotransferase (ALT) activity, an indication of liver damage, was measured (Fig. 7 A, right). There was no significant difference in ALT levels between vehicle-treated Mkp-1+/+ and Mkp-1−/− mice (25.8 ± 2.4 vs. 17.9 ± 2.4 U/liters, respectively). Although LPS challenge did not significantly change blood ALT levels in Mkp-1+/+ mice, challenge of Mkp-1−/− mice with LPS resulted in a significant increase in blood ALT levels (Fig. 7 A, right). Although the ALT levels in LPS-challenged wild-type mice were 22.7 ± 2.8 U/liters, ALT levels in LPS-challenged Mkp-1−/− mice were increased to 149.2 ± 3.6 U/liters.
Figure 7.
LPS causes renal, heptic, and pulmonary damages in Mkp-1−/− mice. Mice were challenged with LPS (1.5 mg/kg body weight) and killed 24 h later. Plasma BUN and ALT activity were measured. Lungs were perfused and fixed with formalin, and 4-μm sections were stained with hematoxylin and eosin. (A) BUN and ALT activities in vehicle- and LPS-challenged mice. Data are presented as mean ± SEM of 8–13 independent experiments. *, P < 0.001, one-way ANOVA comparing Mkp-1 +/+ with Mkp-1 −/− groups. (B) Representative images of lung sections from LPS-challenged wild-type and Mkp-1 −/− mice.
Because respiratory failure is often associated with septic shock syndrome, we examined the effect of Mkp-1 deficiency on lung histology. Lung tissues were fixed with formalin at a constant distending pressure of 25 cm H2O. The lung sections were stained with hematoxylin and eosin. The lungs from Mkp-1 −/− mice appeared normal, and there were no differences observed in lung histology between vehicle-treated Mkp-1 +/+ and Mkp-1 −/− mice (not depicted). However, after LPS challenge, massive infiltration of leukocytes in the interstitial spaces, marked thickening of the alveolar septa, and pulmonary edema were observed in lungs from Mkp-1 −/− mice, but not in lungs from Mkp-1 +/+ mice (Fig. 7 B). Collectively, our results indicate that Mkp-1 −/− but not Mkp-1 +/+ mice are highly susceptible to the development of multiple organ failure syndrome after LPS administration.
Mkp-1 knockout mice develop severe hypotension in response to LPS challenge
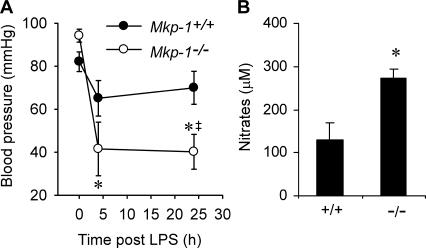
Hypotension is a clinical characteristic of severe sepsis and plays an important role in the pathophysiology of septic shock and multiple organ failure syndrome. To understand the role of MKP-1 in the regulation of vasculature function, we measured systemic blood pressure in both Mkp-1 +/+ and Mkp-1 −/− mice after LPS challenge (Fig. 8 A). For Mkp-1 +/+ mice, LPS challenge at a dose of 1.5 mg/kg resulted in no significant change in systolic blood pressure. In contrast, LPS challenge led to a substantial decrease in systolic blood pressure in Mkp-1 −/− mice. By 4 h after LPS challenge, the systolic pressure in Mkp-1 −/− mice decreased from 94.2 ± 3.0 mmHg to 41.5 ± 12.5 mmHg (P < 0.005). Furthermore, the severe hypotension in the LPS-challenged Mkp-1 −/− mice persisted at 24 h (Fig. 8 A). Nitric oxide plays a critical role in the regulation of vasculature function. Blood samples were collected from both wild-type and Mkp-1 −/− mice 24 h after LPS challenge (1.5 mg/kg body weight), and plasma nitrate levels were measured (Fig. 8 B). Plasma nitrate levels were significantly higher in the Mkp-1 −/− mice than in Mkp-1 +/+ mice after LPS challenge. Collectively, these observations indicate a critical regulatory role of MKP-1 in modulating the inflammatory responses to LPS and demonstrate that lack of MKP-1 markedly sensitizes mice to septic shock.
Figure 8.
LPS challenge results in hypotension and increased nitric oxide production in Mkp-1−/− mice. Mkp-1 +/+ and Mkp-1 −/− mice were injected i.p. with LPS (1.5 mg/kg body weight). (A) Systolic blood pressure in mice injected with LPS. Data are presented as mean ± SEM of six independent experiments. *, 4 and 24 h different from control (0 h), P < 0.001; †, Mkp-1 −/− different from Mkp-1 +/+ at same time point, P < 0.05. (B) Plasma nitrate levels. Mkp-1 +/+ and Mkp-1 −/− mice were injected i.p. with LPS and killed 24 h later. Plasma nitrate levels were measured by chemiluminescence. Data are presented as the mean ± SEM of four independent experiments. *, Mkp-1 −/− different from Mkp-1 +/+, P < 0.05.
DISCUSSION
In this report, we have demonstrated that MKP-1 is a critical negative regulator in the innate immune response to LPS. We have found that MKP-1 is induced by LPS and plays a critical role in the attenuation of both JNK and p38 in peritoneal macrophages (Fig. 1). We showed that deletion of the Mkp-1 gene resulted in a prolonged activation of JNK and p38 after LPS stimulation, leading to augmented production of the proinflammatory cytokines TNF-α and IL-6 in macrophages (Fig. 3). Although the prolonged JNK and p38 activation was only demonstrated experimentally in thioglycollate-elicited peritoneal macrophages, we found that LPS stimulation also resulted in a substantial increase in the production of TNF-α and IL-6 in Mkp-1–deficient splenocytes and dendritic cells (Fig. 4). This observation strongly suggests that MKP-1 plays a similar regulatory role in these cell types. The fact that Mkp-1–deficient mice produced dramatically more TNF-α and IL-6 after LPS challenge further validates the critical role of MKP-1 in the control of these two proinflammatory cytokines in vivo (Fig. 5). The substantial increase in mortality after LPS challenge in Mkp-1–deficient mice illustrates the critical importance of the MKP-1–dependent regulatory mechanism in host defense (Fig. 6). The severe hypotension (Fig. 8) and dysfunction of vital organs (Fig. 7) in LPS-challenged Mkp-1–deficient mice are consistent with the clinical symptoms of septic shock. These results clearly indicate that MKP-1 acts as a vital suppressor of the inflammatory responses and thereby protects the host from shock, multiple organ failure, and mortality upon exposure to LPS. Our results also raise the possibility that variations in the MKP-1 gene may represent a susceptibility factor for septic shock.
What is the mechanism through which MKP-1 controls the inflammatory cascade and prevents shock and multiple organ failure in the host? First, by inactivating JNK and p38, MKP-1 determines the window of synthesis of proinflammatory cytokines, including TNF-α. In this sense, MKP-1 serves as a servocontrol mechanism for TNF-α production. In the absence of the MKP-1–mediated servocontrol mechanism, the signal directing TNF-α synthesis is not appropriately down-regulated, thus resulting in the overproduction of proinflammatory cytokines. Previously, it has been shown that both JNK and p38 positively regulate TNF-α biosynthesis by stabilizing TNF-α mRNA and enhancing its translation (12, 23). Thus, it is plausible that both the stability and the translation of TNF-α mRNA are enhanced as the result of sustained JNK and p38 activation in Mkp-1 −/− cells, explaining the prolonged TNF-α biosynthesis in Mkp-1 −/− mice after LPS challenge (Fig. 5 B). A potential explanation for the shock and multiple organ dysfunction is that the excessive TNF-α triggers a considerable elevation in nitric oxide synthase activity (24, 25), resulting in severe hypotension that leads to hypoperfusion and multiple organ failure (Fig. 8).
A balance between activation and subsequent deactivation of the immune system is of critical importance in the host immunological defenses. Although activation of the signal transduction cascades is critical for mounting an aggressive immune response to eliminate invading pathogens, deactivation of the signaling pathways restrains the potentially devastating actions of the immunological system on the host, thus preventing self-destruction. A variety of negative regulators operate at various steps in the critical signal transduction pathways downstream of TLRs. These negative regulators modulate the strength and duration of the transduced signals and control the production of inflammatory cytokines (26). It has been shown that TLR4 is transiently suppressed in response to LPS (27). In addition to the modulation at the receptor level, several antiinflammatory proteins are also induced, which include IL-1 receptor–associated kinase (IRAK)-M, suppressor of cytokine signaling (SOCS)-1, inhibitor-κB, MKP-1, and antiinflammatory cytokines such as IL-10 (26). Through these inhibitory proteins, cells not only terminate the signaling cascade at the cell surface, but also switch off downstream mediators, thus silencing the signaling pathways leading to the production of proinflammatory cytokines. Therefore, a timely “switch-off” of the signaling events is crucial, as it not only prevents the overproduction of the potentially harmful cytokines, but also prepares the cells for responding to subsequent pathogenic infection. The discovery of MKP-1 as a crucial negative regulator of the innate immune responses both in vivo and in vitro places it in the center of the complex negative regulatory mechanism dictating endotoxin tolerance. Although similarities exist between phenotypes of Mkp-1 knockout mice and mice lacking other negative regulators, there are also important differences. It appears that knockout of Irak-m or Socs-1 leads to generalized hyperresponses of innate cells to LPS challenge (28, 29), whereas deletion in the Mkp-1 gene changes the pattern of innate immune responses. For example, knockout of either Irak-m or Socs-1 resulted in an increase in IL-12 production after LPS stimulation (28, 29). In contrast, knockout of Mkp-1 leads to decreased LPS-induced IL-12 production (Figs. 3 and 4). The phenotypical differences between Mkp-1 knockout mice and Irak-m or Socs-1 knockout mice likely reflect the different mechanisms on which these regulators operate.
There are at least 11 MKPs in mammalian cells (30). Although this group of phosphatases exhibit differential substrate specificities toward different MAP kinases, many of the phosphatases also share substrates. For example, MKP-1 has been shown to prefer p38 and JNK as substrates (31), although it was originally characterized as an ERK phosphatase (32). In contrast, phosphatase of activated cells 1, an MKP predominantly expressed in hematopoietic cells, preferentially inactivates ERK and p38 (33). In macrophages, at least four MKPs are expressed: MKP-1, MKP-2, phosphatase of activated cells 1, and MKP-5/MKP-M (13, 34). It is possible that multiple MKPs act cooperatively to control the MAP kinase cascades. In the absence of MKP-1 protein, JNK and p38 will be eventually inactivated by other MKPs, albeit at a much slower rate. This is consistent with the observation that deactivation of p38 and JNK in LPS-stimulated Mkp-1 −/− macrophages was delayed. The finding that inactivation of ERK was not affected by the knockout of the Mkp-1 gene (Fig. 1) further supports the notion that MKP-1 does not play a significant role in the inactivation of ERK in our system. Recently, Zhang et al. (35) demonstrated that MKP-5 acts as a JNK phosphatase and plays an important role in the regulation of both innate and adaptive immune responses. Whether knockout of Mkp-1 has an appreciable impact on the adaptive immunity remains to be addressed. Although it has not been reported whether knockout of Mkp-5 also sensitizes mice to endotoxin shock, it is tempting to speculate that the endotoxin-induced phenotype of Mkp-5 knockout may be less severe than that of Mkp-1 knockout. MKP-5 has been found to only regulate the JNK pathway, whereas MKP-1 regulates both the JNK and p38 pathways. Considering that the JNK and the p38 pathways regulate both related and distinct cellular functions (36, 37), knockout of Mkp-1 may perturb more cellular processes and have a more severe consequence than the Mkp-5 deletion. Perhaps reflecting different severity, the differences in blood TNF-α levels between Mkp-5 knockout mice and their wild-type littermates were relatively modest (less than twofold; reference 35). In contrast, the difference between the blood TNF-α levels in Mkp-1–deficient mice and their wild-type littermates after LPS challenge was dramatic (almost 30-fold; Fig. 5).
Another intriguing finding from this study is that Mkp-1 deficiency also leads to elevated production of IL-10 both in isolated cells and in vivo. These results indicate that IL-10 is regulated in a fashion similar to TNF-α, suggesting that the IL-10–mediated antiinflammatory mechanism is hardwired in the cells to counterbalance the actions of proinflammatory cytokines. Interestingly, it has been shown that p38 is crucial for the production of IL-10 during LPS stimulation (38, 39). Therefore, the increase in IL-10 production in Mkp-1 −/− cells may be a reflection of the increase in p38 activity. The down-regulation of IL-12 and IFN-γ observed in Mkp-1–deficient cells was also an interesting and unexpected finding (Figs. 3 and 4). Although the mechanisms mediating their down-regulation are unclear, two potential mechanisms may be involved. First, IL-10 is a potent antiinflammatory cytokine (20, 40). The profound increase in IL-10 secretion after LPS stimulation may down-regulate the expression of the classic Th1 cytokines IL-12 (Fig. 3 D) and IFN-γ through an autocrine system. Indeed, it has been reported that IL-10 inhibits IL-12 expression in dendritic cells (41). Second, although less likely, JNK and/or p38 may negatively regulate the expression of IL-12 and IFN-γ. Thus, loss of MKP-1–mediated attenuation of these pathways may lead to the down-regulation of these cytokines. It is worth noting that IFN-γ can also influence Mkp-1 expression (Fig. 2). Such a regulation likely has important biological significance. It has long been known that IFN-γ can boost the antimicrobial activity of macrophages and substantially enhance the secretion of TNF-α by macrophages (16). We found that IFN-γ can attenuate LPS-induced Mkp-1 expression and prolong the activation of both JNK and p38. It is plausible that the prolongation of these MAP kinase pathways contributes to the biological activities of IFN-γ.
In summary, we found that Mkp-1 knockout prolonged the activation of p38 and JNK in LPS-stimulated macrophages. This prolonged activation of p38 and JNK was associated with substantially elevated production of TNF-α and IL-6 in macrophages. Furthermore, Mkp-1 knockout mice demonstrated a substantial increase in mortality after LPS treatment compared with wild-type mice. LPS treatment of Mkp-1 knockout mice resulted in renal, hepatic, and pulmonary dysfunction. Collectively, our findings demonstrate the essential role of Mkp-1 in containing the host immune response to bacterial products. The unexpected findings that Mkp-1 deficiency resulted in decreased IL-12 and IFN-γ but increased IL-10 production suggest that Mkp-1 regulation of the immune response is more complex than simply down-regulation of proinflammatory cytokine production. We speculate that Mkp-1 may represent a pharmacological target for treatment of patients with endotoxic shock, and that polymorphisms in the MKP-1 gene may result in greater susceptibility to endotoxic shock.
MATERIALS AND METHODS
Animals.
Cryopreserved embryos of Mkp-1 knockout mice (Mkp-1 +/− and Mkp-1 −/−) were provided by Bristol-Myers Squibb Pharmaceutical Research Institute and regenerated into mice at The Jackson Laboratory. These mice were bred in-house to yield both Mkp-1 +/+ and Mkp-1 −/− mice. All animals received humane care and all animal-related work was performed in accordance with National Institutes of Health guidelines. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Columbus Children's Research Institute.
Isolation of peritoneal macrophages, splenocytes, and dendritic cells.
Peritoneal macrophages were isolated from naive Mkp-1 +/+ or Mkp-1 −/− mice by peritoneal lavage. The resident peritoneal macrophages were cultured overnight in RPMI 1640 medium (Mediatech) supplemented with 5% FBS (Hyclone Laboratories) and 50 U/ml IFN-γ (Calbiochem) before being stimulated with 100 ng/ml LPS (Escherichia coli O127:B8) purchased from Sigma-Aldrich. To isolate elicited peritoneal macrophages, mice were injected i.p. (2 ml/mouse) with 3% Brewer Thioglycollate Medium (BD Diagnostic) and cells were harvested 4 d later. Splenocytes were isolated, cultured in RPMI 1640 medium containing 10% FBS, and stimulated with 100 ng/ml LPS. Bone marrow–derived dendritic cells were prepared as described previously (42). In brief, bone marrow cells depleted of T and B cells were cultured for 5 d in RPMI 1640 medium supplemented with 5% FBS and 10 ng/ml of recombinant murine GM-CSF (BD Biosciences). Cells were replated and matured in 1 μg/ml LPS (Escherichia coli O55:B55; Sigma-Aldrich) for 24 h.
Western blotting, immune complex kinase assays, and ELISA.
Western blot analysis was performed using antibodies against MKP-1 (Santa Cruz Biotechnology, Inc.), phosphorylated JNK, p38, and ERK, as well as phosphorylated MK2 (Cell Signaling Technology). MK-2 activity was measured by immune complex kinase assays using [γ-32P]ATP and recombinant mouse Hsp25 (StressGen Biotechnologies) as a substrate as described previously (14). TNF-α, IL-6, IL-10, IL-12, and IFN-γ in the culture medium were determined using ELISA as described previously (43).
qRT-PCR.
Resident peritoneal macrophages isolated from naive Mkp-1 +/+ or Mkp-1 −/− mice were primed overnight with 50 U/ml IFN-γ and then stimulated with 100 ng/ml LPS (Escherichia coli O127:B8) for 4 h. Total RNA was isolated using Trizol (Invitrogen), digested with RNase-free DNase I, and purified with RNeasy MinElute Cleanup kit (QIAGEN). First-strand cDNA was synthesized with 1.5 μg total RNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen). qRT-PCR was performed by the comparative threshold cycle (ΔCT) method and normalized to GAPDH. The primers used for GAPDH, IL-6, IL-12p35, and IL-12p40 were as described previously (41). The following primers were used for TNF-α: 5′-CCCCAAAGGGATGAGAAGTT-3′ (forward) and 5′-CACTTGGTGGTTTGCTACGA-3′ (reverse).
LPS injection and endotoxin shock.
Mkp-1 +/+ or Mkp-1 −/− mice were injected i.p. with PBS or the designated doses of LPS dissolved in LPS. Plasma or serum was assayed for cytokine levels using ELISA. Plasma BUN and ALT activity was measured using Infinity Urea and ALT kits (Thermo Electron), respectively. Lungs were first perfused with 10% formalin at constant distending pressure of 25 cm H2O for 10 min, excised from the animals, and then placed in 10% formalin overnight at 4°C. 4-μm sections were prepared and stained with hematoxylin and eosin. Systolic blood pressures were determined noninvasively by tail cuff monitor (44). Plasma nitrate levels were measured by chemiluminescence (45).
Statistic analysis.
The in vitro cytokine production was compared between Mkp-1 +/+ and Mkp-1 −/− cells using one-way analysis of variance (ANOVA). Plasma ALT levels were log-transformed before analysis using one-way ANOVA. One-way ANOVA was used to analyze the differences in plasma BUN and nitrate levels as well as in systolic blood pressures between Mkp-1 +/+ and Mkp-1 −/− mice. Plasma or serum cytokine concentrations between Mkp-1 +/+ and Mkp-1 −/− mice with or without in vivo LPS challenge were analyzed using two-way ANOVA. When ANOVA demonstrated differences, a modified t test was used to identify differences. Differences in survival between Mkp-1 +/+ and Mkp-1 −/− mice after LPS challenge were determined by Kaplan-Meier analysis. All tests were performed using SPSS 13.01 software (SPSS Inc.). A p-value <0.05 was considered significant.
Acknowledgments
We thank Bristol-Myers Squibb Pharmaceutical Research Institute for providing Mkp-1 knockout mice. We are grateful to J. Landry for providing us with the MK2 antibody and to J. Hayes for statistical analysis. We thank Drs. Jing Yang and Lei Zheng for assistance and valuable discussions.
This work is supported in part by the Columbus Children's Research Institute and grants from the National Institutes of Health (AI057798 to Y. Liu, AI045811 to C.-H. Chang, GM044263 to C.V. Smith, and HL063067 to J.A. Bauer).
The authors have no conflicting financial interests.
Abbreviations used: ALT, alanine aminotransferase; ANOVA, analysis of variance; BUN, blood urea nitrogen; ERK, extracellular signal–regulated kinase; IRAK, IL-1 receptor–associated kinase; JNK, c-Jun NH2-terminal kinase; MAP, mitogen-activated protein; MK2, MAP kinase–activated protein kinase 2; MKP, MAP kinase phosphatase; qRT-PCR, quantitative real-time RT-PCR; SOCS, suppressor of cytokine signaling; TLR, Toll-like receptor.
References
- 1.Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Martin, G.S., D.M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. [DOI] [PubMed] [Google Scholar]
- 4.Parrillo, J.E. 1993. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328:1471–1477. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov, R., and C.A. Janeway Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 91:295–298. [DOI] [PubMed] [Google Scholar]
- 6.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20–26. [DOI] [PubMed] [Google Scholar]
- 7.Dumitru, C.D., J.D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J.H. Lin, C. Patriotis, N.A. Jenkins, N.G. Copeland, G. Kollias, and P.N. Tsichlis. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 103:1071–1083. [DOI] [PubMed] [Google Scholar]
- 8.Han, J., J.D. Lee, L. Bibbs, and R.J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 265:808–811. [DOI] [PubMed] [Google Scholar]
- 9.Lee, J.C., J.T. Laydon, P.C. McDonnell, T.F. Gallagher, S. Kumar, D. Green, D. McNulty, M.J. Blumenthal, J.R. Heys, and S.W. Landvatter. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 372:739–746. [DOI] [PubMed] [Google Scholar]
- 10.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H.D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1:94–97. [DOI] [PubMed] [Google Scholar]
- 11.Lehner, M.D., F. Schwoebel, A. Kotlyarov, M. Leist, M. Gaestel, and T. Hartung. 2002. Mitogen-activated protein kinase-activated protein kinase 2-deficient mice show increased susceptibility to Listeria monocytogenes infection. J. Immunol. 168:4667–4673. [DOI] [PubMed] [Google Scholar]
- 12.Neininger, A., D. Kontoyiannis, A. Kotlyarov, R. Winzen, R. Eckert, H.D. Volk, H. Holtmann, G. Kollias, and M. Gaestel. 2002. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 277:3065–3068. [DOI] [PubMed] [Google Scholar]
- 13.Chen, P., J. Li, J. Barnes, G.C. Kokkonen, J.C. Lee, and Y. Liu. 2002. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 169:6408–6416. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd, E.G., Q. Zhao, S.E. Welty, T.N. Hansen, C.V. Smith, and Y. Liu. 2004. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J. Biol. Chem. 279:54023–54031. [DOI] [PubMed] [Google Scholar]
- 15.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A.R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 78:1027–1037. [DOI] [PubMed] [Google Scholar]
- 16.Collart, M.A., D. Belin, J.D. Vassalli, S. de Kossodo, and P. Vassalli. 1986. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J. Exp. Med. 164:2113–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong, C., R.J. Davis, and R.A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55–72. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh, C.S., S.E. Macatonia, C.S. Tripp, S.F. Wolf, A. O'Garra, and K.M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 260:547–549. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 20.D'Andrea, A., M. Aste-Amezaga, N.M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer, K., T. Matsuyama, T.M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P.S. Ohashi, M. Kronke, and T.W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73:457–467. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis, D., M. Pasparakis, T.T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 10:387–398. [DOI] [PubMed] [Google Scholar]
- 24.Goureau, O., F. Amiot, F. Dautry, and Y. Courtois. 1997. Control of nitric oxide production by endogenous TNF-alpha in mouse retinal pigmented epithelial and Muller glial cells. Biochem. Biophys. Res. Commun. 240:132–135. [DOI] [PubMed] [Google Scholar]
- 25.Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. The enigma of sepsis. J. Clin. Invest. 112:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan, H., and J.A. Cook. 2004. Molecular mechanisms of endotoxin tolerance. J. Endotoxin Res. 10:71–84. [DOI] [PubMed] [Google Scholar]
- 27.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164:3476–3479. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi, K., L.D. Hernandez, J.E. Galan, C.A. Janeway Jr., R. Medzhitov, and R.A. Flavell. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 110:191–202. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa, R., T. Naka, H. Tsutsui, M. Fujimoto, A. Kimura, T. Abe, E. Seki, S. Sato, O. Takeuchi, K. Takeda, et al. 2002. SOCS-1 participates in negative regulation of LPS responses. Immunity. 17:677–687. [DOI] [PubMed] [Google Scholar]
- 30.Keyse, S.M. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186–192. [DOI] [PubMed] [Google Scholar]
- 31.Franklin, C.C., and A.S. Kraft. 1997. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 272:16917–16923. [DOI] [PubMed] [Google Scholar]
- 32.Sun, H., C.H. Charles, L.F. Lau, and N.K. Tonks. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 75:487–493. [DOI] [PubMed] [Google Scholar]
- 33.Chu, Y., P.A. Solski, R. Khosravi-Far, C.J. Der, and K. Kelly. 1996. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 271:6497–6501. [DOI] [PubMed] [Google Scholar]
- 34.Matsuguchi, T., T. Musikacharoen, T.R. Johnson, A.S. Kraft, and Y. Yoshikai. 2001. A novel mitogen-activated protein kinase phosphatase is an important negative regulator of lipopolysaccharide-mediated c-Jun N-terminal kinase activation in mouse macrophage cell lines. Mol. Cell. Biol. 21:6999–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Y., J.N. Blattman, N.J. Kennedy, J. Duong, T. Nguyen, Y. Wang, R.J. Davis, P.D. Greenberg, R.A. Flavell, and C. Dong. 2004. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 430:793–797. [DOI] [PubMed] [Google Scholar]
- 36.Johnson, G.L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 298:1911–1912. [DOI] [PubMed] [Google Scholar]
- 37.Raingeaud, J., A.J. Whitmarsh, T. Barrett, B. Derijard, and R.J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo, X., R.E. Gerl, and J.W. Schrader. 2003. Defining the involvement of p38alpha MAPK in the production of anti- and proinflammatory cytokines using an SB 203580-resistant form of the kinase. J. Biol. Chem. 278:22237–22242. [DOI] [PubMed] [Google Scholar]
- 39.Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276:13664–13674. [DOI] [PubMed] [Google Scholar]
- 40.Lang, R., R.L. Rutschman, D.R. Greaves, and P.J. Murray. 2002. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 168:3402–3411. [DOI] [PubMed] [Google Scholar]
- 41.Yao, Y., W. Li, M.H. Kaplan, and C.H. Chang. 2005. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J. Exp. Med. 201:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yee, C.S., Y. Yao, Q. Xu, B. McCarthy, D. Sun-Lin, M. Tone, H. Waldmann, and C.H. Chang. 2005. Enhanced production of IL-10 by dendritic cells deficient in CIITA. J. Immunol. 174:1222–1229. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, Q., E.G. Shepherd, M.E. Manson, L.D. Nelin, A. Sorokin, and Y. Liu. 2005. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J. Biol. Chem. 280:8101–8108. [DOI] [PubMed] [Google Scholar]
- 44.Chaves, A.A., M.J. Mihm, B.L. Schanbacher, A. Basuray, C. Liu, L.W. Ayers, and J.A. Bauer. 2003. Cardiomyopathy in a murine model of AIDS: evidence of reactive nitrogen species and corroboration in human HIV/AIDS cardiac tissues. Cardiovasc. Res. 60:108–118. [DOI] [PubMed] [Google Scholar]
- 45.Chicoine, L.G., M.L. Paffett, T.L. Young, and L.D. Nelin. 2004. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L60–L68. [DOI] [PubMed] [Google Scholar]