Abstract
A majority of the antibodies expressed by nascent B cells in healthy humans are self-reactive, but most of these antibodies are removed from the repertoire during B cell development. In contrast, untreated systemic lupus erythematosus (SLE) patients fail to remove many of the self-reactive and polyreactive antibodies from the naive repertoire. Here, we report that SLE patients in clinical remission continue to produce elevated numbers of self-reactive and polyreactive antibodies in the mature naive B cell compartment, but the number of B cells expressing these antibodies is lower than in patients with active disease. Our finding that abnormal levels of self-reactive mature naive B cells persist in the majority of patients in clinical remission suggests that early checkpoint abnormalities are an integral feature of SLE.
In healthy humans, self-reactive antibodies are removed from the B cell repertoire during the transition from early immature to immature B cells in the bone marrow and from the new emigrant to the mature naive B cell compartment in the periphery (1). Untreated systemic lupus erythematosus (SLE) patients show defective early B cell tolerance checkpoints and therefore accumulate self-reactive and polyreactive antibodies in the circulating mature naive B cell compartment (2). Mature naive B cells, however, do not secrete antibodies and are unlikely to play a direct role in autoimmune pathology, but they are precursors of antibody-secreting cells and therefore aberrant self-tolerance in this compartment would predispose to the development of the high affinity autoantibodies characteristic of SLE (3, 4).
Immunosuppressive or cytotoxic drugs and anti-CD20–mediated B cell depletion can induce long-term remission in patients with SLE (5). However, current treatment protocols frequently fail to prevent relapses (5). Furthermore, SLE patients show high levels of serum antinuclear antibodies (ANAs) long before the onset of disease. The absence of clinical signs and symptoms does not necessarily correlate with the absence of serum autoantibodies (6). Therefore, SLE patients may fail to establish or maintain B cell self-tolerance independent of their clinical status. Whether this persistent tolerance defect involves early stages in B cell development has not been examined.
To determine the status of early B cell tolerance in SLE patients in clinical remission, we cloned 278 antibodies from mature naive B cells from six such patients and tested them for binding to HEp-2 cell lysates and for polyreactivity against a panel of purified antigens. Here, we report that SLE patients in clinical remission continue to show increased numbers of self-reactive and polyreactive antibodies in the mature naive B cell compartment, but they have fewer B cells expressing these antibodies than patients with active disease.
RESULTS AND DISCUSSION
Ig repertoire in SLE patients in remission
To study early B cell tolerance in SLE patients during clinical remission, we cloned, expressed, and tested antibodies from mature naive B cells from six adolescent patients (SLE100CR, SLE101CR, SLE122CR, SLE14CR, SLE21CR, and SLE33CR; Table I) and compared them to recombinant antibodies cloned from three previously published healthy controls (1, 7). Three of the remission patients described here had been studied at the time of diagnosis before any therapeutic intervention (SLE100, SLE101, and SLE122; reference 2). All patients met the Revised Criteria of the American College of Rheumatology, and their treatment is summarized (Table I; reference 2). Remission was defined by resolution of clinical symptoms, normalization of laboratory findings, and minimal maintenance therapy, but we did not assay for remission of organ damage (Table I). Samples were obtained at least 3 mo after initial remission, and 278 antibodies were cloned from cDNA libraries created from single mature naive B cells purified on the basis of surface markers (CD19+CD10−IgM+CD27−; Tables S1–S6 and Fig. S1, which are available at http://www.jem.org/cgi/content/full/jem.20061446/DC1; reference 8). Sequence analysis confirmed that all clones were unrelated and derived from naive B cells because they lacked somatic hypermutation (8).
Table I.
Patient characteristics
| Patient | SLE100 | SLE101 | SLE122 | SLE14 | SLE21 | SLE33 | |||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Female | Female | Female | |||
| Age at diagnosis |
15 yr | 15 yr | 9 yr | 11 yr | 17 yr | 7 yr | |||
| Date of diagnosis |
10/2002
|
10/2002
|
2/2003
|
11/1998 | 10/1999 | 7/2001 | |||
| Sample | SLE100 | SLE100CR | SLE101 | SLE101CR | SLE122 | SLE122CR | SLE14CR | SLE21CR | SLE33CR |
| Sample date | 10/10/2002 | 2/12/2004 | 10/24/2002 | 3/3/2004 | 2/22/2003 | 9/14/2004 | 6/1/2005 | 7/21/2005 | 8/8/2005 |
| Clinical course |
Arthritis, nephritis IIB |
Clinical remission |
Myocarditis, nephritis III |
Clinical remission |
Chorea, nephritis IIB |
Clinical remission |
Clinical remission |
Clinical remission |
Clinical remission |
| Therapy | None | Initially IV steroids (stopped ∼3 mo), continues on low dose oral steroids, PL, aspirin |
None | Initially IV steroids, CPH (stopped ∼10 mo), continues on IV steroids, MMF, PL |
None | Initially IV steroids (stopped ∼10 mo), continues on low dose oral steroids |
Initially IV CPH and steroids (stopped ∼4 yr), continues on PL |
Initially IV steroids (stopped ∼5 yr), continues on PL |
Initially IV steroids (stopped ∼3 yr), continues on PL |
| Hematology | L, A, T | Normal | A | Normal | L, A | Normal | Normal | Normal | Normal |
| CD19+ cells ×103/ml |
284 | 245 | 168 | 378 | 145 | 210 | |||
| Renal | Abnormal | Normal | Abnormal | Normal | Borderline | Normal | Normal | Normal | Normal |
| ANA | + | + | + | + | + | + | NA | NA | NA |
| Serology | D, P, Sm, RNP |
Normal | P | Normal | D | Borderline D | D | Normal | Borderline D |
| ESR, mm/hr | 11 | 25 | >120 | 11 | NA | 41 | 50 | 10 | 12 |
| Complement | Low | Normal | Low | Normal | Low | Normal | Normal | Normal | Normal |
A, anemia; CPH, cyclophosphamide; D, anti–double-stranded DNA antibody; ESR, erythrocyte sedimentation rate; L, lymphopenia; MMF, mycophenolatemofetil; NA, not available; P, antiphospholipid antibody; PL, Plaquenil; RNP, anti-RNP antibody; Sm, anti-Smith antibody; T, thrombocytopenia.
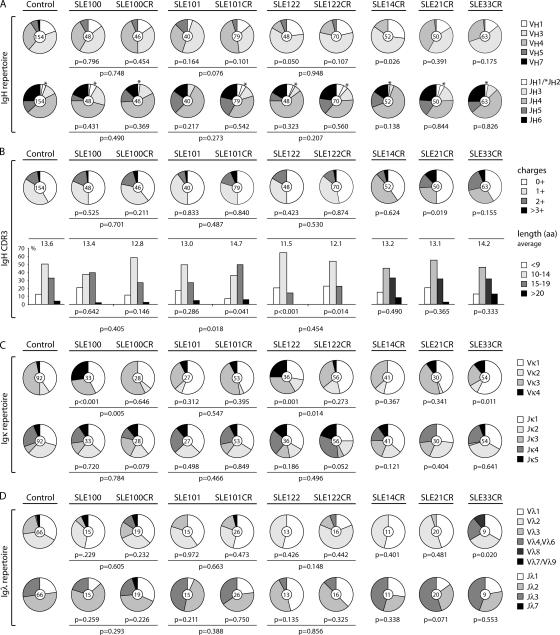
Several different abnormalities in Ig gene usage have been reported for patients with SLE, including bias toward VH3, VH4-34, Vκ1, and Vκ4 gene family usage (2, 9). We found some of these abnormalities, but no consistent abnormalities and no consistent differences between active disease and remission (Fig. 1). For example, SLE122 initially showed increased VH3 gene family representation and unusually short IgH CDR3 regions, and this pattern remained unchanged after treatment (P = 0.948 for VH repertoire, P = 0.454 for IgH CDR3 length), but these abnormalities were not found in other patients (Fig. 1, A and B; reference 2). In contrast, overrepresentation of Vκ4-1 was found in active disease in SLE100 and SLE122, but not in remission (Fig. 1 C; reference 2). We conclude that there are no consistent abnormalities in the IgH or IgL repertoires in SLE patients with active disease or in remission, and that although some specific features such as long CDR3s are associated with increased self-reactivity, they are not predictive.
Figure 1.
Ig heavy and light chain gene features. The absolute number of sequences analyzed is indicated in the center of each pie chart. (A) VH and JH repertoire. (B) IgH CDR3+ charges and length. Pie charts show percent of IgH CDR3s with zero, one, two, or three positively charged amino acid (aa) residues. Bar graphs show frequency of IgH CDR3s with ≤9 aa (white bars), 10–14 aa (light gray bars), 15–19 aa (dark gray bars), and 20 aa (black bars). (C) Igκ V and J gene family usage. (D) Igλ V and J gene family usage. p-values indicated below the charts or below horizontal lines are in comparison with healthy controls or data obtained from the same SLE patient during active disease, respectively (references 1, 2, and 7). Values for controls (GO, JB, and JH) and untreated SLE patients (SLE100, SLE101, and SLE122) in this and other figures were previously published and are shown here for comparison (references 1, 2, and 7).
Autoreactive antibodies
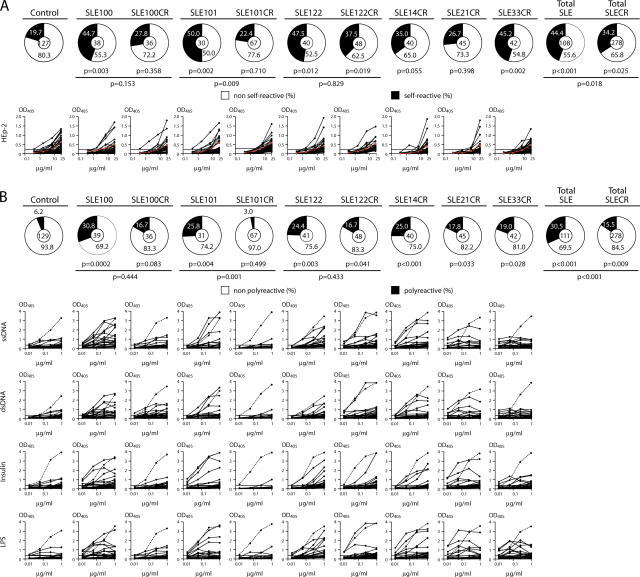
Antibodies reactive with HEp-2 cell lysates as measured in ELISA assays (used to detect ANAs, ANA ELISA) are significantly enriched in mature naive B cells from untreated SLE patients compared with healthy controls (2). To measure the frequency of these antibodies in clinical remission, we tested the cloned antibodies in HEp-2 cell ELISA assays and confirmed positives by indirect immunofluorescence assay on HEp-2 cell–coated slides (Fig. 2 A, Tables S1–S6, and Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20061446/DC1; references 1 and 2). Although there was individual variation, antibodies cloned from mature naive B cells from SLE patients in remission remained more self-reactive in the HEp-2 ELISA than healthy controls (34.25 vs. 19.7%, respectively, P = 0.025; Fig. 2 A; references 1 and 7). In all three cases where paired samples were available, the frequency of HEp-2 reactive antibodies was lower in remission than in crisis, but the difference only reached statistical significance in SLE101, who showed near normal levels of HEp-2–reactive antibodies in clinical remission (22.4% in SLE101CR vs. 19.7% in control, P = 0.710, and vs. 50.0% in SLE101, P = 0.009). We conclude that there is variability in the HEp-2 reactivity of the antibodies produced by mature naive B cells among SLE patients in crisis and remission, but most SLE patients in clinical remission show persistent abnormalities in this respect.
Figure 2.
Autoreactive antibodies. (A) Pie charts summarize self-reactivity as measured by HEp-2 cell ELISA and HEp-2 cell immunofluorescence assay (Tables S1–S6, Fig. S2, and not depicted) with the number of tested antibodies indicated in the center of each pie chart. Graphs show HEp-2 cell ELISA results. Red lines show the low positive serum control and horizontal lines indicate cutoff OD405 levels for positive reactivity. (B) Polyreactivity was measured by ELISA with single-stranded DNA, double-stranded DNA, insulin, and LPS. The frequency of polyreactive clones is summarized in the pie charts with the number of tested antibodies indicated in the pie chart centers. Graphs show polyreactivity ELISA results. Dotted lines show ED38+ control (references 1 and 29) and horizontal lines indicate cutoff OD405 levels for reactivity. p-values indicated below the pie charts or below the horizontal lines are in comparison with healthy controls or data obtained from the same SLE patient with active disease, respectively (references 1, 2, and 7).
Polyreactivity, i.e., antibody binding to diverse nonrelated antigens including self-antigens such as DNA and insulin, is another measure of self-reactivity. In normal humans, polyreactive antibodies comprise ∼6% of antibodies in the circulating mature naive B cell compartment, whereas in untreated SLE patients, these antibodies account for ∼31% of the repertoire (1, 2, 7). To determine whether this abnormality persists during remission, we tested our collection of remission-derived antibodies for binding with single-stranded DNA, double-stranded DNA, insulin, and LPS (Fig. 2 B). Although the number of polyreactive antibodies varied between individuals, all but one (SLE101CR) of the clinical remission patients showed significantly elevated levels (16.7% in SLE100CR and SLE122CR, P = 0.083 and 0.041, respectively; 17.8% in SLE21CR, P = 0.033; 19.0% in SLE33CR, P = 0.028; and 25.0% in SLE14CR, P < 0.001 vs. 6.2% in healthy controls; reference 1). The one patient that differed from the rest, SLE101, had higher than normal levels of polyreactivity before treatment (25.8%, P = 0.004; reference 2) but reverted to normal levels of polyreactivity in clinical remission (3.0% in SLE101CR vs. 6.2% in control, P = 0.499). The other paired samples showed decreased polyreactivity in remission, but the difference did not reach statistical significance (30.8% in SLE100 and 16.7% in SLE100CR, P = 0.444; 24.4% in SLE122 and 16.7% in SLE122CR, P = 0.433). Finally, when all remission samples, including SLE101CR, were combined, the overall level of polyreactivity in the mature naive B cell compartment was significantly greater than in healthy controls (15.5 vs. 6.2%, respectively, P = 0.009), but the frequency of polyreactive antibodies in clinical remission was significantly lower than at the time of diagnosis (15.5 vs. 31.5%, respectively, P < 0.001; Fig. 2 B).
The abnormalities in early B cell self-tolerance in active SLE might be secondary to disease activity per se because lymphopenia and mediators of acute inflammation such as type I IFNs and TNF-α can directly alter B cell development in vivo (10–12). Indeed, we found a decrease in the number of HEp-2–reactive and polyreactive antibodies expressed by circulating mature naive B cells during remission. However, despite normal numbers of circulating lymphoid and myeloid cells (Table I), the majority of patients in clinical remission continued to show persistent abnormalities in antibody tolerance in the mature naive B cell compartment, suggesting that defects in early tolerance defects are in part independent of active disease.
Ig gene usage and antibody self-reactivity
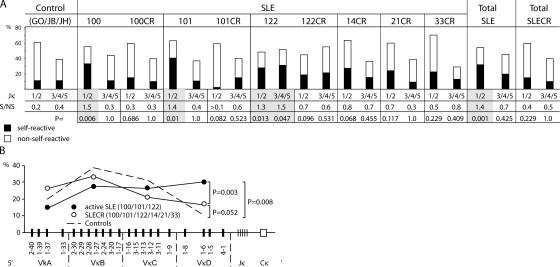
Primary Ig gene rearrangements frequently involve 3′ Vκ genes and 5′ Jκ genes (13–15). This allows for replacement of self-reactive antibodies by secondary recombination between 5′ Vκ genes and 3′ Jκ genes during receptor editing (13–15). Abnormalities in IgL chain receptor editing based on sequence analyses have been reported in patients with autoimmune diseases including SLE (9, 16, 17), but these reports did not discriminate between patients with active disease and patients in clinical remission. To determine whether receptor editing associated genomic alterations occurs in the Igκ locus in SLE patients, we analyzed the Vκ and Jκ usage of self-reactive versus nonself-reactive antibodies (Fig. 3). Vκ genes were divided into four groups according to their 5′ to 3′ orientation in the Igκ locus (VκA–VκD). Jκ genes were grouped into 5′ (Jκ1/2) and 3′ (Jκ3/4/5) genes. In healthy controls, few self-reactive or polyreactive antibodies were present in the mature naive B cell compartment (1, 7), and there was no apparent difference in Jκ usage between self-reactive and nonself-reactive antibodies (0.2 and 0.4, respectively; Fig. 3 A). In contrast, untreated SLE patients with active disease showed high levels of self-reactive antibodies that used 5′ Jκ genes (ratio of self-reactive/nonself-reactive: 1.5 for SLE100, P = 0.006; 1.4 for SLE101, P = 0.01; and 1.3 for SLE122, P = 0.013 when compared with healthy controls; references 1 and 7) and a variable level of self-reactive antibodies that used 3′ Jκ genes (0.3 for SLE100, P = 1.0; 0.4 for SLE101, P = 1.0; and 1.5 for SLE122, P = 0.047). However, SLE patients in remission resembled healthy controls in terms of frequency of self-reactive antibodies that used 5′ Jκ genes and were significantly different from patients with active disease (0.4 in total SLECR vs. 1.4 in total SLE, P = 0.001; 0.4 in total SLECR vs. 0.2 in controls, P = 0.229; references 1, 2, and 7). We found a significant bias toward 3′ Vκ gene usage in self-reactive antibodies in active patients, but not in remission (Fig. 3 B; P = 0.003 and 0.052, respectively; references 1 and 2). In summary, mature naive B cells from SLE patients with active disease express self-reactive antibodies that are biased to contain 3′ Vκ's in association with 5′ Jκ's, whereas those from patients in remission do not. Such antibodies are more likely to originate from primary rearrangements (13–15), suggesting that active SLE is associated with increased expression of unedited self-reactive antibodies by mature naive B cells.
Figure 3.
Ig gene usage and antibody self-reactivity of mature naive B cells from SLE patients in clinical remission. (A) Jκ genes were grouped according to their 5′ (Jκ1) and 3′ (Jκ3/4/5) position, and the proportion of self-reactive (black) versus nonself-reactive antibodies (white) is indicated as a bar graph for each group with the ratio of self-reactive versus nonself-reactive (S/NS) antibodies indicated below. p-values are in comparison to antibodies of the corresponding Jκ group from healthy controls. (B) Vκ genes were divided into four groups according to their 5′ to 3′ orientation in the Igκ locus (VκA–VκD). The proportion of self-reactive antibodies in each Vκ group for healthy controls (references 1 and 7), SLE patients before treatment (reference 2), or SLE patients in clinical remission is indicated.
B cell tolerance is regulated by distinct checkpoints in the bone marrow and in the periphery. Either of these might be altered in SLE (for review see reference 18). Elegant studies with transgenic mice have demonstrated that nascent self-reactive B cells can be regulated by receptor editing, deletion, or anergy, and that receptor editing appears to be the preferred mechanism to establish early B cell self-tolerance (13, 14, 19). Consistent with the primary importance of editing in shaping the B cell repertoire, this mechanism produces 25–50% of all antibodies (20, 21). In patients with active SLE or rheumatoid arthritis, and in mice with chronic autoimmune disease, the increase in self-reactive antibodies is consistent with abnormalities in editing and/or selection (16, 17, 22). In SLE patients in clinical remission, normalization of Igκ gene usage is consistent with an overall decrease in the number of self-reactive antibodies in these patients.
Less is known about how B cell tolerance is established in the periphery. Both positive and negative selection mechanisms have been proposed (18), and several genetic loci have been implicated in disease etiology (23). Under normal conditions, one important determinant appears to be decreased expression of BAFF receptor on autoreactive B cells rendering them less able to compete for limiting amounts of BAFF, which is a key B cell survival factor (24). Consistent with this idea, excess BAFF expression leads to autoimmunity in mice (25–27), and patients with SLE show high levels of BAFF that could enable self-reactive B cells to survive in the mature naive B cell repertoire (28).
In conclusion, patients with SLE have increased numbers of circulating mature naive B cells that express self-reactive and polyreactive antibodies regardless of disease status. We speculate that these cells may increase disease susceptibility by serving as precursors for cells that produce pathogenic lupus antibodies.
MATERIALS AND METHODS
Patient samples and single B cell sorting.
The study was performed in accordance with institutional review board–reviewed protocols of the UT Southwestern Medical Center (IRB no. 0199-017) and the Rockefeller University (SYU-0571-1005), and all samples were obtained after signed informed consent at the Division of Pediatric Rheumatology of UT Southwestern Medical Center. Control data and data from SLE100, SLE101, and SLE122 before treatment were previously published and are shown for direct comparison. Single B cell isolation was performed as described previously (1, 2, 7).
PCR amplification and expression vector cloning.
Single cell cDNA synthesis and RT-PCR reactions were performed as described previously (1, 2, 7). All sequences matched published germline sequences of human Ig genes or could be attributed to polymorphisms (not depicted).
Antibody production, ELISA, and indirect immunofluorescence assay.
Antibodies were expressed in vitro as described previously (1, 2, 7). Antibody concentrations were determined by ELISA (1, 2, 7). ELISAs were performed as described previously (1, 2, 7), and dilutions are indicated in the figures. Samples from SLE patients were considered negative if the OD405 did not exceed a threshold value as indicated in each graph at any of the four dilutions in at least three independent experiments (Tables S1–S6). Threshold values for reactivity with specific antigens were set in all assays using antibodies from healthy donors and included our previously published control antibodies, mGO53 (negative), eiJB40 (low positive), and ED38 (strong positive; references 1 and 29). HEp-2 ELISAs were performed as described previously (1, 2, 7). The threshold OD405 levels below which samples were considered negative are indicated in the graphs. Positive and negative controls included sera from patients and healthy individuals (INOVA Diagnostics) and were included in every experiment.
Indirect immunofluorescence assays were performed as described previously (1). Controls included ED38 (1) and positive and negative sera (Bion Enterprises, Ltd.).
Statistics.
p-values for Ig gene repertoire analyses, analysis of positive charges in IgH CDR3, and antibody reactivity were calculated by 2 × 2 or 2 × 5 Fisher's Exact test or Chi-square test. p-values for IgH CDR3 length were calculated by Student's t test.
Online supplemental material.
Fig. S1 shows representative FACS profiles of B cells from SLE patients in clinical remission. Fig. S2 shows antibody staining patterns in HEp-2 cell immunofluorescence assay. Tables S1–S6 show IgH and IgL chain characteristics and antibody reactivity for mature naive B cells of SLE patients in clinical remission. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061446/DC1.
Supplemental Material
Acknowledgments
We thank all members of the Nussenzweig laboratory, Dr. E. Besmer, and Dr. M. Mazumdar for help with the manuscript.
This work is supported by grants from the National Institutes of Health (NIH; to M.C. Nussenzweig) and the Dana Foundation (to H. Wardemann). M.C. Nussenzweig is a Howard Hughes Medical Institute investigator. S. Yurasov is supported by career development grants from NIH and the New York Chapter of the Arthritis Foundation.
The authors have no conflicting financial interests.
H. Wardemann and M.C. Nussenzweig contributed equally to this work.
References
- 1.Wardemann, H., S. Yurasov, A. Schaefer, J.W. Young, E. Meffre, and M.C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 2.Yurasov, S., H. Wardemann, J. Hammersen, M. Tsuiji, E. Meffre, V. Pascual, and M.C. Nussenzweig. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan, E.M. 1989. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 44:93–151. [DOI] [PubMed] [Google Scholar]
- 4.Davidson, A., and B. Diamond. 2001. Autoimmune diseases. N. Engl. J. Med. 345:340–350. [DOI] [PubMed] [Google Scholar]
- 5.Stichweh, D., E. Arce, and V. Pascual. 2004. Update on pediatric systemic lupus erythematosus. Curr. Opin. Rheumatol. 16:577–587. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle, M.R., M.T. McClain, M.V. Rubertone, R.H. Scofield, G.J. Dennis, J.A. James, and J.B. Harley. 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349:1526–1533. [DOI] [PubMed] [Google Scholar]
- 7.Tsuiji, M., S. Yurasov, K. Velinzon, S. Thomas, M.C. Nussenzweig, and H. Wardemann. 2006. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 203:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorner, T., and P.E. Lipsky. 2001. Immunoglobulin variable-region gene usage in systemic autoimmune diseases. Arthritis Rheum. 44:2715–2727. [DOI] [PubMed] [Google Scholar]
- 10.Maciejewski, J.P., F.F. Weichold, and N.S. Young. 1994. HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha. J. Immunol. 153:4303–4310. [PubMed] [Google Scholar]
- 11.Binder, D., J. Fehr, H. Hengartner, and R.M. Zinkernagel. 1997. Virus-induced transient bone marrow aplasia: major role of interferon-α/β during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J. Exp. Med. 185:517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett, L., A.K. Palucka, E. Arce, V. Cantrell, J. Borvak, J. Banchereau, and V. Pascual. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiegs, S.L., D.M. Russell, and D. Nemazee. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gay, D., T. Saunders, S. Camper, and M. Weigert. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prak, E.L., and M. Weigert. 1995. Light chain replacement: a new model for antibody gene rearrangement. J. Exp. Med. 182:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bensimon, C., P. Chastagner, and M. Zouali. 1994. Human lupus anti-DNA autoantibodies undergo essentially primary V kappa gene rearrangements. EMBO J. 13:2951–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuels, J., Y.S. Ng, C. Coupillaud, D. Paget, and E. Meffre. 2005. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melchers, F., and A.R. Rolink. 2006. B cell tolerance–how to make it and how to break it. Curr. Top. Microbiol. Immunol. 305:1–23. [DOI] [PubMed] [Google Scholar]
- 19.Halverson, R., R.M. Torres, and R. Pelanda. 2004. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 5:645–650. [DOI] [PubMed] [Google Scholar]
- 20.Retter, M.W., and D. Nemazee. 1998. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casellas, R., T.A. Shih, M. Kleinewietfeld, J. Rakonjac, D. Nemazee, K. Rajewsky, and M.C. Nussenzweig. 2001. Contribution of receptor editing to the antibody repertoire. Science. 291:1541–1544. [DOI] [PubMed] [Google Scholar]
- 22.Witsch, E.J., H. Cao, H. Fukuyama, and M. Weigert. 2006. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J. Exp. Med. 203:1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakeland, E.K., K. Liu, R.R. Graham, and T.W. Behrens. 2001. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 15:397–408. [DOI] [PubMed] [Google Scholar]
- 24.Lesley, R., Y. Xu, S.L. Kalled, D.M. Hess, S.R. Schwab, H.B. Shu, and J.G. Cyster. 2004. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 20:441–453. [DOI] [PubMed] [Google Scholar]
- 25.Mackay, F., S.A. Woodcock, P. Lawton, C. Ambrose, M. Baetscher, P. Schneider, J. Tschopp, and J.L. Browning. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross, J.A., J. Johnston, S. Mudri, R. Enselman, S.R. Dillon, K. Madden, W. Xu, J. Parrish-Novak, D. Foster, C. Lofton-Day, et al. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 404:995–999. [DOI] [PubMed] [Google Scholar]
- 27.Thien, M., T.G. Phan, S. Gardam, M. Amesbury, A. Basten, F. Mackay, and R. Brink. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 20:785–798. [DOI] [PubMed] [Google Scholar]
- 28.Stohl, W., S. Metyas, S.M. Tan, G.S. Cheema, B. Oamar, D. Xu, V. Roschke, Y. Wu, K.P. Baker, and D.M. Hilbert. 2003. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 48:3475–3486. [DOI] [PubMed] [Google Scholar]
- 29.Meffre, E., A. Schaefer, H. Wardemann, P. Wilson, E. Davis, and M.C. Nussenzweig. 2004. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J. Exp. Med. 199:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.