Abstract
The mechanisms by which glucocorticoid receptor (GR) mediates glucocorticoid (GC)-induced apoptosis are unknown. We studied the role of mitochondrial GR in this process. Dexamethasone induces GR translocation to the mitochondria in GC-sensitive, but not in GC-resistant, T cell lines. In contrast, nuclear GR translocation occurs in all cell types. Thymic epithelial cells, which cause apoptosis of the PD1.6 T cell line in a GR-dependent manner, induce GR translocation to the mitochondria, but not to the nucleus, suggesting a role for mitochondrial GR in eliciting apoptosis. This hypothesis is corroborated by the finding that a GR variant exclusively expressed in the mitochondria elicits apoptosis of several cancer cell lines. A putative mitochondrial localization signal was defined to amino acids 558–580 of human GR, which lies within the NH2-terminal part of the ligand-binding domain. Altogether, our data show that mitochondrial and nuclear translocations of GR are differentially regulated, and that mitochondrial GR translocation correlates with susceptibility to GC-induced apoptosis.
Glucocorticoids (GCs) are commonly used for therapy of various hematologic malignancies, most notably acute lymphoblastic leukemia, multiple myeloma, and chronic lymphocytic leukemia (1, 2). The effectiveness of this approach is based on the ability of GCs to induce apoptosis of leukemic cells. Yet, the mechanisms by which GC causes apoptosis remain obscure (2, 3).
Apoptosis by GC requires its binding to the glucocorticoid receptor (GR). Several GR isoforms have been identified (4), the GRα isoform being the predominant active form. The GR protein contains two transactivation domains, a zinc-finger DNA-binding domain (DBD) and a ligand-binding domain (LBD) (5, 6). The DBD consists of two highly conserved zinc fingers, which are crucial for the binding to glucocorticoid response element (GRE) sequences. In addition, the first zinc-finger of DBD binds NFκB and AP-1 (7, 8), and the second DBD zinc finger mediates receptor dimerization (9). The LBD binds GC as well as heat-shock proteins (10) and is also involved in receptor dimerization (6).
Numerous studies indicate that in the absence of a ligand, GR is sequestered in the cytosol bound to a large heteromeric complex of heat-shock proteins and immunophilins (10) or to 14–3-3σ (11). Upon ligand-binding, GR translocates to the nucleus where it transactivates and transrepresses multiple genes (3, 5). Transactivation occurs through interaction with GREs, whereas transrepression follows binding and inactivation of AP-1 and NFκB. It is unknown which of these alterations in gene expression are essential for the apoptotic effect of GC.
Some differences have been observed in the spectrum of genes affected by GC in GC-sensitive versus GC-resistant cells (12). Of note are the up-regulation of the pro-apoptotic gene Bim in GC-sensitive cells (13) and the down-regulation of the survival gene c-Myc (3). It has been suggested that the many effects of GC may shift the balance between prosurvival and proapoptotic factors, ultimately leading to cell death (2, 3). As AP-1 and NFκB control several survival pathways, the interaction of GR with these factors is believed to play a major role in GC-mediated apoptosis (3, 5). This notion is supported by the observation that a GR mutant (GR-LS7) compromised in transactivation, but normal in transrepression, is as effective as the WT receptor in inducing apoptosis (14). However, a dimerization-defective GR is unable to mediate GC-induced apoptosis, although it causes transrepression through interaction with AP-1 and NFκB (9). Thus, interference with AP-1 and NFκB functions per se is insufficient for inducing apoptosis. The role of transrepression is further questioned by the study of Tao et al. (7), who showed that a GR mutant deficient in transrepression is still able to induce apoptosis. Because dimerization is required for DNA binding (9), it was suggested that DNA binding is a prerequisite for eliciting the apoptotic response. However, a DNA-binding defective variant of GR, which is mainly localized in the cytosol, still induces apoptosis (15). These data suggest that GC may induce apoptosis by a yet unknown pathway, which is independent of the nuclear effects of GR.
Although GR is predominantly cytosolic, a plasma membrane form of GR (mGR) has been detected in some lymphoid cells (16, 17). Expression of mGR was found to be associated with GR mRNA transcript 1A (18), which is one of several GR transcripts expressed most abundantly in hematopoietic cell lines sensitive to GC-induced apoptosis (19, 20). It was thus suggested that mGR may be responsible for GC-induced apoptosis. Other studies, however, did not show correlation between expression of mGR and sensitivity to GC (2).
Several reports have shown that GR translocates to the mitochondria in addition to its well-characterized nuclear translocation (21–24). As GC-induced apoptosis is mediated via the mitochondrial pathway (2), we addressed the question whether GR translocation to the mitochondria is essential for GC-induced apoptosis. We found correlation between mitochondrial GR translocation and sensitivity of cells to GC-induced apoptosis. Moreover, a GR variant that is exclusively expressed in the mitochondria enabled apoptosis of various cancer cell lines. Altogether, our data indicate a role for mitochondrial GR in eliciting apoptosis.
RESULTS
GC-sensitive PD1.6 and 2B4 cells do not express plasma membrane GR
We used a series of lymphoma and leukemia cells to study the mechanisms of GC-induced apoptosis. Susceptibility to GC-induced apoptosis was determined by appearance of subdiploid cells and caspase 3 activation. PD1.6 and 2B4 cells readily apoptose in response to GC, whereas B10, S49, NB4, and Jurkat cells are resistant to GC-induced apoptosis (Fig. 1, A–C). It should be noted that the S49 variant used in this study (25) differs from the S49 variant described by Gametchu et al. (16) in being resistant to GC-induced apoptosis. Because it has been proposed that mGR is a mediator of GC-induced apoptosis (18), we compared mGR expression on GC-sensitive and resistant cells. It appears that S49, but not PD1.6, B10, 2B4, or Jurkat cells, express mGR (Fig. 1 D, subpanels A vs. C, E, G, and I, respectively). Also, the macrophage cell line RAW264.7 expresses a high level of mGR and is resistant to GC-induced apoptosis (unpublished data). Treatment with 100 nM dexamethasone (Dex) for 2 h did not induce expression of mGR on otherwise mGR-negative cells (Fig. 1 D, subpanels D, F, H, and J). The fact that GC-resistant S49 and RAW264.7 cells express mGR indicates that expression of mGR per se does not impose susceptibility to GC. The observation that GC-sensitive PD1.6 and 2B4 cells do not express mGR suggests that another mechanism is involved in this apoptotic process.
Figure 1.
(A) Sensitivity of various lymphoma and leukemia cell lines to Dex-induced apoptosis. Cells were incubated with 100 nM Dex for 20 h, and the DNA content measured by flow cytometry using propidium iodide. Percentage of subdiploid cells is given. (B) Dose–response to Dex. Cells were incubated with various concentrations of Dex and processed as in A. (C) Caspase 3 activation. Untreated or Dex-treated cells were stained for activated caspase 3 as described in Materials and methods. Percentage of positive cells is given. (D) Expression of mGR on lymphoma and leukemia cells. Untreated cells or cells treated with 100 nM Dex for 2 h were incubated with M20 antibodies to GR and FITC-conjugated goat anti–rabbit IgG (dashed line) or FITC-conjugated goat anti–rabbit IgG only (solid line). The fluorescence intensity was measured by flow cytometry.
Translocation of GR to the mitochondria and sensitivity to GC-induced apoptosis
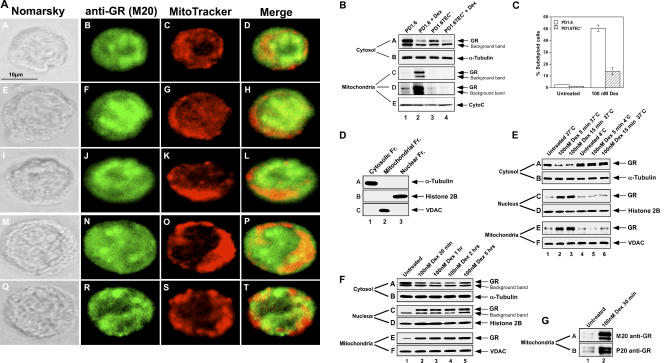
It has recently been shown that GR may be localized to the mitochondria (24). As GC-evoked apoptosis is mediated via the mitochondrial pathway (2), we asked whether GR translocation to the mitochondria may be a trigger of apoptosis. To answer this question, we analyzed the intracellular trafficking of GR in GC-sensitive and resistant cells after treatment with Dex. Dex-induced GR translocation to the mitochondria was demonstrated in GC-sensitive PD1.6 cells using two different methods (Fig. 2, A, B, and E). First, immunofluorescence studies using M20 antibodies to GR and red mitotracker to visualize the mitochondria show that a certain fraction of GR localizes to the mitochondria in Dex-treated PD1.6 cells (Fig. 2 A). Second, PD1.6 cells treated with 100 nM Dex for 2 h were fractionated using the Oncogene cytosol/mitochondria fractionation kit. The quantities of GR in the cytosolic and mitochondrial fractions were analyzed by Western blotting (Fig. 2 B). The PA1-511A antibody to GR (epitope aa 346–367) resolves two major bands on Western blots. The top one is GRα, whereas the bottom band is of unknown character and considered in Fig. 2 as a “background band.” GR expression in the mitochondrial fraction of PD1.6 cells is increased after Dex treatment (Fig. 2 B, subpanels C and D, lane 2 vs. lane 1), indicating mitochondrial translocation of GR. Contamination of the mitochondrial fraction with mGR is excluded, because PD1.6 cells do not express mGR even after Dex treatment (Fig. 1 D, subpanels C and D). As expected, the amount of GR in the cytosolic fraction is reduced by Dex (Fig. 2 B, subpanel A, lane 2 vs. lane 1). We also determined the GR expression in cytosolic and mitochondrial extracts of PD1.6TEC− cells that display reduced sensitivity to Dex-induced apoptosis (Fig. 2 C). In contrast with the GC-sensitive parental PD1.6 cells, the GR level was not increased in the mitochondrial fraction of PD1.6TEC− cells treated with Dex (Fig. 2 B, subpanels C and D, lane 4 vs. lane 3). These data indicate that selection of PD1.6 cells toward apoptotic resistance in response to Dex is accompanied with reduced mitochondrial translocation of GR.
Figure 2.
(A) Intracellular staining of GR. PD1.6 cells were treated with 100 nM Dex for 2 h and the mitochondria were stained with 50 nM mitotracker. Rehydrated methanol-fixed cells were incubated with M20 antibodies to GR and FITC-conjugated goat anti–rabbit IgG, and visualized under confocal microscope. Five different cells are presented. (B) Dex induces mitochondrial translocation of GR in PD1.6, but not in PD1.6TEC− cells. PD1.6 cells were incubated in the absence or presence of 100 nM Dex for 2 h before subcellular fractionation using the Oncogene fractionation kit. GR was detected by Western blotting using the PA1-511A antibody to GR (A, C, and D). (C and D) Data were obtained by two different exposure times. The blots were reprobed with antibodies to α-tubulin (B) and CytoC (D). CytoC could be used as a mitochondrial marker because the cells were harvested before any CytoC release. (C) Sensitivity of PD1.6 and PD1.6TEC− cells to Dex-induced apoptosis. Cells were either untreated or treated with 100 nM Dex for 20 h, and percentage of apoptotic cells was determined as in Fig. 1 A. (D) The mitochondrial fraction is not contaminated by cytosolic or nuclear proteins. Subcellular fractions were run side by side on the same gel and analyzed by Western blotting using antibodies to α-tubulin (A), histone 2B (B), and VDAC (C), which react with cytosolic, nuclear, and mitochondrial fractions, respectively. (E) Dex induces a rapid, temperature-dependent translocation of GR to the nucleus and mitochondria in PD1.6 cells. PD1.6 cells were incubated in the absence or presence of 100 nM Dex for 5 and 15 min at 37°C (lanes 1–3) or 4°C (lanes 4–6) before subcellular fractionation as described in Materials and methods. GR was detected by Western blotting using the PA1-511A antibody to GR (A, C, and E). The blots were reprobed with antibodies to α-tubulin (B), histone H2B (D), and VDAC (F). (F) Dex induces sustained GR translocation to the nucleus and the mitochondria in PD1.6 cells. PD1.6 cells were incubated in the absence or presence of 100 nM Dex for 30 min to 5 h and processed as in E. (G) The mitochondrial GR is also immunoreactive to the M20 and P20 antibodies to GR. The mitochondrial samples 1 and 2 of F were rerun on gel and probed with either M20 or P20 antibodies.
Because the Oncogene kit does not yield nuclear extracts, we applied a cellular fractionation protocol that enabled comparison between mitochondrial and nuclear translocations of GR. Cross-contamination between the cytosolic, nuclear, and mitochondrial fractions was ruled out by using antibodies against α-tubulin, histone H2B, and VDAC, respectively (Fig. 2 D). As with the Oncogene kit, this method detected mitochondrial translocation of GR in Dex-treated PD1.6 cells (Fig. 2 E, subpanel E, lanes 2–3 vs. lane 1). A longitudinal follow-up (Fig. 2, E and F) revealed mitochondrial translocation of GR as early as 5 min after addition of Dex, long before the onset of apoptosis. Thereafter, the mitochondrial GR level did not increase further with time (Fig. 2 E [subpanel E, compare lanes 2 and 3] and F [subpanel E, compare lanes 2–5]). Also, the amount of nuclear GR already peaked at 5 min (Fig. 2, E and F, subpanel C). Similar results were obtained with PD1.6 cells treated with the naturally occurring GC corticosterone (unpublished data). The mitochondrial GR could also be detected on Western blot using the NH2-terminal (aa 5–20)-reacting M20 and the COOH-terminal (aa 750–769)-reacting P20 antibodies (Fig. 2 G), indicating that this GR is in full-length. To exclude the possibility of nonspecific GR binding to the mitochondria, we studied whether this translocation is temperature sensitive. To this end, PD1.6 cells were exposed to Dex at 4°C for 5–15 min before subcellular fractionation. GR did not translocate to either the mitochondria or the nucleus at 4°C (Fig. 2 E, compare lanes 5 and 6 with lanes 2 and 3), indicating that the GR mitochondrial localization is specific and temperature dependent.
Next, we compared GR translocation to the mitochondria in GC-sensitive PD1.6 cells and GC-insensitive B10 cells (Fig. 3 A). PD1.6 and B10 cells are both derived from a thymic lymphoma, and express similar GR levels. The cells were exposed to 5 and 100 nM Dex for 4 h before subcellular fractionation. Dex induced GR translocation to the nucleus in both PD1.6 and B10 cells (Fig. 3 A, subpanel C, lanes 2, 3, 5, and 6). A dose-dependent mitochondrial translocation of GR was observed in PD1.6 cells (Fig. 3 A, subpanel E, lanes 2 and 3), but not in B10 cells (Fig. 3 A, subpanel E, lanes 5 and 6). In some experiments, a slight increase in GR expression was seen in mitochondrial extracts of B10 cells treated with 100 nM Dex (unpublished data), which reflects residual sensitivity (10–12%) of these cells to Dex (Fig. 1 B). Moreover, Dex induced GR translocation to both the nucleus and the mitochondria in GC-sensitive 2B4 cells (Fig. 3 B) and thymocytes (Fig. 3 C). In 2B4 cells, nuclear translocation of GR was induced by as low as 1 nM Dex, a nontoxic concentration (Fig. 3 B, subpanel C, lane 2). This Dex concentration was insufficient to cause mitochondrial GR translocation in the same cells (Fig. 3 B, subpanel E, lane 2). However, at toxic Dex concentrations (10 and 100 nM), GR translocated to the mitochondria as well (Fig. 3 B, subpanel E, lanes 3 and 4 vs. lane 1).
Figure 3.
(A–E) Dex induces GR translocation to the mitochondria in GC-sensitive PD1.6 cells (A), 2B4 cells (B), and thymocytes (C), but not in GC-resistant B10 (A), NB4 (D), S49 (E), and Jurkat (E) cells. Cells were incubated in the absence or presence of various Dex concentrations for 4 h before subcellular fractionation and Western blotting. In addition, NB4 cells were treated with 1 μM As2O3 (D, lanes 5 and 6), which is known to stabilize PML in these cells (reference 26).
Contrary to GC-sensitive cells, GC-resistant NB4 (Fig. 3 D, lanes 3–6) and S49 (Fig. 3 E, lanes 1–3) cells responded to Dex with GR translocation to the nucleus, but not to the mitochondria. We also treated NB4 cells with As2O3, which stabilizes PML and degrades the PML–RAR fusion protein (26). As2O3 stabilized GR in these cells (Fig. 3 D, subpanel A, lane 5 vs. lane 3) to a level comparable with that of PD1.6. Even after As2O3 treatment, Dex did not cause mitochondrial translocation of GR in NB4 cells (Fig. 3 D, subpanel E, lane 6). Although S49 cells express a high basal level of GR (Fig. 3 E, subpanel A, lane 1), no GR translocation to the mitochondria is seen (Fig. 3 E, subpanel E, lanes 2 and 3). Thus, mitochondrial GR translocation does not correlate with GR expression but rather with the apoptotic sensitivity to GC.
Jurkat cells express a very low level of GR (Fig. 3 E, subpanel A, lane 4), which may explain their unresponsiveness to the apoptotic effects of GC. It is well documented that GC-induced apoptosis requires a threshold level of GR (24, 27). Although GR translocates to the nucleus in Dex-treated Jurkat cells (Fig. 3 E, subpanel C, lanes 5 and 6), GR is barely seen in the mitochondria (Fig. 3 E, subpanel E, lanes 5 and 6). Moreover, Jurkat cells express one WT and one mutated (R477H) GR allele, as do the GC-sensitive CCRF–CEM cells (28).
It should be noted that in most of the GR-expressing cell lines tested, a certain basal level of GR is detected in the mitochondria (e.g., Fig. 3 A [subpanel E, lanes 1 and 4], B [subpanel E, lane 1], and E [subpanel E, lane 1]). Altogether, the data demonstrate correlation between GR translocation to the mitochondria and sensitivity to GC-induced apoptosis.
Mitochondrial translocation of GR can occur without concomitant apoptosis
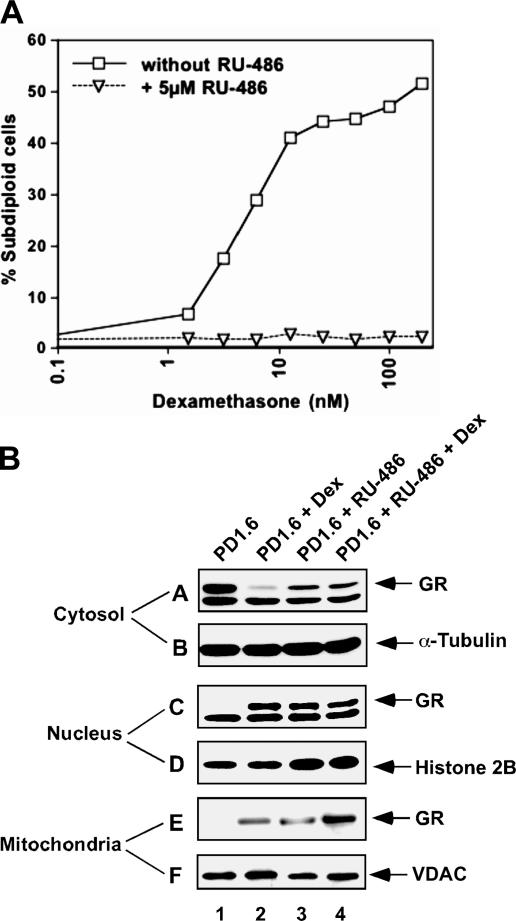
The GR antagonist RU486 induces a different conformational change of GR than GC agonists (29). It causes nuclear translocation of GR, but prevents its binding to GREs, thereby avoiding transactivation. We sought to find out whether RU486 affects mitochondrial translocation of GR. Initially, we analyzed the effect of RU486 on Dex-induced apoptosis of PD1.6 cells. As expected, RU486 prevented this apoptotic response (Fig. 4 A). When looking on the intracellular trafficking of GR, we found that RU486 induced both nuclear and mitochondrial translocation of GR in PD1.6 cells (Fig. 4 B, subpanel C and E, respectively, lane 3). A combination of RU486 and Dex had an additive effect on GR translocation to the mitochondria (Fig. 4 B, subpanel E, lane 4). These data indicate that a mere localization of GR to the mitochondria is insufficient for inducing apoptosis, which apparently requires a particular GR conformation.
Figure 4.
(A) RU-486 prevents Dex-induced apoptosis of PD1.6 cells. PD1.6 cells were incubated with various concentrations of Dex in the absence or presence of 5 μM RU-486 for 20 h. Percentage of apoptosis was determined as in Fig. 1 A. (B) RU-486 induces mitochondrial translocation of GR in PD1.6 cells. Cells were incubated in the absence or presence of 5 μM RU-486 and/or 100 nM Dex for 2 h followed by subcellular fractionation and Western blotting.
TEC induces mitochondrial, but not nuclear, translocation of GR in PD1.6 cells
We have recently shown that thymic epithelial cells (TECs) induce apoptosis of PD1.6 cells in a GR-dependent manner (30). It was therefore of interest to study the intracellular trafficking of GR in PD1.6 cells cocultured with TECs. For this purpose, PD1.6 cells were incubated alone or on a TEC monolayer for 4 h. In PD1.6 cells grown alone, most of the GR is localized in the cytosol (Fig. 5 A, lane 1). After cocultivation with TECs, GR is found in the mitochondrial fraction, but, surprisingly, not in the nuclear fraction (Fig. 5, compare lane 2 in subpanel E vs. subpanel C). PD1.6 cells treated with 100 nM Dex for 4 h were used as a positive control for nuclear translocation. As expected, this treatment caused both nuclear and mitochondrial translocation of GR (Fig. 5, lane 7 in subpanels C and E, respectively). Cocultivation on TEC did not cause a reduction in the cytosolic GR level as seen with Dex (Fig. 5 A, compare lanes 2 and 7 with lane 1). It should be noted that a similar quantity of GR was observed in the mitochondrial fractions of PD1.6 cells cocultivated with TECs and of PD1.6 cells treated with 100 nM Dex (Fig. 5 E, compare lanes 2 and 7). This outcome is consistent with the similar extent of apoptosis induced by these two stimuli (30). To exclude the possibility that GR detected in PD1.6 cells cocultured with TECs is due to TEC contamination, we included in the experiment Dex-resistant PD1.6Dex− cells that express minute amount of GR (30). No GR could be seen in the mitochondrial or nuclear fractions of these cells after cocultivation with TECs (Fig. 5, lane 4 in subpanels E and C, respectively). As a negative control, we used TEC-resistant PD1.6TEC− cells (30), which show only a slight reduction in GR expression level in comparison to the parental PD1.6 cells. Interestingly, TECs induced neither mitochondrial nor nuclear translocation of GR in PD1.6TEC− cells (Fig. 5, lane 6 in subpanels E and C, respectively). Altogether, our data demonstrate that TEC induces GR translocation to the mitochondria in TEC-sensitive PD1.6 cells, but not in TEC-resistant PD1.6TEC− cells. Because TEC causes apoptosis of PD1.6 cells in a GR-dependent manner (30) and induces mitochondrial (but not nuclear) GR translocation, we propose that GR translocated to the mitochondria is a trigger of apoptosis. Our findings also show that the nuclear and mitochondrial translocations of GR are differentially regulated.
Figure 5.
TEC induces mitochondrial, but not nuclear, translocation of GR in TEC-sensitive PD1.6 cells. PD1.6, PD1.6TEC−, and GR-deficient PD1.6Dex− cells were incubated alone or with TEC for 4 h. Nonadherent lymphoma cells were harvested and subjected to subcellular fractionation and Western blotting. The samples were harvested at an early stage after cocultivation with TEC, long before the onset of the apoptotic process. Thus, CytoC could be used as a marker for the mitochondria.
The mitochondrial localization signal (MLS) and the nuclear localization signal (NLS) are located at different domains of GR
Our data showing that, depending on circumstances, GR translocates either to the nucleus or to the mitochondria, suggest that nuclear and mitochondrial GR translocations are differentially regulated. We therefore searched for signals directing the intracellular trafficking of GR. To this end, we transfected GR-negative 293 cells with WT or various deletion mutants of human GR (hGR). In the absence of ligand, the transfected GR is expressed in cytosol, nucleus, and mitochondria (Fig. 6 A, lane 1). Addition of Dex did not alter the intracellular distribution of GR in these cells (unpublished data). This pattern of exogenous GR expression enabled the search for NLS and MLS. Using 293 cells transfected with GR deletion mutants, we found that NH2-terminal GR fragments (1–488, 1–515, and 1–550) translocated to the nucleus, but not to the mitochondria (Fig. 6 A, lanes 2–4), indicating that these mutants contain NLS but not MLS. GRΔ428-490 (ΔDBD) and GRΔ490-515 translocated to the mitochondria, but barely to the nucleus (Fig. 6 A, lanes 5 and 6), indicating that NLS resides within aa 428–515 of hGR, which is in accordance with the three NLS described previously (31). GRΔ550-600 translocated to the nucleus, but barely to the mitochondria (Fig. 6 A, lane 7), indicating that MLS resides, at least in part, within aa 550–600. A residual mitochondrial translocation observed with GRΔ550-600 suggests that the 550–600 domain may act in concert with another site within the COOH-terminal region of GR. Similar to WT GR, the Δ727-777 mutant distributed to both the nucleus and the mitochondria (Fig. 6 A, lane 8). Also, the GRΔ77-262 deletion mutant translocated to both the mitochondria and nucleus (unpublished data). Hence, NLS and MLS are located within different domains of the GR protein.
Figure 6.
(A) MLS and NLS are located within different domains of GR. GR-negative 293 epithelial cells were transfected with plasmids encoding the indicated human GR variants. After 20 h, the cells were subjected to subcellular fractionation and GR was detected on Western blot using the PA1-511 antibody to GR. (B) α-Helix wheel model of the putative MLS located within aa 558–580 of human GR. The positive-charged arginine and lysine (red) and the hydrophilic threonine (orange) are located on the one side of the α-helix, whereas the hydrophobic aa leucine, isoleucine, valine, and tryptophane (blue) are located on the opposite side of the α-helix. Amino acids interacting with GC are labeled with gray numbers. This putative MLS is the α-Helix 3 of LBD. (C) The α-Helix 3 (aa 558–580) as it appears in the 1M2Z crystal structure. The same color labeling of aa is used as in B. (D) The MLS of GR resembles that of cytochrome C oxidase (COX). Amino acid sequence alignment between MLS of GR and MLS of COX. (E) The R564G and R575G mutants show reduced ability to enter the mitochondria. Mouse GFP-GR was point-mutated at aa 564 or 575 corresponding to the human R558 and R569, and the ability of these mutants to enter the mitochondria was determined as described in A. (F) The MLS (H3) of GR in the LBD crystal structure. The putative MLS is labeled in red in the 1M2Z crystal structure of a dimer complex of the human GR LBD (aa 521–777) bound to Dex and a Tif2 coactivator motif (reference 6).
The 550–600 domain of GR comprises the α-Helix 3 (aa 558–580) of the LBD. This α-helix is characterized by a series of positively charged aa (arginine/lysine), hydrophilic aa (threonine), and hydrophobic aa (valine, isoleucine, and leucine), but lacks negatively charged aa. The positively charged and hydrophilic aa locate on the one side of the α-helix, whereas the hydrophobic aa locate on its other side (Fig. 6, B and C). This arrangement is compatible with the requirements for an MLS (32). It shows some sequence similarity to the MLS of cytochrome C oxidase (COX) (Fig. 6 D). To verify that the MLS resides within aa 558–580 stretch, Arg564 and Arg575 of mouse GFP-GR (which correspond to human GRArg558 and Arg569, respectively) were each mutated to glycine. Arg575 was also mutated to aspartate. The ability of these mutants to translocate to the mitochondria was compared with that of GFP-GR WT. R564G and R575G showed reduced ability to enter the mitochondria, whereas R575D behaved as WT in this respect (Fig. 6 E). These data indicate that the secondary structure created by R564 and R575, rather than their charge, is important for the MLS integrity. When this MLS is placed in the crystal structure of dimerized ligand-bound LBD, the two MLS appear adjacent (Fig. 6 F). The differential location of MLS and NLS may explain the dissociated nuclear and mitochondrial translocations of GR observed in various cell types in response to diverse stimuli.
GR targeted to the mitochondria induces apoptosis
Our data suggest a role for mitochondrial GR in mediating apoptosis. To verify this hypothesis, we sought to distinguish between the effects exerted by GR in the mitochondria and in other intracellular compartments. To this end, we constructed a GR variant (MLSCOX-GFP-GR) that exclusively localizes to the mitochondria. This variant was attained by adding the MLS of COX upstream to GFP-GR. We initially analyzed the intracellular localization of MLSCOX-GFP-GR in the absence or presence of Dex. HeLa, H1299, and PC3 cells transfected with GFP-GR or MLSCOX-GFP-GR were either untreated or treated with 100 nM Dex for 2 h. Thereafter, the cells were stained with red mitotracker to visualize the mitochondria and analyzed by confocal microscopy. The MLSCOX-GFP-GR exclusively localized to the mitochondria both in the absence and in the presence of Dex (Fig. 7, A and B). Hence, this construct was indeed useful for studying the effect of mitochondrial GR on apoptosis. For this purpose, we transfected HeLa cervical carcinoma, PC-3 prostate adenocarcinoma, and L929 E8.2 A3 fibroblast-like cells with plasmids encoding either GFP-GR or MLSCOX-GFP-GR. After 48 h, the percentage of apoptotic transfectants was compared with that of nontransfectants from the same sample. GFP-GR induced apoptosis of HeLa and L929 E8.2 A3 cells, but not of PC-3 cells (Fig. 7 C). MLSCOX-GFP-GR induced apoptosis of HeLa and L929 E8.2 A3 cells more efficiently than GFP-GR (Fig. 7 C). Interestingly, PC-3 cells, which do not undergo apoptosis by GFP-GR, were sensitive to MLSCOX-GFP-GR (Fig. 7 C). These results demonstrate that when GR is directed to the mitochondria, it is capable of inducing apoptosis.
Figure 7.
(A and B) MLSCOX-GFP-GR is exclusively localized to the mitochondria. The mitochondria-directed GFP-GR variant was prepared by inserting the MLS of COX upstream to GFP-GR. HeLa cells (A), H1299 (B), or PC-3 (B) cells were transfected with either GFP-GR or MLSCOX-GFP-GR and stained with red mitrotracker to visualize the mitochondria before confocal microscopy. (C) MLSCOX-GFP-GR is more efficient in inducing apoptosis than GFP-GR. HeLa, L929 E8.2 A3, and PC-3 cells were transfected with plasmids encoding MLSCOX-GFP-GR or GFP-GR. After 48 h, the percentage of apoptotic GFP-positive cells (transfectants) was compared with that of GFP-negative cells (nontransfectants) of the same sample. (D) NLS-defective GRK513-515A induces apoptosis of HeLa cells. Cells were transfected with plasmids encoding GFP-GRwt, GFP-GRK513-515A, or pEGFP-F. After 48 h, the percentage of apoptotic cells was determined as in C. (E and F) A nucleus-directed GFP-GR variant (NLSTAg-GFP-GR) induces apoptosis of HeLa (E) and H1299 (F) cells. Cells were transfected with plasmids encoding GFP-GR, MLSCOX-GFP-GR, NLSTAg-GFP-GR, or pEGFP-F. After 48 h, the percentage of apoptotic cells was assessed as in C.
NLS-defective GR and a nuclear-directed GR possess proapoptotic properties
The aforementioned data indicate that mitochondrial GR triggers apoptosis induced by GC. We cannot, however, exclude the possibility that GR may act at additional intracellular sites. To further study in which intracellular compartments GR may induce apoptosis, we analyzed the apoptotic ability of an NLS-defective GR mutant (pEGFP-ratGRK513-515A) and a nucleus-only directed GR variant (NLSTAg-GFP-GR). The latter was constructed by adding the NLS of SV40 T antigen in triplet [(DPKKRKV)3] upstream to GFP-GR of mouse origin.
The apoptotic ability of pEGFP-GRK513-515A was compared with that of the parental pEGFP-GR WT in HeLa cells. It should be noted that pEGFP-GRK513-515A is unable to translocate to the nucleus (31). We observed that both GR WT and the NLS-defective GR variant induced apoptosis of HeLa cells (Fig. 7 D). pEGFP-F, used as a negative control, did not cause apoptosis of HeLa cells (Fig. 7 D). The ability of pEGFP-GRK513-515A to trigger apoptosis demonstrates that GR may mediate apoptosis by a nucleus-independent manner.
Last, we compared the apoptotic ability of the nucleus-directed NLSTAg-GFP-GR to those of MLSCOX-GFP-GR and parental GFP-GR. For this purpose, HeLa and H1299 cells were transfected with the respective plasmids. Both NLSTAg-GFP-GR and MLSCOX-GFP-GR were more efficient than GFP-GR in inducing apoptosis of these cells (Fig. 7, E and F). pEGFP-F did not induce apoptosis under the same circumstances (Fig. 7, E and F). These results suggest that an overexpressed GR may induce apoptosis when present either in the mitochondria or in the nucleus. Under physiological conditions, we anticipate that the mitochondrial GR cooperates with nuclear GR in inducing apoptosis.
DISCUSSION
Numerous studies have been performed to elucidate the mechanisms by which GC induces apoptosis (2, 3, 5, 27). Several biochemical changes occurring immediately after exposure to GC have been characterized. These include Ca2+ mobilization, activation of Src and Cdk2 kinases, and activation of phosphatidylinositol-specific phospholipase C and acidic sphingomyelinase with subsequent ceramide generation (3, 33–35). Downstream effector mechanisms of GC-induced apoptosis have also been defined. These involve the mitochondria apoptotic pathway mediated by Bax, Bak, Bim, and tBid, and antagonized by Bcl-2 and Bcl-XL (3, 36). Dissipation of the mitochondrial membrane potential (ΔΨm) is followed by release of cytochrome C and Smac/Diablo to the cytosol (3, 36), which in turn leads to the activation of caspase-9, caspase-3, and endonucleases (3, 36). With the good knowledge of downstream effectors in GC-induced apoptosis, little is known about the role and fate of GR in this response, as well as the reasons why some GR-expressing cells are sensitive, whereas others are not.
GR expression above a threshold level is necessary, but is not sufficient to activate GC-mediated apoptosis (2, 5, 27). Several studies have indicated that GC-resistant cells may express similar GR levels as GC-sensitive cells (2, 37–39). Likewise, in the present study, we show that GC-sensitive (PD1.6, 2B4, thymocytes) and some GC-resistant (B10, S49) cells express high GR levels. These observations suggest that the apoptotic response is regulated by additional factors acting downstream to ligand–receptor interaction. One possibility is that only a certain isotype of GR can initiate apoptosis. Indeed, various mRNA transcripts of GR have been observed in both mouse and human cells (19, 40, 41); among them, the 1A variant is most abundantly expressed in hematopoietic cells (19, 20). The 1A transcript is mainly translated to a ∼90-kD GR and a small proportion of a higher molecular mass GR (∼150 kD) (18). The various cells analyzed in our study mainly express the ∼90-kD form corresponding to GRα. We also detected a small amount of ∼150-kD GR in the cytosolic fraction of PD1.6, 2B4, B10, and S49 cells (unpublished data). Hence, the ∼150-kD GR form is not expressed exclusively in cells sensitive to GC, but in GC-resistant cells as well.
Another factor that could affect GC-mediated apoptosis is the intracellular trafficking of GR. GC causes nuclear translocation of GR in both GC-sensitive and GC-resistant cells. Both transactivation-deficient and transrepression-deficient GR variants have been shown to restore GC sensitivity in human T-ALL cells (7, 14, 42, 43). These data together with our present finding that an NLS-deficient GR mutant is proapoptotic suggest that nuclear-independent mechanisms are likely involved in this death pathway.
It has been suggested that mGR is involved in GC-induced apoptosis based on the finding that a cDNA derived from full-length 1A transcript, imparted both mGR expression and GC sensitivity to some GC-resistant cells (18). However, this cDNA also caused GR overexpression in the cytosol (18), which may have contributed to the GC sensitivity of the cells. In the present paper, we have further studied the role of mGR in GC-induced apoptosis by analyzing mGR expression in GC-sensitive and resistant lymphoid cells. mGR was expressed on some cell types that were GC resistant, but not on the GC-sensitive cells studied. Thus, mGR is not required for GC-induced apoptosis, and its mere presence does not impose susceptibility to GC. Our findings are compatible with those of Gametchu et al. (17), showing that mGR-negative lymphoma cells may be GC sensitive.
Another possibility could be that mitochondrial GR is the trigger of apoptosis. GR has been shown to be located within the mitochondria in some cell types (22–24). In these studies, GR was detected in the mitochondrial membranes and in the matrix space. Likewise, we also observed a basal mitochondrial GR expression in most of the lymphoma cells analyzed. However, Dex induces GR translocation to the mitochondria in GC-sensitive, but not in GC-resistant, cells. This is in contrast with nuclear translocation of GR that takes place in all cell types. This is the first qualitative difference in GR behavior described that distinguishes between GC-sensitive and resistant cells, suggesting a role for mitochondrial GR in apoptosis.
In GC-sensitive cells, mitochondrial and nuclear translocations of GR occur simultaneously within the first minutes after exposure to Dex. After its initial translocation, the amount of GR in the mitochondria was maintained at a steady state. Thus, the elevated mitochondrial GR level is sustained in GC-sensitive cells, which is in contrast with the transient residence of GR in the mitochondria (5–30 min) in HeLa cells and with the reduced amount of mitochondrial GR in a glioma cell line after Dex treatment (24, 44). The sustained expression of GR in the mitochondria of GC-sensitive cells may account for the apoptotic effects of GC. Another support for a role of mitochondrial GR in mediating apoptosis comes from the observation that TECs, which trigger apoptosis of PD1.6 in a GR-dependent manner (30), induces GR translocation to the mitochondria, but not to the nucleus. Thus, GR translocation to the nucleus is not necessary for the apoptotic response. However, we cannot exclude the possibility that mitochondrial GR cooperates with nuclear GR in inducing apoptosis. The fact that a mitochondria-directed GR induces apoptosis suggests that exclusive expression of GR in the mitochondria is sufficient for triggering apoptosis. Conversely, a nucleus-directed GR is also proapoptotic. It should be noted that the latter data were obtained by a transient transfection assay where the proteins were overexpressed. Thus, the GR effects are more pronounced than at physiological GR levels. Nevertheless, it is a valuable approach that provides information on GR function. Altogether, our data show that mitochondrial translocation of GR correlates with apoptotic sensitivity.
This behavior of GR resembles that of the proapoptotic p53 and Nur77 proteins. These proteins have recently been shown to have a direct apoptogenic role at the mitochondria, besides their nuclear effects (45, 46). Our results add GR to the growing list of proteins that mediate apoptosis when localized to the mitochondria. Also, the thyroid, estrogen β and retinoid X receptors have been shown to be located in the mitochondria (24, 47, 48). Thus far, however, the mitochondrial localization of these receptors has not been implicated in apoptosis.
The mechanism by which mitochondrial GR mediates apoptosis is a matter for further study. GC has been shown to regulate mitochondrial transcription and energy production (24), and GRE elements have been found in the mitochondrial genome (49). It should be mentioned in this regard that some of the apoptotic effects of p53 and Nur77 occur independently of their transcriptional activities (45, 46). p53 and Nur77 interact with the protective Bcl-XL and Bcl-2 proteins in the mitochondria (46, 50). It would, therefore, be interesting to find out whether mitochondrial GR has similar effects.
Another important finding of our study is that nuclear and mitochondrial GR translocations are differentially regulated. For instance, Dex induces both mitochondrial and nuclear GR translocations in GC-sensitive lymphoid cells, but only nuclear translocation in GC-resistant cells. In contrast, TEC induces mitochondrial, but not nuclear, GR translocation. Furthermore, we have partially characterized a putative MLS comprising the α-Helix 3 (aa 558–580) of LBD. This domain lies COOH terminally to the three NLSs (aa 428–515) (31), and possesses several traits of an MLS (32). The hydrophobic and positively charged residues are partitioned on opposite sides of the helix. Most of the proteins destined to the mitochondria contain an NH2-terminal mitochondrial transfer peptide that is cleaved off after entering the mitochondrial matrix (32). Some mitochondrial proteins, however, have a noncleavable internal MLS (32). The GR detected by us in the mitochondria is of a similar size as cytosolic GR, indicating that it does not undergo cleavage upon mitochondrial translocation. Hence, GR contains a noncleavable internal mitochondrial targeting sequence possessing an amphipathic presequence-type helix. This target sequence resembles the internal MLS of BCS1 (51).
It is conceivable that GR is transported to the mitochondria by a heat-shock protein, as its 558–580 domain overlaps with one of several characterized Hsp90-binding sites (5), and both Hsp90 and Hsp70 function as chaperones that interact with the mitochondrial protein import receptor Tom 70 (52). Further studies are required to verify the role of heat shock proteins in GR trafficking.
In summary, we conclude that mitochondrial GR acts independently of nuclear GR in inducing apoptosis, and that mitochondrial and nuclear GR translocations are differentially regulated. Given the multiple nuclear adverse effects of GC therapy, our findings propose further research focusing on the development of therapeutic modalities that preferentially direct GR to the mitochondria.
MATERIALS AND METHODS
Cells.
PD1.6 thymic lymphoma (30), B10 thymic lymphoma (30), S49 T lymphoma (provided by J. Hochman, The Hebrew University of Jerusalem, Jerusalem, Israel), TEC (provided by A. Kruisbeek, The Netherlands Cancer Institute, Amsterdam, Netherlands), 293 kidney epithelial cells, HeLa cervical carcinoma, and L929 E8.2 A3 fibroblast-like cells (provided by W.V. Vedeckis, Louisiana State University, New Orleans, LA) were grown in DMEM supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 10 mM Hepes, 1 mM sodium pyruvate, nonessential aa, antibiotics, and 50 μM β-mercaptoethanol. 2B4 T cell hybridoma, 4B2 PML, Jurkat ALL, thymocytes, H1299 lung adenocarcinoma, and PC-3 prostate adenocarcinoma were cultured in RPMI 1640 with the same supplements as for DMEM. PD1.6Dex− cells were derived from PD1.6 cells by repeated exposure to increasing concentrations of Dex and PD1.6TEC− cells were derived from PD1.6 cells repeatedly cocultured with TECs (30).
Plasmids.
The following plasmids were used: GFP-mouse GR (provided by L.J. Muglia, Washington University in St. Louis, St. Louis, MO); hGRΔDBD and hGRΔ77-262 (provided by W. Doppler, Universität Innsbruck, Innsbruck, Austria); hGR WT, hGR1-488, hGR1-515, hGR1-550, hGR418-777, hGRΔ490-515, hGRΔ550-600, hGR550-777, and hGRΔ727-777 (provided by T.D. Gelehrter, University of Michigan, Ann Arbor, MI); pEGFP-rat GR and pEGFP-ratGR K513-515A (provided by K.R. Yamamoto, University of California, San Francisco, CA); pCMV/myc/mito (Invitrogen) (provided by S. Ostrand-Rosenberg, University of Maryland, Baltimore, MD); pCMV/myc/nuc (Invitrogen) (provided by D. Bensi, Mayo Clinic, Rochester, MN); farnesylated GFP (pEGFP-F; CLONTECH) and pEGFP-N1 (CLONTECH) (provided by Y. Haupt, The Hebrew University of Jerusalem, Jerusalem, Israel). A mitochondria-directed GFP-GR variant (MLSCOX-GFP-GR) was prepared by inserting the MLS of COX (MSVLTPLLLRGLTGSARRLPVPRAKIHSL) NH2-terminal to GFP-GR. A PCR product containing this MLS was obtained from pCMV/myc/mito using 5′-primer CTAGCTAGCTGACGCAAATGGGCGGTAGGCGTG-3′ harboring a NheI site and 3′-primer CCCACCGGTTTGGCCCCATTCAGATCCTCTTC-5′ harboring a AgeI site. After double digestion with NheI and AgeI, the PCR product was inserted within the NheI/AgeI sites of GFP-GR. A nucleus-directed GFP-GR variant (NLSTAg-GFP-GR) was prepared by inserting the NLS of SV40 T antigen in triplet ([DPKKRKV)3]) NH2-terminal to GFP-GR. pCMV/myc/nuc was used as template for the aforementioned primers. Mutations in GR were introduced by site-directed mutagenesis (QuickChange kit; Stratagene). Sequencing of plasmids was done at the DNA Sequencing Facility of The Hebrew University of Jerusalem, Israel.
Indirect immunofluorescence.
Cell surface staining of GR was performed by incubating cells with M20 antibody to GR (Santa Cruz Biotechnology) followed by FITC-conjugated AffiniPure F(ab)2-fragment of goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories). Intracellular GR staining was performed on rehydrated methanol-fixed cells.
Determination of apoptosis.
Apoptosis was assessed by cell cycle analysis and caspase 3 activation. For cell cycle distribution, cells were fixed with ice-cold methanol, rehydrated in PBS, and treated with 50 μg/ml RNase. After adding 5 μg/ml propidium iodide, the DNA content was analyzed by flow cytometry (FACSCalibur; Becton Dickinson). Subdiploid cells are regarded as apoptotic cells, and presented as percentage of the whole cell population. Caspase 3 activation was analyzed by incubating rehydrated methanol-fixed cells with antibody to cleaved caspase 3 (Asp 175; Cell Signaling Technologies) followed by FITC-conjugated AffiniPure F(ab)2-fragment of goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories).
Subcellular fractionation and Western blot.
Cell pellets were gently resuspended in cytoplasmic buffer (10 mM Hepes, pH 7.4; 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 10 mM Na2MoO4, 2 mM PMSF, 20 μg/ml aprotinin, 0.1% NP-40; 25 mM NaF, and 0.2 mM Na3VO4) and kept on ice for 10 min before centrifugation at 900 g for 10 min. The nuclear pellets were processed as described below. The cytoplasmic supernatant was recentrifuged at 900 g to ensure complete removal of nuclear material. The resulting supernatant was centrifuged at 10,000 g for 30 min. The cytosolic supernatant was processed for Western blot by adding protein sample buffer (PSB) × 4.5. The mitochondrial pellet was washed with cytoplasmic buffer, recentrifuged at 10,000 g, and dissolved in PSB × 1.5. The nuclear pellet was washed with cytoplasmic buffer and recentrifuged at 900 g before their extraction in nuclear buffer (20 mM Hepes, pH 7.4, 1.5 mM MgCl2, 420 mM NaCl, 25% glycerol (vol/vol), 0.2 mM EDTA, 0.5 mM DTT, 10 mM Na2MoO4, 2 mM PMSF, 20 μg/ml aprotinin, 25 mM NaF, and 0.2 mM Na3VO4). The nuclear extracts were cleared at 20,000 g for 10 min, and processed for Western blot by adding PSB × 4.5. GR was detected on Western blot by using the PA1-511A (Affinity BioReagent), M20, or P20 antibodies to GR (Santa Cruz Biotechnology). The blots were reprobed with antibodies to α-tubulin (Sigma-Aldrich), cytochrome C (CytoC; Santa Cruz Biotechnology), VDAC (Oncogene Research Products), or histone H2B (LG2-2; provided by M. Monestier, Temple University, Philadelphia, PA) (53). The Oncogene cytosol/mitochondria fractionation kit (QIA88; Oncogene Research Products) was used according to the manufacturer's instructions.
Cocultivation of PD1.6 cells with TECs.
5 × 106 TECs were seeded in 75 cm2 tissue culture bottles (Nunc). On the following day, 107 PD1.6 cells were added to the TEC monolayer in a 40-ml medium. After 4 h, nonadherent PD1.6 cells were harvested and processed for cell fractionation as described before.
Transient transfection assay.
Cells were transfected with the given plasmids by using the calcium phosphate precipitation method. For apoptosis assay, the adherent cells were trypsinized and collected in culture supernatants. Flow cytometry was performed while gating on GFP positive or negative cells. Percentage of apoptotic transfectants (GFP-positive) was compared with that of nontransfectants (GFP-negative) from the same culture. For confocal microscopy, the cells were incubated with red mitotracker (50 nM; Invitrogen) during the last 30 min of incubation. For cell fractionation studies, adherent transfected cells were harvested with rubber policeman and centrifuged at 720 g for 5 min.
Acknowledgments
We thank Dr. M. Tarshish for excellent assistance with confocal microscopy.
This work was supported by The Concern Foundation, CA.
The authors have no conflicting financial interests.
Abbreviations used: ALL, acute lymphoblastic leukemia; COX, cytochrome C oxidase; DBD, DNA-binding domain; Dex, dexamethasone; GC, glucocorticoid; GR, glucocorticoid receptor; GRE, glucocorticoid response element; hGR, human GR; LBD, ligand binding domain; mGR, membrane glucocorticoid receptor; MLS, mitochondrial localization signal; NLS, nuclear localization signal; PML, promyelocytic leukemia; TEC, thymic epithelial cell.
References
- 1.Haarman, E.G., G.J. Kaspers, and A.J. Veerman. 2003. Glucocorticoid resistance in childhood leukaemia: mechanisms and modulation. Br. J. Haematol. 120:919–929. [DOI] [PubMed] [Google Scholar]
- 2.Tissing, W.J., J.P. Meijerink, M.L. den Boer, and R. Pieters. 2003. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 17:17–25. [DOI] [PubMed] [Google Scholar]
- 3.Greenstein, S., K. Ghias, N.L. Krett, and S.T. Rosen. 2002. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin. Cancer Res. 8:1681–1694. [PubMed] [Google Scholar]
- 4.Lu, N.Z., and J.A. Cidlowski. 2004. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann. NY Acad. Sci. 1024:102–123. [DOI] [PubMed] [Google Scholar]
- 5.Schaaf, M.J., and J.A. Cidlowski. 2002. Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 83:37–48. [DOI] [PubMed] [Google Scholar]
- 6.Bledsoe, R.K., V.G. Montana, T.B. Stanley, C.J. Delves, C.J. Apolito, D.D. McKee, T.G. Consler, D.J. Parks, E.L. Stewart, T.M. Willson, et al. 2002. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 110:93–105. [DOI] [PubMed] [Google Scholar]
- 7.Tao, Y., C. Williams-Skipp, and R.I. Scheinman. 2001. Mapping of glucocorticoid receptor DNA binding domain surfaces contributing to transrepression of NF-κB and induction of apoptosis. J. Biol. Chem. 276:2329–2332. [DOI] [PubMed] [Google Scholar]
- 8.Liden, J., F. Delaunay, I. Rafter, J. Gustafsson, and S. Okret. 1997. A new function for the C-terminal zinc finger of the glucocorticoid receptor. Repression of RelA transactivation. J. Biol. Chem. 272:21467–21472. [DOI] [PubMed] [Google Scholar]
- 9.Reichardt, H.M., K.H. Kaestner, J. Tuckermann, O. Kretz, O. Wessely, R. Bock, P. Gass, W. Schmid, P. Herrlich, P. Angel, and G. Schutz. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 93:531–541. [DOI] [PubMed] [Google Scholar]
- 10.Kanelakis, K.C., and W.B. Pratt. 2003. Regulation of glucocorticoid receptor ligand-binding activity by the hsp90/hsp70-based chaperone machinery. Methods Enzymol. 364:159–173. [DOI] [PubMed] [Google Scholar]
- 11.Kino, T., E. Souvatzoglou, M.U. De Martino, M. Tsopanomihalu, Y. Wan, and G.P. Chrousos. 2003. Protein 14-3-3σ interacts with and favors cytoplasmic subcellular localization of the glucocorticoid receptor, acting as a negative regulator of the glucocorticoid signaling pathway. J. Biol. Chem. 278:25651–25656. [DOI] [PubMed] [Google Scholar]
- 12.Thompson, E.B., and B.H. Johnson. 2003. Regulation of a distinctive set of genes in glucocorticoid-evoked apoptosis in CEM human lymphoid cells. Recent Prog. Horm. Res. 58:175–197. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L., and P.A. Insel. 2004. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J. Biol. Chem. 279:20858–20865. [DOI] [PubMed] [Google Scholar]
- 14.Helmberg, A., N. Auphan, C. Caelles, and M. Karin. 1995. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 14:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Naghy, M., B.H. Johnson, H. Chen, N.H. Ansari, W. Zhang, P. Moller, Y. Ji, and E.B. Thompson. 2001. The pathway of leukemic cell death caused by glucocorticoid receptor fragment 465*. Exp. Cell Res. 270:166–175. [DOI] [PubMed] [Google Scholar]
- 16.Gametchu, B., F. Chen, F. Sackey, C. Powell, and C.S. Watson. 1999. Plasma membrane-resident glucocorticoid receptors in rodent lymphoma and human leukemia models. Steroids. 64:107–119. [DOI] [PubMed] [Google Scholar]
- 17.Gametchu, B. 1987. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 236:456–461. [DOI] [PubMed] [Google Scholar]
- 18.Chen, F., C.S. Watson, and B. Gametchu. 1999. Association of the glucocorticoid receptor alternatively-spliced transcript 1A with the presence of the high molecular weight membrane glucocorticoid receptor in mouse lymphoma cells. J. Cell. Biochem. 74:430–446. [PubMed] [Google Scholar]
- 19.Breslin, M.B., C.D. Geng, and W.V. Vedeckis. 2001. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol. Endocrinol. 15:1381–1395. [DOI] [PubMed] [Google Scholar]
- 20.Purton, J.F., J.A. Monk, D.R. Liddicoat, K. Kyparissoudis, S. Sakkal, S.J. Richardson, D.I. Godfrey, and T.J. Cole. 2004. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J. Immunol. 173:3816–3824. [DOI] [PubMed] [Google Scholar]
- 21.Demonacos, C., N.C. Tsawdaroglou, R. Djordjevic-Markovic, M. Papalopoulou, V. Galanopoulos, S. Papadogeorgaki, and C.E. Sekeris. 1993. Import of the glucocorticoid receptor into rat liver mitochondria in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 46:401–413. [DOI] [PubMed] [Google Scholar]
- 22.Moutsatsou, P., A.M. Psarra, A. Tsiapara, H. Paraskevakou, P. Davaris, and C.E. Sekeris. 2001. Localization of the glucocorticoid receptor in rat brain mitochondria. Arch. Biochem. Biophys. 386:69–78. [DOI] [PubMed] [Google Scholar]
- 23.Scheller, K., C.E. Sekeris, G. Krohne, R. Hock, I.A. Hansen, and U. Scheer. 2000. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol. 79:299–307. [DOI] [PubMed] [Google Scholar]
- 24.Scheller, K., P. Seibel, and C.E. Sekeris. 2003. Glucocorticoid and thyroid hormone receptors in mitochondria of animal cells. Int. Rev. Cytol. 222:1–61. [DOI] [PubMed] [Google Scholar]
- 25.Hochman, J., E. Levy, N. Mador, M.M. Gottesman, G.M. Shearer, and E. Okon. 1984. Cell adhesiveness is related to tumorigenicity in malignant lymphoid cells. J. Cell Biol. 99:1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, S., W.H. Miller Jr., and A. Dejean. 1998. Trivalent antimonials induce degradation of the PML-RAR oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood. 92:4308–4316. [PubMed] [Google Scholar]
- 27.Kofler, R., S. Schmidt, A. Kofler, and M.J. Ausserlechner. 2003. Resistance to glucocorticoid-induced apoptosis in lymphoblastic leukemia. J. Endocrinol. 178:19–27. [DOI] [PubMed] [Google Scholar]
- 28.Riml, S., S. Schmidt, M.J. Ausserlechner, S. Geley, and R. Kofler. 2004. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 11:S65–S72. [DOI] [PubMed] [Google Scholar]
- 29.Kauppi, B., C. Jakob, M. Farnegardh, J. Yang, H. Ahola, M. Alarcon, K. Calles, O. Engstrom, J. Harlan, S. Muchmore, et al. 2003. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J. Biol. Chem. 278:22748–22754. [DOI] [PubMed] [Google Scholar]
- 30.Zilberman, Y., E. Zafrir, H. Ovadia, E. Yefenof, R. Guy, and R.V. Sionov. 2004. The glucocorticoid receptor mediates the thymic epithelial cell-induced apoptosis of CD4+8+ thymic lymphoma cells. Cell. Immunol. 227:12–23. [DOI] [PubMed] [Google Scholar]
- 31.Freedman, N.D., and K.R. Yamamoto. 2004. Importin 7 and importin α/importin β are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell. 15:2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfanner, N., and A. Geissler. 2001. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2:339–349. [DOI] [PubMed] [Google Scholar]
- 33.Granes, F., M.B. Roig, H.J. Brady, and G. Gil-Gomez. 2004. Cdk2 activation acts upstream of the mitochondrion during glucocorticoid induced thymocyte apoptosis. Eur. J. Immunol. 34:2781–2790. [DOI] [PubMed] [Google Scholar]
- 34.Lepine, S., B. Lakatos, M.P. Courageot, H. Le Stunff, J.C. Sulpice, and F. Giraud. 2004. Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J. Immunol. 173:3783–3790. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti, M.C., B. Di Marco, G. Cifone, G. Migliorati, and C. Riccardi. 2003. Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood. 101:585–593. [DOI] [PubMed] [Google Scholar]
- 36.Almawi, W.Y., O.K. Melemedjian, and M.M. Jaoude. 2004. On the link between Bcl-2 family proteins and glucocorticoid-induced apoptosis. J. Leukoc. Biol. 76:7–14. [DOI] [PubMed] [Google Scholar]
- 37.Berki, T., L. Palinkas, F. Boldizsar, and P. Nemeth. 2002. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int. Immunol. 14:463–469. [DOI] [PubMed] [Google Scholar]
- 38.Gupta, V., E.B. Thompson, D. Stock-Novack, S.E. Salmon, H.I. Pierce, J.D. Bonnet, D. Chilton, and J. Beckford. 1994. Efficacy of prednisone in refractory multiple myeloma and measurement of glucocorticoid receptors. A Southwest Oncology Group study. Invest. New Drugs. 12:121–128. [DOI] [PubMed] [Google Scholar]
- 39.Wiegers, G.J., M. Knoflach, G. Bock, H. Niederegger, H. Dietrich, A. Falus, R. Boyd, and G. Wick. 2001. CD4+CD8+TCRlow thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur. J. Immunol. 31:2293–2301. [DOI] [PubMed] [Google Scholar]
- 40.Chen, F., C.S. Watson, and B. Gametchu. 1999. Multiple glucocorticoid receptor transcripts in membrane glucocorticoid receptor-enriched S-49 mouse lymphoma cells. J. Cell. Biochem. 74:418–429. [PubMed] [Google Scholar]
- 41.Strähle, U., A. Schmidt, G. Kelsey, A.F. Stewart, T.J. Cole, W. Schmid, and G. Schutz. 1992. At least three promoters direct expression of the mouse glucocorticoid receptor gene. Proc. Natl. Acad. Sci. USA. 89:6731–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazareth, L.V., D.V. Harbour, and E.B. Thompson. 1991. Mapping the human glucocorticoid receptor for leukemic cell death. J. Biol. Chem. 266:12976–12980. [PubMed] [Google Scholar]
- 43.Thompson, E.B., L.V. Nazareth, R. Thulasi, J. Ashraf, D. Harbour, and B.H. Johnson. 1992. Glucocorticoids in malignant lymphoid cells: gene regulation and the minimum receptor fragment for lysis. J. Steroid Biochem. Mol. Biol. 41:273–282. [DOI] [PubMed] [Google Scholar]
- 44.Koufali, M.M., P. Moutsatsou, C.E. Sekeris, and K.C. Breen. 2003. The dynamic localization of the glucocorticoid receptor in rat C6 glioma cell mitochondria. Mol. Cell. Endocrinol. 209:51–60. [DOI] [PubMed] [Google Scholar]
- 45.Li, H., S.K. Kolluri, J. Gu, M.I. Dawson, X. Cao, P.D. Hobbs, B. Lin, G. Chen, J. Lu, F. Lin, et al. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR 3. Science. 289:1159–1164. [DOI] [PubMed] [Google Scholar]
- 46.Mihara, M., S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancoska, and U.M. Moll. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 11:577–590. [DOI] [PubMed] [Google Scholar]
- 47.Casas, F., L. Daury, S. Grandemange, M. Busson, P. Seyer, R. Hatier, A. Carazo, G. Cabello, and C. Wrutniak-Cabello. 2003. Endocrine regulation of mitochondrial activity: involvement of truncated RXRα and c-Erb Aα1 proteins. FASEB J. 17:426–436. [DOI] [PubMed] [Google Scholar]
- 48.Yang, S.H., R. Liu, E.J. Perez, Y. Wen, S.M. Stevens Jr., T. Valencia, A.M. Brun-Zinkernagel, L. Prokai, Y. Will, J. Dykens, et al. 2004. Mitochondrial localization of estrogen receptor β. Proc. Natl. Acad. Sci. USA. 101:4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demonacos, C.V., N. Karayanni, E. Hatzoglou, C. Tsiriyiotis, D.A. Spandidos, and C.E. Sekeris. 1996. Mitochondrial genes as sites of primary action of steroid hormones. Steroids. 61:226–232. [DOI] [PubMed] [Google Scholar]
- 50.Lin, B., S.K. Kolluri, F. Lin, W. Liu, Y.H. Han, X. Cao, M.I. Dawson, J.C. Reed, and X.K. Zhang. 2004. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR 3. Cell. 116:527–540. [DOI] [PubMed] [Google Scholar]
- 51.Folsch, H., B. Guiard, W. Neupert, and R.A. Stuart. 1996. Internal targeting signal of the BCS1 protein: a novel mechanism of import into mitochondria. EMBO J. 15:479–487. [PMC free article] [PubMed] [Google Scholar]
- 52.Voos, W. 2003. A new connection: chaperones meet a mitochondrial receptor. Mol. Cell. 11:1–3. [DOI] [PubMed] [Google Scholar]
- 53.Monestier, M., P. Decker, J.P. Briand, J.L. Gabriel, and S. Muller. 2000. Molecular and structural properties of three autoimmune IgG monoclonal antibodies to histone H2B. J. Biol. Chem. 275:13558–13563. [DOI] [PubMed] [Google Scholar]