Abstract
Here, we report on the expression of programmed death (PD)-1 on human virus-specific CD8+ T cells and the effect of manipulating signaling through PD-1 on the survival, proliferation, and cytokine function of these cells. PD-1 expression was found to be low on naive CD8+ T cells and increased on memory CD8+ T cells according to antigen specificity. Memory CD8+ T cells specific for poorly controlled chronic persistent virus (HIV) more frequently expressed PD-1 than memory CD8+ T cells specific for well-controlled persistent virus (cytomegalovirus) or acute (vaccinia) viruses. PD-1 expression was independent of maturational markers on memory CD8+ T cells and was not directly associated with an inability to produce cytokines. Importantly, the level of PD-1 surface expression was the primary determinant of apoptosis sensitivity of virus-specific CD8+ T cells. Manipulation of PD-1 led to changes in the ability of the cells to survive and expand, which, over several days, affected the number of cells expressing cytokines. Therefore, PD-1 is a major regulator of apoptosis that can impact the frequency of antiviral T cells in chronic infections such as HIV, and could be manipulated to improve HIV-specific CD8+ T cell numbers, but possibly not all functions in vivo.
Cellular immune responses play a pivotal role in the ability of HIV-infected individuals to control viral replication (1). The immune system, however, ultimately fails to control the virus and the majority of HIV-infected patients develop AIDS. Several mechanisms of viral immune evasion have been proposed (2–4). Accumulated data suggest that the functional capacity of HIV-specific CD8+ T cells is adversely affected by HIV infection. Under chronic antigen stimulation HIV-specific CD8+ T cells, which lack CD4+ T cell help, express an impaired ability to produce cytokines (termed “exhaustion”) (5). Part of the CD8+ T cell profile in HIV infection also involves an impaired ability to survive and proliferate (6) that could further contribute to the overall impairment in cytokine production. Therefore, therapeutic interventions that enhance the survival of these cells could also augment their proliferative and cytokine functions, thereby leading to improved immune control of HIV infection.
A variety of manipulations can augment HIV-specific CD8+ T cell responses in vitro. Treatment with cytokines (i.e., IL-2 and IL-15) can increase survival and effector function of these cells (7, 8). Furthermore, cross-linking of costimulatory T cell receptors such as 4-1BB, CD80, and OX40 has been previously described to enhance HIV-specific CD8+ T cell responses either directly (9, 10) or indirectly (11). However, blocking of negative T cell regulators offers an alternative approach to restoring T cell function. Emerging data indicate that the outcome of a T cell response is, at least in part, dependent on the interplay between positive and negative costimulatory molecules expressed on T cells and antigen-presenting cells (12, 13). Recently, restoration of exhausted virus-specific CD8+ T cells in a mouse model of chronic lymphocytic choriomeningitis virus infection was achieved by blocking programmed death (PD)-1, but not CTLA-4 (14), two negative regulators of T cell activation and function (15). PD-1 is the newest member of the CD28 family, expressed on activated CD4+ and CD8+ T cells, B cells, and macrophages (16). Although there is some evidence showing delivery of positive signals by the ligands of PD-1, programmed death ligand (PD-L)1 and PD-L2, (17, 18) the vast majority of the data point to negative regulation of T cell activation, proliferation, and cytokine production by these ligands (19–21). In support of this negative role, in vitro studies using primary human T cells have shown that PD-1 ligation can inhibit TCR-mediated signaling by reducing phosphorylation of ZAP70, activation of PKCθ (22), and activation of Akt (23). PD-1 was originally cloned from cell lines exhibiting high sensitivity to apoptosis (24). More recently, induction of apoptosis of tumor-specific CD8+ T cells by PD-L1 was described (25), a process mediated by other receptors in addition to PD-1 (25). Therefore, induction of apoptosis could be a key mechanism used by the PD-1–PD-L system to affect the outcome of a virus-specific T cell response.
Importantly, blockade of PD-1 has been shown to be effective in enhancing virus-specific CD8+ T cell responses in mice lacking CD4+ T cell help (14), raising the possibility that, if such negative regulation of CD8+ T cell function were operative in HIV infection, blocking this pathway might enhance HIV-specific CD8+ T cell function. Here, we report on the expression of PD-1 on HIV-specific CD8+ T cells, and CD8+ T cells specific for other viruses, and the consequences of manipulating the PD-1–PD-L system on survival, proliferation, and cytokine production by these cells. We found that HIV-specific CD8+ T cells express high levels of PD-1 that may play a critical role for their survival, and that the control of survival is the predominant mechanism through which PD-1 affects HIV-specific T cell function.
RESULTS
HIV-specific CD8+ T cells express high levels of PD-1 independently of their maturational status
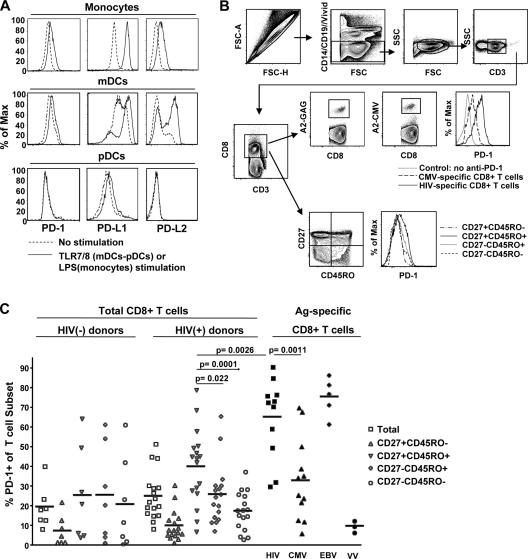
PD-1 has two known ligands, PD-L1 and PD-L2 (26). We first investigated the expression of PD-1 and its ligands on antigen-presenting cells: monocytes, myeloid dendritic cells (mDCs) (27), and plasmacytoid dendritic cells (pDCs) (28–30). We purified human CD11c+ mDCs and CD123+ pDCs from elutriated monocytes (31, 32). In vitro activation of purified monocytes with LPS resulted in up-regulation of PD-L1, whereas PD-1 and PD-L2 were less affected (Fig. 1 A). Cross-linking of toll-like receptor (TLR)7 or 8 (with the TLR7/8 ligand Resiquimod) on mDCs substantially stimulated the expression of both PD-L1 and PD-L2 and caused a slight induction in PD-1 expression (Fig. 1 A). In marked contrast, TLR7/8-mediated activation of pDCs had no effect on either PD-1 or its ligands, suggesting that pDCs are not involved in regulating CD8+ T cell function via PD-1–PD-L interactions (Fig. 1 A). Our data indicate that mDCs and monocytes, in addition to presenting antigen and costimulatory signals to virus-specific CD8+ T cells, may also serve to regulate CD8+ T cell function through the expression of PD-1–specific ligands.
Figure 1.
PD-1 is highly expressed on HIV-specific CD8+ T cells. (A) Representative histograms depicting PD-1, PD-L1, and PD-L2 expression in monocytes, mDCs, and pDCs from one subject tested. Cell preparations from at least three donors were tested. (B) The polychromatic flow cytometry gating scheme for identification of cell populations is shown. Histograms depict the PD-1 expression in HIV- and CMV-specific CD8+ T cells from the same sample. Memory subsets identified by CD27 and CD45RO staining of total CD8+ T cells are presented. (C) Pooled data showing the percentage of CD8+ T cells expressing PD-1+ phenotype in total CD8+ T cells from healthy donors (n = 7) and HIV+ individuals (n = 17), HIV-specific (n = 11), CMV-specific (n = 12), and EBV-specific (n = 5) CD8+ T cells from HIV+ individuals, and VV-specific CD8+ T cells from vaccinated healthy donors (n = 3). PD-1 expression in memory subsets of total CD8+ T cells from HIV+ and healthy donors is also shown. Horizontal lines depict mean values. The p values were calculated using Student's t test.
We next assessed the expression of PD-1 on naive, memory, and virus-specific CD8+ T cells using polychromatic flow cytometry. The gating scheme for identification of the various CD8+ T cell subsets is shown in Fig. 1 B. Our results show that PD-1 expression on naive (CD27+CD45RO−) CD8+ T cells is infrequent in both HIV− and HIV+ donors, consistent with previous studies (19, 33) (Fig. 1 C). However, in HIV+ subjects, PD-1 expression was more frequent on CD27+CD45RO+ memory CD8+ T cells than on the other memory CD8+ subsets (P = 0.02 and 0.0001 versus CD27−CD45RO+ and CD27−CD45RO− memory CD8+ T cells, respectively). This distinction was not present within memory CD8+ T cells from HIV− subjects. We next assessed PD-1 expression on virus-specific CD8+ T cells after staining with HIV-, CMV-, EBV-, and vaccinia virus (VV)–specific tetramers (example shown in Fig. 1 B). We found different levels of PD-1 expression on memory CD8+ T cells according to their specificity. In general, HIV-specific and EBV-specific CD8+ T cells were found to express PD-1 more frequently than CMV-specific CD8+ T cells, and VV-specific CD8+ T cells rarely expressed PD-1 (Fig. 1 C). Similar patterns of PD-1 expression with respect to virus antigen specificity were obtained when PD-1 expression was analyzed by mean fluorescence intensity, rather than the frequency of cells that express a given level of PD-1 (unpublished data). We did not see a correlation between plasma viral load and PD-1 expression on HIV-specific CD8+ T cells in this small group of subjects (unpublished data), although in larger cohort studies such an association has been clearly demonstrated (34, 35).
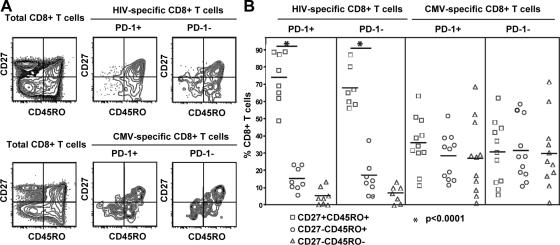
We asked whether PD-1 expression on antigen-specific cells is related to their altered maturational status (6, 36). The vast majority of HIV-specific CD8+ T cells was found to express a CD27+ phenotype, in agreement with previously published data (37). There was no difference in the maturational phenotype of the PD-1+ and PD-1− fractions of HIV-specific CD8+ T cells (Fig. 2, A and B). CMV-specific CD8+ T cells were evenly distributed among the three memory phenotypes defined by CD27 and CD45RO; furthermore, PD-1+ and PD-1− CMV-specific CD8+ T cells exhibited the same distribution of these three memory phenotypes (Fig. 2, A and B). We also found that there was no difference in the expression of other homing and maturational markers (CCR7 and CD57) between PD-1+ and PD-1− antigen-specific CD8+ T cells (unpublished data). Our data therefore indicate that PD-1 expression is independent of the maturational state of antigen-specific memory CD8+ T cells.
Figure 2.
PD-1 expression is independent of the maturational status of HIV- and CMV-specific CD8+ T cells. (A) Representative flow cytometry showing the distribution of PD-1+ and PD-1− HIV- and CMV-specific CD8+ T cells in memory CD8+ T cell subsets. Cells were gated as in Fig. 1. The distribution of total CD8+ T cells is also shown. (B) Pooled data showing the percentage of CD8+ T cells in memory populations for PD-1+ and PD-1− subsets of HIV-specific (n = 8) and CMV-specific (n = 11) CD8+ T cells from HIV-infected individuals. The p values were calculated using Student's t test.
Expression of PD-1 does not directly affect the ability of HIV-specific CD8+ T cells to produce cytokines
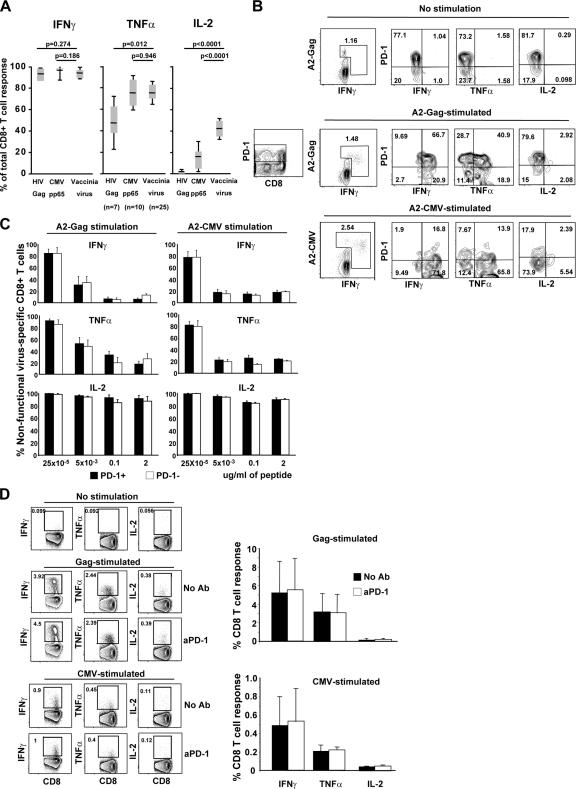
Knowing that sustained high levels of PD-1 are associated with a diminished ability of LCMV-specific CD8+ T cells to produce cytokine in chronically infected mice (14), we hypothesize that PD-1 expression on human HIV-specific CD8+ T cells would similarly result in impaired cytokine production. We first confirmed that the ability of CD8+ T cells to produce cytokines in response to antigen stimulation was impaired in those T cells known to express high levels of PD-1. We measured IFN-γ, TNF-α, and IL-2 production by CD8+ T cells after HIV Gag, CMV pp65, and VV stimulation, and assessed the proportion of the cells that were able to produce any one of the three cytokines. As shown in Fig. 3 A, there was no difference in the proportion of the HIV Gag–, CMV pp65–, or VV-specific CD8+ T cells that were able to produce IFN-γ, but there was lower production of TNF-α and IL-2 by CD8+ T cells known to express higher levels of PD-1 (HIV > CMV > VV). This confirmed that PD-1 expression is associated with impaired cytokine (TNF-α and IL-2) production by antigen-specific CD8+ T cells.
Figure 3.
No direct association between PD-1 expression and cytokine production by virus-specific CD8+ T cells. (A) Pooled data depicting the percentage of antigen-specific CD8+ T cells that produce IFN-γ (left), TNF-α (middle), or IL-2 (right) after stimulation with HIV Gag peptides, CMV pp65 peptides, or VV. Cells from HIV+ (n = 7), CMV+ (n = 10), and healthy individuals immunized with modified vaccinia virus Ankara (n = 25) were tested. (B) PBMCs from an HIV and CMV coinfected subject were stimulated with A2-Gag or A2-CMV peptides, and epitope-specific CD8+ T cells were gated according to their expression of tetramer and/or production of cytokine, and the production of cytokine in the PD-1+ and PD-1− subsets was assessed. The left panel shows the PD-1 staining of CD8+ cells to demonstrate how the gating of PD-1+ and PD-1− cells was chosen. (C) Pooled data showing the percentage of nonfunctional virus-specific CD8+ T cells from three HIV-infected individuals off antiretroviral therapy [(tetramer+cytokine−) × 100/(tetramer+cytokine−) + (tetramer+cytokine+)] at the different peptide concentrations for both PD-1+ and PD-1− compartments are shown. (D) Flow cytometry showing the production of IFN-γ, TNF-α, or IL-2 from CD8+ T cells after stimulation with Gag or CMV peptides for 6 h in the absence or presence of anti–human PD-1 antibody (left). Pooled data are shown on the right. Samples from three HIV+ individuals were tested.
We next asked if the impaired cytokine function resulted directly from the expression of PD-1, or whether the two phenomena were only indirectly linked. The expression of PD-1 in relation to production of cytokines under short (6 h) stimulation was assessed in HIV- and CMV-specific CD8+ T cells. Cells were stimulated with peptides corresponding to HLA-A2–defined optimal HIV and CMV epitopes, and the production of IFN-γ, TNF-α, and IL-2 was measured within tetramer+ cells according to PD-1 expression. The data demonstrate that IFN-γ and TNF-α are readily produced from PD-1+ cells, whereas IL-2 production is rarely produced from either PD-1+ or PD-1− CMV- or HIV-specific CD8+ T cells (Fig. 3 B). We next gated on the PD-1+ and PD-1− subsets of antigen-specific cells (defined by cytokine production and/or tetramer staining) and calculated the proportion of the cells that were nonfunctional for cytokine production after stimulation with a range of peptide concentrations (Fig. 3 C). For both HIV- and CMV-specific CD8+ T cells at all levels of antigen stimulation, there was no difference in the production (or lack thereof) of IFN-γ, TNF-α, or IL-2 between PD-1+ and PD-1− cells. Therefore, PD-1 expression does not appear to be directly responsible for the inability of some CMV- and HIV-specific CD8+ T cells to produce these cytokines.
We postulated, however, that PD-1 may not be adequately engaged by its ligands during the ex vivo stimulations. We therefore performed 6-h peptide stimulations in the presence and absence of anti–PD-1 antibody to directly engage the PD-1 pathway during the time of antigen stimulation. Raw data for one subject and compiled data for three subjects are shown in the left and right panels, respectively, of Fig. 3 D, and show that engagement of PD-1 during antigen stimulation had no effect on the ability of the antigen-specific T cells to produce IFN-γ, TNF-α, or IL-2. Collectively, these data demonstrate that PD-1 does not directly influence the capacity of HIV- or CMV-specific CD8+ T cells to produce cytokines upon stimulation.
PD-1–PD-L1 manipulation alters virus-specific CD8+ T cell proliferation and cytokine production by regulating their survival
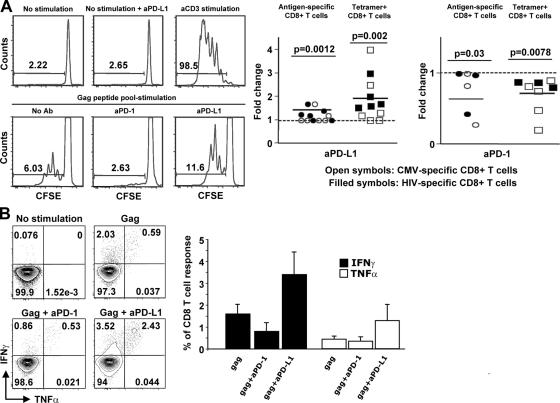
The major effect of PD-1 blockade in mice was on antigen-specific CD8+ T cell proliferation (14). HIV-specific CD8+ T cells are characterized by limited proliferative capacity in chronic HIV infection (38, 39), a defect that is linked to the depletion of IL-2–secreting HIV-specific CD4+ and/or CD8+ T cells (7, 39, 40). Therefore, we investigated whether stimulation through, or interference with, the PD-1–PD-L1 pathway could affect the proliferation of HIV- and CMV-specific CD8+ T cells. CFSE-labeled cells were stimulated with HIV- or CMV-specific peptide pools in the presence or absence of antibodies against either PD-1 or its ligand PD-L1. After 6 d, the frequency of CFSElow cells, in either all CD8+ or tetramer+ cells, was compared between cultures that had received no blocking antibody, an anti–PD-1 antibody (that acts as a PD-1 agonist), or an anti–PD-L1 antibody (clone MIH1; reference 41). Representative flow cytometry plots are shown in the left panel of Fig. 4 A and composite data from multiple experiments are shown in the right panel. The data show that stimulation of PD-1, in both HIV- and CMV-specific CD8+ T cells, resulted in a decrease in proliferative capacity. Alternatively, blocking of the PD-1–PD-L1 interaction with an anti–PD-L1 antibody resulted in increased proliferation of both HIV- and CMV- specific CD8+ T cells. There was variation in the amount of inhibition or augmentation of proliferation that was not directly associated with the simple level of PD-1 expression, suggesting that PD-1 is not the only factor regulating the ability of antigen-specific T cells to proliferate.
Figure 4.
PD-1–PD-L1 manipulation alters virus-specific CD8+ T cell proliferation and cytokine production. (A) Representative histograms depicting the CFSE profile of CD8+ T cells from an HIV+ donor. Pooled data showing the fold change of the percentage of CFSE low cells under treatment with aPD-L1 or aPD-1 antibody (right). The percentage of cells that divided in the absence of antibody treatment was assigned a value of 1. The ratio of the percentage in the presence and absence of antibody treatment for each response was calculated. The fold change for antigen-specific CD8+ (those that diluted CFSE in response to antigen; • and ◯) and gated antigen-specific tetramer+ CD8+ T cells (□ and ▪) is shown. The p values were calculated using Wilcoxon's paired t test. (B) Flow cytometry showing the CD8+ T cells secreting IFN-γ and TNF-α after stimulation with Gag peptides in the absence or presence of anti–PD-1 or anti–PD-L1 antibodies. Cells cultured for 6 d were restimulated with Gag peptides for the last 6 h (left). Pooled data showing the percentage of CD8+ T cells producing IFN-γ and TNF-α under these conditions (right). At least four patients were tested for each treatment.
We postulated that the effect of PD-1 on proliferation of antigen-specific T cells would affect the number of cytokine- producing cells in a multiday assay. Therefore, the ability of cells to produce cytokines after antigen stimulation and 6 d of culture was examined. PD-1 cross-linking in the presence of HIV Gag peptide reduced the frequency of CD8+ T cells producing IFN-γ and had minimal effect on the frequency of cells producing TNF-α (Fig. 4 B). On the other hand, anti–PD-L1 treatment resulted in an increased frequency of cells capable of producing both cytokines (Fig. 4 B). Collectively, these data indicate that any effect of PD-1 engagement on cytokine expression in a multiday assay is secondary to enhanced proliferation of cytokine-producing cells.
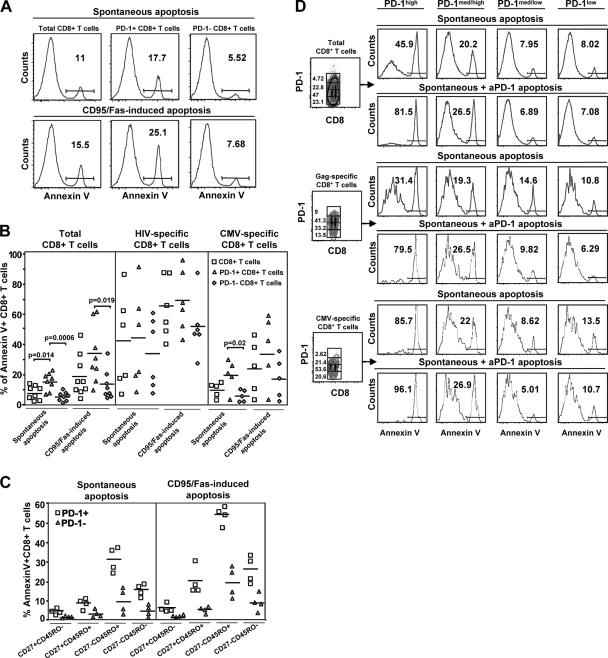
We next sought to determine if the previously described effects of PD-1 on apoptosis were responsible for the observed effects on proliferation. PD-1 expression was consistently associated with higher sensitivity of total CD8+ T cells to both spontaneous and CD95/Fas-induced apoptosis, estimated by annexin V surface exposure (Fig. 5 A, representative data; Fig. 5 B, compiled data). Apoptosis sensitivity was found to be significantly higher in HIV-specific compared with CMV-specific CD8+ T cells, in agreement with previously published data (6, 42), and PD-1+ CD8+ T cells were always more sensitive to apoptosis than PD-1− CD8+ T cells irrespective of antigen specificity (Fig. 5 B). Of interest, PD-1− HIV-specific CD8+ T cells often were more sensitive to apoptosis than PD-1+ CMV-specific CD8+ T cells (Fig. 5 B). We therefore asked whether this difference could be secondary to the different maturational phenotypes of HIV- and CMV-specific CD8+ T cells. In fact we found that PD-1+CD8+ T cells were more susceptible to both spontaneous and CD95/Fas-induced apoptosis compared with PD-1−CD8+ T cells in all memory subsets tested, but that sensitivity varied between maturational phenotypes (CD27−CD45RO+ > CD27−CD45RO− > CD27+CD45RO+; Fig. 5 C), suggesting that PD-1 expression augments apoptosis sensitivity upon the background defined by maturation and activation status.
Figure 5.
PD-1 regulates the in vitro survival of virus-specific CD8+ T cells. (A) Representative histograms depicting the annexin V positivity in total, PD-1+ and PD-1− CD8+ T cells from an HIV+ donor under spontaneous or CD95/Fas-induced apoptosis. (B) Pooled data showing the percentage of annexin V+ cells in PD-1+ and PD-1− subsets of total (n = 8), HIV- (n = 6), and CMV-specific (n = 5) CD8+ T cells from HIV+ individuals. (C) Annexin V positivity in PD-1+ versus PD-1− CD8+ T cells in memory subsets defined by CD27 and CD45RO markers. (D) PBMCs were cultured in the absence or presence of plate-bound anti–PD-1, and the percentage of annexin V+ cells was determined for CD8+ T cell subsets with different levels of PD-1 expression. Flow cytometry showing the different levels of spontaneous and anti–PD-1–induced apoptosis according to the level of PD-1 expression (high, medium/high, medium/low, and low) in total, Gag- and CMV-specific CD8+ T cells from the same HIV+ donor.
Another possible explanation for the greater apoptosis sensitivity of HIV-specific CD8+ T cells than CMV-specific CD8+ T cells is the absolute level of PD-1 expression on the cells. Although we had defined CD8+ T cells as being either PD-1 positive or PD-1 negative, it was apparent that PD-1 expression was higher (as measured by mean fluorescence intensity) on HIV- than CMV-specific CD8+ T cells. We therefore asked if the absolute level of PD-1 expression on CD8+ T cells was the primary determinant of apoptosis sensitivity while further determining whether PD-1 ligation directly affected survival of CD8+ T cells. To do this, we assessed apoptosis in the presence and absence of plate-bound anti–PD-1 in samples from six HIV+ donors. The results were consistent for all of the donors, and the results from one are shown in Fig. 5 D. We found that HIV-specific CD8+ T cells had higher expression of PD-1 than total or CMV-specific CD8+ T cells, but that irrespective of antigen specificity, the CD8+ T cells with the highest expression of PD-1 were the most sensitive to apoptosis and were the cells that augmented their apoptosis to the greatest extent upon PD-1 ligation. In fact, at medium/low and low levels of PD-1 expression, there was low spontaneous apoptosis that did not increase with PD-1 ligation, whereas at high levels of PD-1 expression, PD-1 ligation augmented the apoptosis to 80% or greater irrespective of antigen specificity. Therefore, the absolute level of PD-1 expression on antigen-specific CD8+ T cells is the primary indicator of apoptosis sensitivity and the major determinant of sensitivity to apoptosis upon PD-1 ligation. Collectively, our data strongly support that PD-1 is a critical regulator of CD8+ T cell survival in HIV infection.
DISCUSSION
The balance between positive and negative signals delivered by costimulatory molecules to T cells appears to be critical for the ultimate fate of cellular immune responses (13, 43). Recent data (10, 11, 14) suggest that manipulation of T cell costimulatory pathways may present a novel approach for enhancing and restoring virus-specific CD8+ T cell responses, especially in the context of a chronic infection like HIV. We report here on the role of PD-1, a negative costimulatory receptor of T cells, as a regulator of virus-specific CD8+ T cells in HIV infection. To understand how PD-1 can affect the function of CD8+ T cells, it is critical to identify which cells provide the ligand(s) for PD-1. Our data indicate that mDCs and monocytes may use the PD-1–PD-L system to regulate adaptive antiviral immunity. Our findings in this context are very preliminary and much more needs to be investigated. For instance, the relative kinetics of costimulatory molecule expression on mDCs and monocytes after activation and the impact of the “positive” and “negative” signals delivered by these molecules to responding T cells remains to be elucidated. In addition, it will be important to determine whether there is redundancy between the various costimulatory signals affecting CD8+ T cells or if they act independently by stimulating separate intracellular pathways after initiation of virus-specific CD8+ T cell responses.
We found remarkably high expression of PD-1 on HIV-specific CD8+ T cells. The frequency of PD-1 expression on the different virus-specific CD8+ T cells (HIV = EBV > CMV > VV) is consistent with PD-1 regulation according to antigen stimulation. Although it is unlikely that the level of antigen in chronic EBV infection reaches that which occurs in HIV infection, it has been shown that EBV is continuously shed into saliva by induction of the lytic cycle as B cells differentiate into plasma cells, thereby chronically stimulating lytic cycle antigen-specific CD8+ T cells (44). The mechanism by which CD8+ T cells control HIV and EBV may differ. Therefore, it may not follow that expression of PD-1 on EBV- and HIV-specific CD8+ T cells will similarly impact CD8-mediated control of these two different virus infections. Further experiments are needed to clarify the relative role of PD-1 in regulation of EBV-specific CD8+ T cell responses and compare it to the regulation of HIV-specific responses. In summary, although we cannot conclude that chronic antigen stimulation is the sole factor determining PD-1 expression, our data reveal that HIV-specific CD8+ T cells, because of their high expression of PD-1, may be vulnerable to negative signals delivered by PD-1, potentially leading to functional consequences in vivo.
Despite their differential expression of PD-1, no difference in IFN-γ production was found between HIV-, CMV-, and EBV-specific CD8+ T cells. On the other hand, PD-1 expression is associated with significantly lower ability of HIV-specific CD8+ T cells to produce TNF-α and even lower production of IL-2. This is in agreement with the finding that PD-1–PD-L1 blockage has a substantial impact on LCMV-specific CD8+ T cells producing both IFN-γ and TNF-α, whereas it has only a slight effect on single IFN-γ producers. However, we found that PD-1− and PD-1+ antigen-specific CD8+ T cells were equally able to produce cytokines upon antigen stimulation, indicating that PD-1 expression has no direct effect on cytokine production. This was further supported by our finding that ligation of PD-1 during antigen stimulation had no effect on cytokine production by virus-specific CD8+ T cells. Collectively these data clearly demonstrate that PD-1 has no direct effect upon the immediate ability of antigen-specific CD8+ T cells to produce IFN-γ, TNF-α, or IL-2.
Manipulation of the PD-1–PD-L system was found to alter the proliferation of virus-specific CD8+ T cells. This was accompanied by altered percentages of CD8+ T cells producing cytokines. The change in proliferation could result from an altered ability of these cells to either survive or divide. Importantly, we found no relationship between PD-1 expression and the degree of change in proliferative capacity after manipulation of the PD-1–PD-L1 axis. In fact, in some instances where the expression of PD-1 was very high on HIV-specific CD8+ T cells, only minor effects of PD-1 ligation on proliferation were observed. Therefore, although PD-1 has a demonstrable effect on the ability of virus-specific (CMV and HIV) CD8+ T cells to proliferate, it is not the sole factor regulating this function. This is not surprising as other factors (i.e., TCR activation threshold, relative expression of other costimulatory molecules, or levels of adaptor proteins mediating the intracellular signaling delivered by PD-1) could also contribute to the ability of PD-1 ligation to affect proliferative capacity.
At least two interventions have now proven successful in vitro in restoring the proliferation of HIV-specific CD8+ T cells: the addition of IL-2 (or CD4+ T cells producing IL-2) and the manipulation of costimulatory pathways such as PD-1. This raises the question of whether these two different manipulations affect proliferation through overlapping intracellular mechanisms. In addition, whether they can act in a synergistic mode remains to be elucidated. Since IL-2 cannot overcome the proliferative defect in CD57+CD8+ T cells (45), it is of particular interest to examine whether manipulation of PD-1–induced pathways could specifically restore their proliferative capacity.
Our data indicate that the primary mechanism by which PD-1 affects CD8+ T cell function is by regulating the ability of these cells to survive. This is in agreement with the originally described role of PD-1 as an apoptotic factor (24) and the reduced survival that characterizes virus-specific CD8+ T cells under conditions of chronic antigen stimulation (46, 47). We can speculate on how PD-1 expression could affect HIV-specific CD8+ T cell survival. It is possible that stimulation through PD-1 can direct cells into a cell cycle resting state, as has been described for the PD-1–PD-L2 interaction (21). We found that lack of PD-1 expression is associated with similar levels of spontaneous and CD95/Fas-induced apoptosis, whereas CD95/Fas-induced apoptosis is greatly augmented in CD8+ T cells that express PD-1, indicating that there may be cross-talk between the signals induced by these two receptors. Previously published data have shown that the Fas–FasL interaction impacts PD-L1–induced apoptosis of activated T cells (25). Although no direct link between PD-1 and CD95/Fas was described in that work (25), the possibility that PD-1 could prime (especially under conditions of chronic stimulation) CD8+ T cells to undergo CD95/Fas-induced apoptosis cannot be excluded. Therefore, clarification of the intracellular mechanism(s) governing the proapoptotic function of PD-1 is of particular interest. Furthermore, the role of such a function on CD4+ T cell survival in HIV infection would significantly add to our understanding of HIV pathogenesis.
A clear conclusion from our results is that the absolute level of PD-1 expression is a major determinant of spontaneous apoptosis and sensitivity to PD-1 ligation. We conclude this despite our observation that PD-1− HIV-specific CD8+ T cells are often more susceptible to apoptosis than PD-1+ CMV-specific CD8+ T cells (Fig. 5 B). Although it is known that sensitivity to apoptosis is also affected by other factors—specifically the level of T cell activation (defined by CD38 expression; unpublished data) and maturational state, which we have shown is independent of PD-1 expression (Fig. 2, A and B and Fig. 5 C)—our data indicate that PD-1 is a primary determinant of apoptosis sensitivity over and above these other factors. We conclude this because, within any population of CD8+ T cells (defined by activation, maturation, or antigen specificity), the PD-1+ population is more sensitive to apoptosis than the PD-1− population. In addition, although we have described PD-1 expression as either positive or negative in most of our data, expression really represents a continuum, with high expression being associated with greater impact upon PD-1–regulated functions (i.e., apoptosis; Fig. 5 D). When CD8+ T cells of different antigen specificities, but similar levels of PD-1 expression, are analyzed, they exhibit similar levels of spontaneous apoptosis and sensitivity to PD-1 ligation. In addition, cells with moderate to low expression of PD-1 have low levels of spontaneous apoptosis and are not affected by PD-1 ligation. What this means is that within any population of antigen-specific CD8+ T cells, it is the absolute level of PD-1 that primarily dictates the rate of spontaneous apoptosis and sensitivity to PD-1 ligation. Therefore, what is unique about HIV-specific CD8+ T cells is their high level of PD-1 expression leading to a profound (but potentially reversible) survival defect. Although the proliferative capacity of a CD8+ T cell is determined by more than just an ability to resist apoptosis, it is not difficult to visualize how the level of PD-1 and associated sensitivity to apoptosis would impact on the ability of a cell to proliferate.
Overall, our data demonstrate that PD-1 is preferentially expressed on CD8+ T cells specific for chronic viruses, and that PD-1 interaction with its ligands can regulate the ability of these virus-specific CD8+ T cells to survive and proliferate. Therefore, manipulation of this axis may lead to at least partial restoration of antigen-specific cell numbers and function in chronic viral infections such as HIV. It is important to remember that our data do not support the ability of PD-1 manipulation to restore all of the T cell functions that define functional “exhaustion.” For instance, we have no evidence that PD-1 blockade will restore absent cytokine functions, and may only affect CD8+ T cell proliferation to the degree possible in the context of other as yet undetermined defects in HIV-specific CD8+ T cells. Therefore, although our data identify PD-1 as a potential therapeutic target for restoring functional capacity of HIV-specific CD8+ T cell responses, it may not be capable of fully restoring function. In addition, it should be appreciated that the PD-1–PD-L1 axis likely evolved to attenuate potentially harmful CD8+ T cell responses to both self-antigens and chronic pathogens. Given that many non–HIV-specific CD8+ T cells express PD-1 (Fig. 1 C), it is likely that interventions to release all CD8+ T cells from PD-1–mediated suppression will have untoward effects.
MATERIALS AND METHODS
Patients.
PBMCs were obtained from HIV+ and HIV− volunteers and cryopreserved until use. None of the HIV-infected subjects in this study were on antiretroviral therapy, and they had a range of viral loads from <50 to 439,000 per ml. Signed informed consent approved by the relevant Institutional Review Board was obtained. Samples from VV-naive individuals, preimmunized with modified vaccinia virus Ankara followed by scarification with Dryvax, were also used. Samples were taken within 3 mo of Dryvax administration. Purification of DC subsets has been previously described (32).
Antibodies.
The following directly conjugated antibodies were used: CD3-Cy7APC, IFN-γ–FITC, TNF-α–Cy7PE, CD14-FITC, CD11c-PE, CD11c-APC, PD-1–PE, PD-L1–Cy7PE, PD-L2–APC (all from BD Biosciences) and CD45RO-TexasRedPE (Beckman Coulter). Annexin V-Cy5PE and the following antibodies were conjugated in our laboratory: IL-2–FITC, IL-2–APC, CD14–Cascade blue, CD19–Cascade blue, CD8-Qdot 705, and CD27-Cy5PE. The unconjugated mAbs were obtained from BD Biosciences. Cascade blue was obtained from Molecular Probes. Cy5 was obtained from BD Biosciences. Quantum Dots were obtained from the Quantum Dot Corporation. The following APC-labeled tetramers were prepared as previously described (48): A2-Gag (SLYNTVATYL), A2-CMV (NLVPVMTV), A2-Vaccinia (KVDDTFYYV), B8-Nef (FLKEKGGL), and A2-EBV (GLCTLVAML).
Cell stimulation.
PBMCs were thawed and rested overnight at 37°C. Viability by Trypan blue exclusion was typically ≥90%. 2 × 106 PBMCs were diluted to 1 ml with medium containing costimulatory antibodies (αCD28 and αCD49d) (1 μg/ml) (Becton Dickinson), monensin (0.7 μg/ml; BD Biosciences), brefeldin A (10 μg/ml; Sigma-Aldrich), in the absence or presence of indicated amounts (μg/μl) of A2-Gag (SLYNTVATL) or A2-CMV (NLVPVMTV) epitope peptides and incubated for 6 h. Cells were washed and incubated with pretitrated amounts of APC-labeled A2-gag or A2-Cmv tetramer for 15 min at 37°C. After washing, cells were surface stained with PD-1–PE, CD8–Qdot 705, CD14– and CD19–cascade blue, and 156 ng/ml violet amine reactive viability dye (vivid; InVitrogen). Following permeabilization (Cytofix/Cytoperm kit; BD Biosciences), cells were stained with IFN-γ–FITC or IL-2–FITC, TNF-α–PECy7, and CD3-Cy7APC. Alternatively, cells were left untreated or preincubated for 30 min at 37°C with an anti–human PD-1 antibody (AF 1086; R&D Systems) (20 μg/ml) and subsequently stimulated with peptides (15mers overlapping by 11) corresponding to full-length HIV-1 Gag (2 μg/ml each peptide, 5 μl/ml; National Institutes of Health AIDS Research and Reference Reagent Program) or cytomegalovirus antigen (60 μl/ml; Microbix Biosystems Inc.) for 6 h. After a washing step, cells were stained with vivid, CD14– and CD19–cascade blue, CD8-Qd705, permeabilized, and stained intracellularly with CD3-Cy7APC, IFN-γ–FITC, IL-2–APC, and TNF-α–Cy7PE. For CFSE studies, PBMCs were washed thoroughly and labeled with 0.25 μM CFSE (Molecular Probes). Cells were adjusted to 1.5 × 106 cells/ml and cultured in the presence of peptides (15mers overlapping by 11) corresponding to full-length HIV-1 Gag (2 μg/ml each peptide, 5 μl/ml; National Institutes of Health AIDS Research and Reference Reagent Program) or cytomegalovirus CF antigen (60 μl/ml; Microbix Biosystems Inc.). aCD28/aCD49d (1 μg/ml) was used for costimulation. An unstimulated and a positive control (culture with 1 μg/ml immobilized anti-CD3, clone UCHT1; R&D Systems) was included in each assay. Antibodies against human PD-1 (AF 1086; R&D Systems) (20 μg/ml) and human PD-L1 (16–5983; eBioscience) (25 μg/ml) were used for cross-linking of PD-1 and PD-L1, respectively. Cells were cultured for 6 d, harvested, and stained first with A2-Gag or A2-CMV tetramer–APC (15 min, 37°C) and subsequently with annexin V–Cy5PE, CD3-Cy7APC, and CD8-PE. 2.5 mM CaCl2 was included in all staining steps. Alternatively, cells were cultured under the same conditions, and on day 6 cells were stained with vivid and CD8-Qd705 and intracellularly with CD3-Cy7APC, IFN-γ–FITC, IL-2–APC, and TNF-α–Cy7PE.
Apoptosis studies.
1–1.5 × 106 PBMCs were cultured in 24-well plates (BD Biosciences) in the absence or presence of plate-bound anti–human CD95/Fas (IgM, CH11; Upstate Biotechnology) (5 μg/ml) or anti–human PD-1 (AF 1086, R&D Systems) (20 μg/ml) for 12 h at 37°C. Cells were harvested, washed, and surface stained with APC-labeled A2-Gag, A2-CMV, or B8-Nef tetramers, and subsequently with annexin V–Cascade blue, CD3-Cy7APC, CD8-Qd705, CD27-CyPE, CD45RO-TRPE, and PD-1–PE and green amine reactive viability dye (grivid; InVitrogen). 2.5 mM CaCl2 was included in all staining steps.
Flow cytometry.
Cells were analyzed with a modified LSRII flow cytometer (BD Immunocytometry Systems). Between 200,000 and 1 million events were collected. Electronic compensation was conducted with antibody capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. Data analysis was performed using FlowJo version 6.0 (TreeStar). Forward scatter area (FSC-A) versus forward scatter height (FSC-H) was used to gate out cell aggregates. CD14+ cells, CD19+ cells, and dead cells were removed from the analysis to reduce background staining. The cells were then gated through a FSC-A versus side scatter height (SSC-H) plot to isolate small lymphocytes. Next, CD3+ cells were selected and PD-1 expression was measured in gated total CD8+ T cells and tetramer+ cells, and in relation to their differentiation level by using the CD27-Cy5PE and CD45RO-TexasRedPE memory markers. Tetramer+ cells were selected and the percentage of PD1+ and PD1−, IFN-γ, and TNF-α–producing cells was determined using gating criteria determined using the total CD3+CD8+ population. For CFSE analysis, after initial gating (FSC-A versus FSC-H), apoptotic (annexin V+) cells were removed and small lymphocytes were identified. CD3+ cells were selected, and the percentage of CFSE low cells was determined in gated total CD8+ T cells and tetramer+ cells. For analysis of monocytes and DCs, the following combinations of titrated antibodies were used: CD11c-APC/CD14-FITC/PD-1–PE, CD11c-PE/CD14-FITC/PD-L1–Cy7PE/PD-L2–APC, and CD123-Cy5PE/PD-1–PE/PD-L1–Cy7PE/PD-L2–APC. PD-1, PD-L1, and PD-L2 levels were determined in CD14+CD11c− (monocytes), CD14−CD11c+ (mDCs), and high CD123 (pDCs) cells.
Statistical analysis.
Statistical analysis was performed using Student's t test and Wilcoxon's paired t test. P values < 0.05 were considered significant. The GraphPad Prism statistical analysis program was used.
Acknowledgments
The authors have no conflicting financial interests.
Abbreviations used: mDC, myeloid dendritic cell; PD, programmed death; pDC, plasmacytoid dendritic cell; PD-L, programmed death ligand; TLR, toll-like receptor; VV, vaccinia virus.
References
- 1.Koup, R.A., J.T. Safrit, Y. Cao, C.A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D.D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gougeon, M.L. 2003. Apoptosis as an HIV strategy to escape immune attack. Nat. Rev. Immunol. 3:392–404. [DOI] [PubMed] [Google Scholar]
- 3.Johnson, W.E., and R.C. Desrosiers. 2002. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499–518. [DOI] [PubMed] [Google Scholar]
- 4.van Kooyk, Y., and T.B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697–709. [DOI] [PubMed] [Google Scholar]
- 5.Betts, M.R., M.C. Nason, S.M. West, S.C. De Rosa, S.A. Migueles, J. Abraham, M.M. Lederman, J.M. Benito, P.A. Goepfert, M. Connors, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller, Y.M., S.C. De Rosa, J.A. Hutton, J. Witek, M. Roederer, J.D. Altman, and P.D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity. 15:871–882. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerli, S.C., A. Harari, C. Cellerai, F. Vallelian, P.A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA. 102:7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller, Y.M., P.M. Bojczuk, E.S. Halstead, A.H. Kim, J. Witek, J.D. Altman, and P.D. Katsikis. 2003. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 101:1024–1029. [DOI] [PubMed] [Google Scholar]
- 9.Serghides, L., J. Bukczynski, T. Wen, C. Wang, J.P. Routy, M.R. Boulassel, R.P. Sekaly, M. Ostrowski, N.F. Bernard, and T.H. Watts. 2005. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J. Immunol. 175:6368–6377. [DOI] [PubMed] [Google Scholar]
- 10.Bukczynski, J., T. Wen, C. Wang, N. Christie, J.P. Routy, M.R. Boulassel, C.M. Kovacs, K.S. Macdonald, M. Ostrowski, R.P. Sekaly, et al. 2005. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J. Immunol. 175:6378–6389. [DOI] [PubMed] [Google Scholar]
- 11.Yu, Q., F.Y. Yue, X.X. Gu, H. Schwartz, C.M. Kovacs, and M.A. Ostrowski. 2006. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J. Immunol. 176:2486–2495. [DOI] [PubMed] [Google Scholar]
- 12.Khoury, S.J., and M.H. Sayegh. 2004. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 20:529–538. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva, R., S. Thomas, T. Nguyen, N. Martin-Orozco, Y. Wang, M.K. Kaja, X.Z. Yu, and C. Dong. 2006. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 25:2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald, R.J., G.J. Freeman, and A.H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki, T., Y. Iwai, and T. Honjo. 2002. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr. Opin. Immunol. 14:779–782. [DOI] [PubMed] [Google Scholar]
- 17.Wang, S., J. Bajorath, D.B. Flies, H. Dong, T. Honjo, and L. Chen. 2003. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 197:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng, S.Y., M. Otsuji, K. Gorski, X. Huang, J.E. Slansky, S.I. Pai, A. Shalabi, T. Shin, D.M. Pardoll, and H. Tsuchiya. 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai, G., A. Karni, E.M. Oliveira, H.L. Weiner, D.A. Hafler, and G.J. Freeman. 2004. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell. Immunol. 230:89–98. [DOI] [PubMed] [Google Scholar]
- 20.Freeman, G.J., A.J. Long, Y. Iwai, K. Bourque, T. Chernova, H. Nishimura, L.J. Fitz, N. Malenkovich, T. Okazaki, M.C. Byrne, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latchman, Y., C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A.J. Long, J.A. Brown, R. Nunes, et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. [DOI] [PubMed] [Google Scholar]
- 22.Sheppard, K.A., L.J. Fitz, J.M. Lee, C. Benander, J.A. George, J. Wooters, Y. Qiu, J.M. Jussif, L.L. Carter, C.R. Wood, and D. Chaudhary. 2004. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 574:37–41. [DOI] [PubMed] [Google Scholar]
- 23.Parry, R.V., J.M. Chemnitz, K.A. Frauwirth, A.R. Lanfranco, I. Braunstein, S.V. Kobayashi, P.S. Linsley, C.B. Thompson, and J.L. Riley. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida, Y., Y. Agata, K. Shibahara, and T. Honjo. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong, H., S.E. Strome, D.R. Salomao, H. Tamura, F. Hirano, D.B. Flies, P.C. Roche, J. Lu, G. Zhu, K. Tamada, et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki, T., and T. Honjo. 2006. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 27:195–201. [DOI] [PubMed] [Google Scholar]
- 27.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonteneau, J.F., M. Larsson, A.S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y.J. Liu, J.D. Lifson, D.R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadowaki, N., S. Antonenko, J.Y. Lau, and Y.J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. [DOI] [PubMed] [Google Scholar]
- 31.Lore, K., M.R. Betts, J.M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R.A. Seder, and R.A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171:4320–4328. [DOI] [PubMed] [Google Scholar]
- 32.Lore, K., A. Smed-Sorensen, J. Vasudevan, J.R. Mascola, and R.A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett, F., D. Luxenberg, V. Ling, I.M. Wang, K. Marquette, D. Lowe, N. Khan, G. Veldman, K.A. Jacobs, V.E. Valge-Archer, et al. 2003. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 170:711–718. [DOI] [PubMed] [Google Scholar]
- 34.Day, C.L., P. Kiepiela, J.A. Brown, E.S. Moodley, S. Reddy, A.J. Leslie, J. Duraiswamy, Q. Eichbaum, M. Altfeld, E.J. Wherry, et al. 2006. PD-1 expression on HIV-specific CD8 T cells is associated with T cell exhaustion and disease progression. Nature. 10.1038/nature05115. [DOI] [PubMed]
- 35.Trautmann, L., L. Janbazian, N. Clomont, E.A. Said, S. Gimmig, B. Bessette, M.R. Boulassel, E. Delwart, H. Sepulveda, R.A. Balderas, et al. 2006. Upregulation of PD-1 expression in HIV specific CD8 T cells leads to reversible immune dysfunction. Nat. Med. 10.1038/nm1482. [DOI] [PubMed]
- 36.Champagne, P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]
- 37.Ochsenbein, A.F., S.R. Riddell, M. Brown, L. Corey, G.M. Baerlocher, P.M. Lansdorp, and P.D. Greenberg. 2004. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J. Exp. Med. 200:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migueles, S.A., A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 39.Lichterfeld, M., D.E. Kaufmann, X.G. Yu, S.K. Mui, M.M. Addo, M.N. Johnston, D. Cohen, G.K. Robbins, E. Pae, G. Alter, et al. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younes, S.A., B. Yassine-Diab, A.R. Dumont, M.R. Boulassel, Z. Grossman, J.P. Routy, and R.P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2–producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saudemont, A., N. Jouy, D. Hetuin, and B. Quesnel. 2005. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 105:2428–2435. [DOI] [PubMed] [Google Scholar]
- 42.Petrovas, C., Y.M. Mueller, I.D. Dimitriou, P.M. Bojczuk, K.C. Mounzer, J. Witek, J.D. Altman, and P.D. Katsikis. 2004. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J. Immunol. 172:4444–4453. [DOI] [PubMed] [Google Scholar]
- 43.Rothstein, D.M., and M.H. Sayegh. 2003. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol. Rev. 196:85–108. [DOI] [PubMed] [Google Scholar]
- 44.Laichalk, L.L., and D.A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenchley, J.M., N.J. Karandikar, M.R. Betts, D.R. Ambrozak, B.J. Hill, L.E. Crotty, J.P. Casazza, J. Kuruppu, S.A. Migueles, M. Connors, et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 101:2711–2720. [DOI] [PubMed] [Google Scholar]
- 46.Yao, S., and L. Chen. 2006. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol. Med. 12:244–246. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76:829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchinson, S.L., L. Wooldridge, S. Tafuro, B. Laugel, M. Glick, J.M. Boulter, B.K. Jakobsen, D.A. Price, and A.K. Sewell. 2003. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J. Biol. Chem. 278:24285–24293. [DOI] [PubMed] [Google Scholar]