Abstract
Glucocorticoids are the most effective antiinflammatory agents for the treatment of chronic inflammatory diseases even though some diseases, such as chronic obstructive pulmonary disease (COPD), are relatively glucocorticoid insensitive. However, the molecular mechanism of this glucocorticoid insensitivity remains uncertain. We show that a defect of glucocorticoid receptor (GR) deacetylation caused by impaired histone deacetylase (HDAC) 2 induces glucocorticoid insensitivity toward nuclear factor (NF)-κB–mediated gene expression. Specific knockdown of HDAC2 by RNA interference resulted in reduced sensitivity to dexamethasone suppression of interleukin 1β–induced granulocyte/macrophage colony-stimulating factor production. Loss of HDAC2 did not reduce GR nuclear translocation, GR binding to glucocorticoid response element (GRE) on DNA, or GR-induced DNA or gene induction but inhibited the association between GR and NF-κB. GR becomes acetylated after ligand binding, and HDAC2-mediated GR deacetylation enables GR binding to the NF-κB complex. Site-directed mutagenesis of K494 and K495 reduced GR acetylation, and the ability to repress NF-κB–dependent gene expression becomes insensitive to histone deacetylase inhibition. In conclusion, we show that overexpression of HDAC2 in glucocorticoid-insensitive alveolar macrophages from patients with COPD is able to restore glucocorticoid sensitivity. Thus, reduction of HDAC2 plays a critical role in glucocorticoid insensitivity in repressing NF-κB–mediated, but not GRE-mediated, gene expression.
In the resting cell, chromatin is tightly compacted to prevent transcription factor accessibility. During activation of the cell this compact inaccessible chromatin is made available to DNA-binding proteins, thus allowing the induction of gene transcription (1, 2). There is compelling evidence that increased gene transcription is associated with an increase in histone acetylation induced by histone acetyltransferase, whereas hypo-acetylation is correlated with reduced transcription or gene silencing (2, 3), which is controlled by histone deacetylases (HDACs).
At least 18 HDACs are now recognized in mammalian cells (4, 5) and are grouped into 4 classes based on similarity of genes in yeast. In previous studies, we have found that total nuclear HDAC activity and HDAC2 expression are decreased in lung macrophages and peripheral lung tissue obtained from chronic obstructive pulmonary disease (COPD) (6, 7) and that the reduction correlates with disease severity.
Glucocorticoids are the most effective therapy for the treatment of many chronic inflammatory diseases such as asthma and inflammatory bowel disease (8). They act by binding to cytosolic glucocorticoid receptors (GRs), which upon binding become activated and rapidly translocate to the nucleus. Within the nucleus, GR either induces transcription of genes, such as secretary leukocyte proteinase inhibitor (SLPI) (8) and mitogen-activated kinase phosphatase–1 (9), or inhibits expression of genes such as IL-6 (10) by binding to specific DNA elements (glucocorticoid response elements [GREs]) at the promoter/enhancer of responsive genes. At lower concentrations, glucocorticoids reduce inflammatory gene transcription induced by NF-κB or AP-1 via association between these factors and GR (11). In addition, Ogawa et al. also show that the GR represses a large set of functionally related inflammatory response genes by disrupting p65–interferon regulatory factor complexes (12). Thus, binding of GR to p65–NF-κB is crucial for transrepression by corticosteroids. However, it is unclear how the GR dissociates its ability to control inflammation by suppressing NF-κB from its ability to directly transactivate genes via binding to GRE.
COPD is a common and debilitating chronic inflammatory disease characterized by progressive airflow limitation that is poorly reversible (13) and glucocorticoid insensitive (14, 15). We previously demonstrated that total HDAC activity negatively correlates with the inhibitory effect of dexamethasone (Dex) on TNF-α–induced IL-8 production (16) in alveolar macrophages from smokers and nonsmokers. We also reported that theophylline might restore glucocorticoid sensitivity via enhancement of HDAC activity in COPD macrophages (7). However, it has not been elucidated which HDAC regulates glucocorticoid sensitivity nor how it modulates glucocorticoid action.
In this paper, we identified an HDAC enzyme involved in glucocorticoid-dependent repression of NF-κB–induced gene expression and provided evidence that HDAC2 overexpression was able to restore glucocorticoid sensitivity in glucocorticoid insensitive diseases such as COPD.
RESULTS AND DISCUSSION
Loss of HDAC2 induced glucocorticoid insensitivity
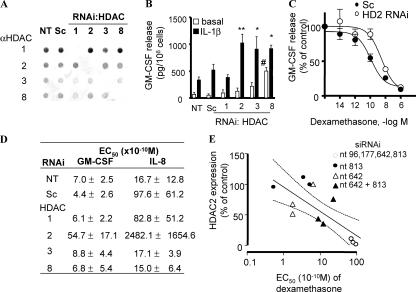
We initially assessed whether loss of specific HDAC enzymes induced glucocorticoid insensitivity on IL-1β–induced GM-CSF production, which is NF-κB mediated, as previously reported (17, 18) and from our data showing concentration-dependent inhibition by IκB kinase 2 inhibitor (AS602868; IC50 = 3.6 × 10−6 M). Class I HDAC enzymes (HDAC1, -2, -3, and -8) (4) were selectively knocked down by RNA interference in A549 cells (Fig. 1 A). When GM-CSF production was normalized to viable cell numbers, HDAC2 (1,057.1 ± 209.3 ng/106 cells), HDAC3 (910.5 ± 230.5 ng/106 cells), and HDAC8 (919.5 ± 64.8 ng/106 cells) knockdown (KD), but not HDAC1 KD, significantly enhanced IL-1β–induced GM-CSF production (vs. 335.0 ± 55.5 ng/106 cells in control; Fig. 1 B). These initial results show that HDAC2, -3, and -8 regulate NF-κB–mediated GM-CSF induction. We previously showed that trichostatin A (TSA), a nonselective HDAC inhibitor, enhanced GM-CSF production in A549 cells (19).
Figure 1.
HDAC2 KD-induced glucocorticoid insensitivity in A549 cells. (A) Dot blot analysis of nuclear extracts from A549 cells 48 h after transfection with siRNA against HDAC1, -2, -3, and -8; Sc; or vehicle only (NT). (B) IL-1β–stimulated (1 ng/ml for 24 h) GM-CSF production. Values represent means ± SEM. * and **, P < 0.05 and P < 0.01, respectively, versus IL-1β control; #, P < 0.05 versus basal control. n = 4∼8 experiments. (C) Concentration response curve of Dex suppression of IL-1β–induced GM-CSF production, and (D) summarized EC50 values of Dex (n = 4∼8). Values in C represent means ± SEM. (E) Correlation between EC50 and HDAC2 expression in HDAC2 RNAi cells. Different levels of HDAC2 expression were induced using individual siRNA or combinations of up to four duplexes against HDAC2. HDAC2 expression evaluated by Western blotting was shown as the percentage of that in nontreated cells. Dashed and continuous lines represent 95% confidence interval.
Furthermore, HDAC2 KD shifted the concentration-dependent inhibition of IL-1β–stimulated GM-CSF release by Dex to the right, indicating a reduction in glucocorticoid action (Fig. 1, C and D). A similar result was seen after treatment with TSA (19). A similar action was also seen with suppression of IL-1β–induced IL-8 production (Fig. 1 D). In contrast, HDAC1, -3, and -8 KD had no effect on Dex action on either GM-CSF or IL-8 production (Fig. 1 D). Using individual short interference RNA (siRNA), or combinations of up to four duplexes against HDAC2, we were able to obtain different levels of HDAC2 reduction in cells (Fig. 1 E). This HDAC2 study showed that there was a significant negative correlation between HDAC2 expression after RNAi and Dex EC50 with respect to IL-1β–induced GM-CSF production (r = −0.771; P = 0.0020; Fig. 1 E). This graded reduction in glucocorticoid responsiveness with reducing HDAC2 expression suggests that HDAC2 plays an important role in the antiinflammatory actions of glucocorticoids. Intriguingly, KD of HDAC2 does not affect the maximal response to Dex seen at very high concentrations (1 μM; Fig. 1 C) and suggests that, at these supraphysiological concentrations, alternative mechanisms of action may be occurring (20).
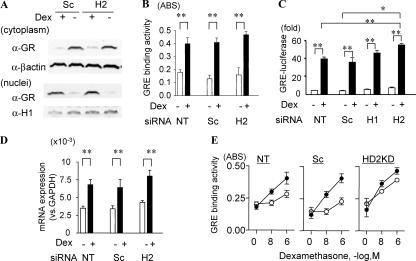
HDAC2 KD did not affect GR expression or nuclear translocation (Fig. 2 A). Loss of HDAC2 did not reduce GR-GRE binding (Fig. 2 B), GRE-dependent luciferase activity (Fig. 2 C), or GRE-mediated SLPI gene expression (Fig. 2 D). In addition, GR-GRE binding after Dex treatment was sustained longer in HDAC2 KD cells than in nontreated cells or scrambled oligonucleotide (Sc)–transfected cells (Fig. 2 E).
Figure 2.
HDAC2 KD does not inhibit GR-mediated gene activation. (A) GR nuclear translocation in Sc and HDAC2 siRNAs (H2) transfected cells stimulated with 10−8 M Dex for 1 h. β-Actin and histone H1 were used as loading controls. GR-GRE binding (B), GRE-luciferase activity (C), and SLPI gene induction (D). * and **, P < 0.05 and P < 0.01, respectively (n = 3 experiments). NT, nontreated. (E) GR-GRE binding 1 h (closed circle) and 8 h (open circle) after Dex stimulation. Values in B–E represent means ± SEM.
Loss of HDAC2 inhibited association of GR and NF-κB
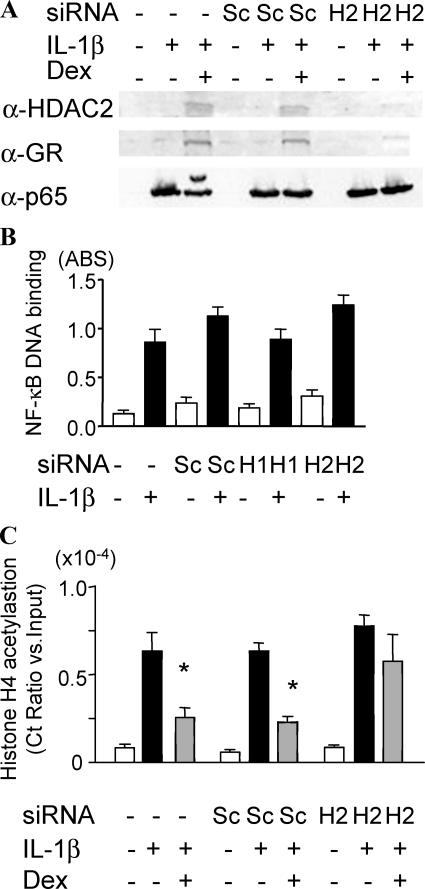
In addition, HDAC2 KD did not affect NF-κB expression, nuclear translocation (Fig. 3 A), or NF-κB–DNA binding (Fig. 3 B). We confirmed previous data showing that Dex increased GR and HDAC2 association with the p65–NF-κB complex (Fig. 3 A) (19). However, GR was not recruited to the p65–NF-κB complex after HDAC2 KD (Fig. 3 A). Chromatin immunoprecipitation (ChIP) analysis showed that Dex failed to inhibit histone 4 acetylation at the p65–NF-κB binding site in the GM-CSF promoter region after HDAC2 KD (Fig. 3 C). Thus, HDAC2 KD did not reduce the ability of GR to induce GR-sensitive gene expression but had a preferential effect on the suppression of NF-κB–mediated inflammatory gene expression.
Figure 3.
HDAC2 KD causes inhibition of GR–NF-κB association. (A) GR and HDAC2 were coimmunoprecipitated with p65–NF-κB in nuclear extracts in the presence or absence of a 30-min treatment of IL-1β with or without 20-min pretreatment with 10−9 M Dex. H2, HDAC2. (B) NF-κB activation measured by NF-κB binding to oligonucleotides, including NF-κB binding site, 30 min after IL-1β treatment (n = 3). (C) Histone 4 acetylation of NF-κB binding site at the GM-CSF promoter region detected by ChIP assay. Ct (threshold) values of PCR products were normalized to those of input samples. *, P < 0.05 versus IL-1β control (n = 3 experiments). Values in B and C represent means ± SEM.
GR is an acetylated protein and deacetylated by HDAC2
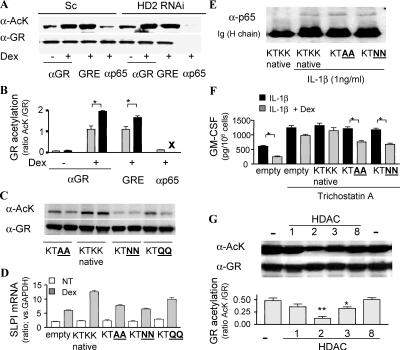
Previous studies have shown that both estrogen receptor α and androgen receptor are acetylated within their hinge/ligand binding domains and that this can modulate hormone-induced gene induction (21, 22). This nuclear receptor acetylation site is conserved among members of related nuclear receptors and, based on these findings (22), there is a potential acetylation site at aa 492–495 (KTKK) within the DNA binding domain/hinge region of GR. Indeed, we show that GR is acetylated after Dex binding (Fig. 4, A and B), and the acetylation levels were decreased when K494 and K495 on GR were mutated to alanine (A), asparagine (N), or glutamine (Q) (Fig. 4 C). These mutants did not further induce SLPI expression by Dex even though overexpression of native GR enhanced SLPI expression by Dex (Fig. 4 D). There was no difference in ability of native or mutant GR to bind to p65–NF-κB (Fig. 4 E). However, GR-mediated suppression of IL-1β–stimulated GM-CSF release was not affected by TSA with the K494 and K495 mutants (Fig. 4 F) even though the repression was attenuated by TSA in native GR overexpression cells. This suggests that GR acetylation negatively regulates Dex-induced repression of NF-κB–dependent gene expression. In addition, we found that p65–NF-κB–associated GR was deacetylated (Fig. 4 A). Because GR is unable to associate with the p65–NF-κB complex in HDAC2 KD cells, this deacetylation must be important for GR-mediated transrepression (Fig. 4, A and B). Importantly, HDAC2 KD does not inhibit GR-GRE binding or SLPI transactivation (Fig. 2, B and D), indicating that the acetylated GR is still able to activate glucocorticoid-responsive genes, which may be involved in some of the deleterious side effects that limit the clinical use of these powerful drugs, as well as some minor antiinflammatory effects via induction of antiinflammatory molecules such as SLPI and MKP-1.
Figure 4.
GR deacetylation by HDAC2 is a prerequisite for p65–NF-κB binding. (A) GR was precipitated after vehicle or Dex treatment with anti-GR antibody in whole cell extracts and with GRE oligonucleotides or anti-p65–NF-κB antibody in the presence of 10−8 M Dex and 1 ng/ml IL-1β. Bands were visualized by anti–acetyl-lysine antibody (α-AcK) and anti-GR antibody (α-GR). (B) Graphical representation of the results shown in A, with the ratio of AcK band to GR band in nontreated (shaded bar) and HDAC2 RNAi cells (closed bar). X represents no GR recruitment to NF-κB. *, P < 0.05. (C) GR acetylation level of each site-directed mutant after treatment with 10−6 M Dex for 1 h. GR were pulled down with anti–His-tag antibody. (D) SLPI mRNA level after treatment with 10−6 M Dex for 4 h. (E) GR binding to p65 under treatment with 10−8 M Dex for 1 h. GR is immunoprecipitated with His-tag antibody 1 h after IL-1β treatment. (F) Effect of 10−8 M Dex on IL-1β–induced GM-CSF production in the presence of 10 nM TSA for 10 min. *, P < 0.05. (G) AcK detection on immunoprecipitated GR in the presence of 10−6 M Dex after incubation with HDAC1, -2, -3, or -8 for 4 h at 30(C. (bottom) The ratio of AcK band to GR band is shown graphically. * and **, P < 0.05 and P < 0.01, respectively, versus control (n = 3 experiments). Values in B, D, F, and G represent means ± SEM.
To determine whether acetylated GR was a substrate for HDAC2, acetylated GR was immunoprecipitated in the presence of Dex and incubated with immunopurified HDAC1, -2, -3, and -8 (adjusted to show the same degree of HDAC activity) in a nonisotopic in vitro assay. Acetylated GR was clearly a substrate for HDAC2, although it is also partially deacetylated by HDAC3 (Fig. 4 G). Thus, acetylated GR is a substrate of HDAC2, and deacetylation of GR by HDAC2 may be prerequisite for GR association with the p65–NF-κB–activated complex and subsequent suppression of inflammatory gene expression. This mechanism provides a molecular explanation for the ability of GR to distinguish between recruitment of coactivator and corepressor proteins, as previously demonstrated for GRIP (23), and the subsequent ability to transactivate or repress gene transcription.
Overexpression of HDAC2 restores glucocorticoid sensitivity in alveolar macrophages from glucocorticoid-insensitive disease (COPD)
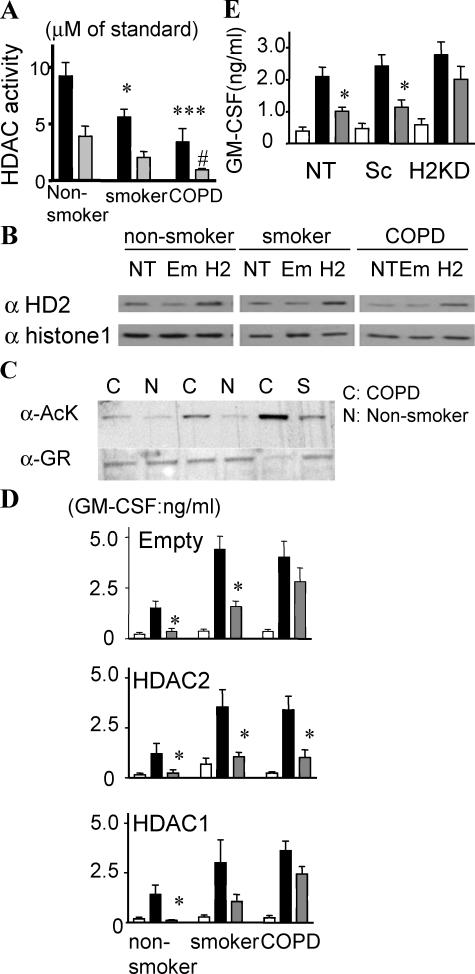
We previously reported that HDAC2 expression and activity is decreased in smokers (16) and patients with COPD (6), who are known to be insensitive to the antiinflammatory effects of glucocorticoids (14). In addition, there is a negative correlation between the repressive effect of Dex on cytokine production and total HDAC activity in alveolar macrophages from smokers and nonsmokers (16). To determine whether HDAC2 is important for Dex actions in primary cells from patients with glucocorticoid-insensitive disease, we obtained alveolar macrophages from healthy nonsmokers, healthy smokers, and patients with COPD. There was no substantial difference in macrophage GR expression between normal and COPD samples (unpublished data). Total HDAC activity, immunoprecipitated HDAC2 activity (Fig. 5 A), and HDAC2 protein expression (Fig. 5 B, NT) were markedly decreased in cells from patients with COPD. The acetylation level in immunoprecipitated nuclear GR after Dex treatment (10−8 M) was increased in alveolar macrophages obtained from patients with COPD (Fig. 5 C). In these cells obtained from COPD patients, Dex did not inhibit NF-κB–dependent (24) LPS-induced GM-CSF production in vitro (Fig. 5 D, top). Overexpression of HDAC2 protein by 6.5 ± 1.1-fold in primary macrophages from COPD patients (Fig. 5 B) restored Dex efficacy toward suppressing LPS-induced GM-CSF release to levels seen in cells from healthy control subjects (Fig. 5 D, middle). In contrast, HDAC2 overexpression by 3.3 ± 0.86-fold and 5.2 ± 1.1-fold in nonsmokers and smokers, respectively, did not further increase Dex efficacy toward LPS-induced GM-CSF production in cells from nonsmokers/smokers, presumably as these cells are already sensitive to Dex actions (Fig. 5 D). HDAC1 overexpression did not affect glucocorticoid sensitivity (Fig. 5 D, bottom). Furthermore, 40% KD of HDAC 2 in sputum macrophages from healthy nonsmokers by RNAi caused a reduction in the inhibitory effect of Dex (10−8 M) from 62 to 36% (Fig. 5 E).
Figure 5.
Overexpression of HDAC2 restores glucocorticoid sensitivity in alveolar macrophages from COPD patients. (A) Total HDAC activity (closed bar) and immunoprecipitated HDAC2 (shaded bar) activity in nuclear extracts. * and **, P < 0.05 and P < 0.001, respectively versus healthy nonsmokers; #, P < 0.05 versus smokers. (B) Representative image of HDAC2 protein expression in nuclear extracts 24 h after transfection with vehicle (NT), empty vector (Em), and HDAC2 vector (H2). (C) Acetylation level of immunoprecipitated GR of alveolar macrophages from normal (N), smoker (S), and COPD patients (C). Cells were stimulated with 10−8 M Dex for 1 h. (D) 100 ng/ml LPS-induced GM-CSF production in the absence (closed bar) or presence (shaded bar) of 10−8 M Dex (for 20 min) 24 h after transfection with each vector. Open bars are unstimulated control samples. *, P < 0.05 versus LPS control (n = 6 experiments). (E) LPS-induced GM-CSF production in nontreated (NT), Sc, or HDAC2 siRNA (H2)–transfected sputum macrophages in the absence (closed bar) or presence (shaded bar) of 10−8 M Dex for 20 min. *, P < 0.05. Values in A, D, and E represent means ± SEM.
Collectively, our results show that HDAC2 is a key protein involved in the suppression of p65–NF-κB–mediated inflammatory gene expression, which occurs at low concentrations of Dex, whereas modulation of HDAC2 expression does not reduce GR-mediated gene induction. HDAC2 acts by deacetylating GR, thereby enabling p65–NF-κB association and subsequent attenuation of proinflammatory gene transcription. The importance of this mechanism in COPD, a glucocorticoid-insensitive disease, is emphasized by overexpression of HDAC2, which restores glucocorticoid sensitivity in primary cells from these patients.
MATERIALS AND METHODS
Cultured cells and alveolar macrophages.
The human lung adenocarcinoma type II cell line (A549 cells) was purchased from American Type Culture Collection. Healthy nonsmokers, current healthy smokers, and patients with stage 2–3 COPD were recruited. The study was approved by the Brompton Harefield and National Heart and Lung Institute Ethics Committees, and all subjects gave signed informed consent. Bronchoscopy, bronchoalveolar lavage, isolation of bronchoalveolar lavage, and sputum macrophages were performed as previously described (24, 25).
RNA interference.
100-nM siRNA sequences were transfected using Gene Silencer (GTS Inc.) for A549 cells and jetSI (Polyplus-transfection SA) for sputum macrophages. siRNAs were prepared with a GeneSilencer kit (Ambion) or purchased from QIAGEN or Dharmacon. Duplexes used were HDAC1 (SMART pool [Dharmacon] and nt 89), HDAC2 (nt 96, 177, 642, and 813), HDAC3 (SMART pool [Dharmacon] and nt 411), and HDAC8 (nt 155 and 373). Nonspecific control duplex (Sc, 47% guanine-cytosine content) was also purchased from Dharmacon.
Overexpression of HDAC.
Plasmids (pcDNA3.1) containing the HDAC1 and HDAC2 genes were donated by S. Georas (Johns Hopkins University, Baltimore, MD). Alveolar macrophages (3 × 105 cells/well) were transfected using jetPEI-Man (Polyplus-transfection SA).
Quantitative RT-PCR.
Total RNA extraction and reverse transcription were performed using an RNeasy kit (QIAGEN) and an Omniscript RT kit (QIAGEN). Gene transcript level of SLPI and GAPDH were quantified by real-time PCR using a QuantiTect SYBR Green PCR kit (QIAGEN) on a Rotor-Gene 3000 (Corbett Research).
Cytokine ELISAs.
IL-8 and GM-CSF concentrations were determined by sandwich ELISA (R&D Systems) and normalized to cell number as determined by MTT assay.
GRE binding assay.
Biotinated oligonucleotide duplexes containing two GREs (26) were incubated in streptavidin-conjugated 96-well plates (Thermo Labsystems). GR binding to oligonucleotides were colormetrically detected in nuclear extracts (19) by an enzyme-immunosorbent method using an anti-GR antibody (Santa Cruz Biotechnology, Inc.).
Western blotting and dot blotting.
Nuclear extracts and immunoprecipitated samples were prepared and evaluated by conventional SDS-PAGE/Western blotting (19). Determination of HDAC protein expression after RNAi was performed by dot blot analysis.
Immuno- or oligonucleotide precipitation of GR.
GR was immunoprecipitated in whole cell extracts with anti-GR antibody–conjugated A/G agarose beads. GR was also precipitated with avidin-agarose beads conjugated with biotinylated oligonucleotides containing 2 × GRE (5′-aagattcaggtcatg-acctgaggaga-3′) or coimmunoprecipitated with p65–NF-κB antibody–conjugated A/G agarose beads in nuclear extracts in the presence of 10−8 M Dex and 1 ng/ml IL-1β.
ChIP assay.
ChIP with panacetylated histone 4 antibody (ChIP assay kit; Upstate Biotechnology) was performed at the GM-CSF promoter region as previously described (19) using the aforementioned real-time PCR system.
Site-directed mutagenesis.
Site-directed mutagenesis was performed using Gene Tailor Site-Directed Mutagenesis system (Invitrogen).
HDAC activity.
HDAC activity was measured with HDAC Fluorescent Activity Assay kit (BIOMOL Research Laboratories, Inc.).
NF-κB activation.
NF-κB activation was determined with NF-κB TransAM kit (Active Motif).
Luciferase assays.
Plasmids containing two GRE-luciferases were donated by J. Bloom (University of Arizona, Tucson, AZ). Plasmids were transfected to A549 cells with pSV–β-galactosidase (Promega) using Lipofectamine 2000 (Invitrogen) as described previously (27).
Statistics.
Results are expressed as means ± SEM. Analysis of variance was performed by Kruskal-Wallis analysis and, when significant comparisons were made, by Mann-Whitney U test using the analysis package SPSS 10.0 (SPSS Inc.). P < 0.05 was considered statistically significant.
Acknowledgments
We are indebted to Dr. Onn Min Kon for assistance in providing clinical samples.
The work in this laboratory was funded by the British Lung Foundation (P00-13), Clinical Research Committee (Royal Brompton Hospital; C/02/15). B. Cosio was the recipient of European Respiratory Society and Separ fellowships. P.J. Barnes, I.M. Adcock, and K. Ito have received nonrestricted funding from Boehringer Ingelheim and GlaxoSmithKline to fund part of this work.
The authors have no conflicting financial interests.
References
- 1.Beato, M. 1996. Chromatin structure and the regulation of gene expression: remodeling at the MMTV promoter. J. Mol. Med. 74:711–724. [DOI] [PubMed] [Google Scholar]
- 2.Wolffe, A.P. 1997. Transcriptional control. Sinful repression. Nature. 387:16–17. [DOI] [PubMed] [Google Scholar]
- 3.Ura, K., H. Kurumizaka, S. Dimitrov, G. Almouzni, and A.P. Wolffe. 1997. Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J. 16:2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ruijter, A.J., A.H. van Gennip, H.N. Caron, S. Kemp, and A.B. van Kuilenburg. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiagalingam, S., K.H. Cheng, H.J. Lee, N. Mineva, A. Thiagalingam, and J.F. Ponte. 2003. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann. NY Acad. Sci. 983:84–100. [DOI] [PubMed] [Google Scholar]
- 6.Ito, K., M. Ito, W.M. Elliott, B. Cosio, G. Caramori, O.M. Kon, A. Barczyk, S. Hayashi, I.M. Adcock, J.C. Hogg, and P.J. Barnes. 2005. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 7.Cosio, B.G., L. Tsaprouni, K. Ito, E. Jazrawi, I.M. Adcock, and P.J. Barnes. 2004. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 200:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbinante-Nissen, J.M., L.G. Simpson, and G.D. Leikauf. 1995. Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am. J. Physiol. 268:L601–L606. [DOI] [PubMed] [Google Scholar]
- 9.Lasa, M., S.M. Abraham, C. Boucheron, J. Saklatvala, and A.R. Clark. 2002. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22:7802–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray, A., K.E. Prefontaine, and P. Ray. 1994. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 269:12940–12946. [PubMed] [Google Scholar]
- 11.Adcock, I.M., and K. Ito. 2000. Molecular mechanisms of corticosteroid actions. Monaldi Arch. Chest Dis. 55:256–266. [PubMed] [Google Scholar]
- 12.Ogawa, S., J. Lozach, C. Benner, G. Pascual, R.K. Tangirala, S. Westin, A. Hoffmann, S. Subramaniam, M. David, M.G. Rosenfeld, and C.K. Glass. 2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 122:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes, P.J. 2000. Mechanisms in COPD: differences from asthma. Chest. 117:10S–14S. [DOI] [PubMed] [Google Scholar]
- 14.Barnes, P.J. 2000. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 161:342–344. [DOI] [PubMed] [Google Scholar]
- 15.Culpitt, S.V., D.F. Rogers, P. Shah, C. De Matos, R.E. Russell, L.E. Donnelly, and P.J. Barnes. 2003. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 167:24–31. [DOI] [PubMed] [Google Scholar]
- 16.Ito, K., S. Lim, G. Caramori, K.F. Chung, P.J. Barnes, and I.M. Adcock. 2001. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 15:1110–1112. [PubMed] [Google Scholar]
- 17.Kagoshima, M., T. Wilcke, K. Ito, L. Tsaprouni, P.J. Barnes, N. Punchard, and I.M. Adcock. 2001. Glucocorticoid-mediated transrepression is regulated by histone acetylation and DNA methylation. Eur. J. Pharmacol. 429:327–334. [DOI] [PubMed] [Google Scholar]
- 18.Kochetkova, M., and M.F. Shannon. 1996. DNA triplex formation selectively inhibits granulocyte-macrophage colony-stimulating factor gene expression in human T cells. J. Biol. Chem. 271:14438–14444. [DOI] [PubMed] [Google Scholar]
- 19.Ito, K., P.J. Barnes, and I.M. Adcock. 2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol. 20:6891–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissen, R.M., and K.R. Yamamoto. 2000. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 14:2314–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, C., M. Fu, R.H. Angeletti, L. Siconolfi-Baez, A.T. Reutens, C. Albanese, M.P. Lisanti, B.S. Katzenellenbogen, S. Kato, T. Hopp, et al. 2001. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J. Biol. Chem. 276:18375–18383. [DOI] [PubMed] [Google Scholar]
- 22.Fu, M., C. Wang, X. Zhang, and R. Pestell. 2003. Nuclear receptor modifications and endocrine cell proliferation. J. Steroid Biochem. Mol. Biol. 85:133–138. [DOI] [PubMed] [Google Scholar]
- 23.Rogatsky, I., H.F. Luecke, D.C. Leitman, and K.R. Yamamoto. 2002. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc. Natl. Acad. Sci. USA. 99:16701–16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosio, B.G., B. Mann, K. Ito, E. Jazrawi, P.J. Barnes, K.F. Chung, and I.M. Adcock. 2004. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am. J. Respir. Crit. Care Med. 170:141–147. [DOI] [PubMed] [Google Scholar]
- 25.Lim, S., N. Roche, B.G. Oliver, W. Mattos, P.J. Barnes, and K.F. Chung. 2000. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am. J. Respir. Crit. Care Med. 162:1355–1360. [DOI] [PubMed] [Google Scholar]
- 26.Eickelberg, O., A. Pansky, R. Mussmann, M. Bihl, M. Tamm, P. Hildebrand, A.P. Perruchoud, and M. Roth. 1999. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J. Biol. Chem. 274:12933–12938. [DOI] [PubMed] [Google Scholar]
- 27.Usmani, O.S., K. Ito, K. Maneechotesuwan, M. Ito, M. Johnson, P.J. Barnes, and I.M. Adcock. 2005. Glucocorticoid Receptor Nuclear Translocation in Airway Cells Following Inhaled Combination Therapy. Am. J. Respir. Crit. Care Med. 172:704–712. [DOI] [PubMed] [Google Scholar]