Abstract
The programmed death (PD)-1–PD-1 ligand (PD-L) pathway, which is part of the B7–CD28 family, consists of the PD-1 receptor and its two ligands PD-L1 and PD-L2. Engagement of PD-1 by its ligands inhibits immune responses, and recent work has shown that PD-1 is highly expressed on exhausted T cells during chronic lymphocytic choriomeningitis virus (LCMV) infection in mice. Blockade of this pathway reinvigorates the exhausted T cells, allowing them to expand and produce effector cytokines, raising the issue of whether this pathway has been exploited by a variety of viruses during chronic infection. New studies now extend these observations to HIV infection and human disease.
PD-1 expression and signaling
PD-1 was isolated as a gene up-regulated in a T cell hybridoma undergoing apoptotic cell death, and was thus named programmed death 1 (1). PD-1 (CD279) is inducibly expressed on CD4 T cells, CD8 T cells, NKT cells, B cells, and monocytes upon activation (for review see references 1, 2).This broad expression of PD-1 contrasts with T cell–specific expression of CD28 and CTLA-4. PD-1 transduces a signal when engaged in combination with T cell receptor (TCR) ligation, but does not transduce a signal when cross-linked alone, similar to other CD28 family members. The cytoplasmic domain of PD-1 contains two tyrosine signaling motifs, both of which may be phosphorylated upon receptor engagement. Phosphorylation of the second tyrosine, an immunoreceptor tyrosine–based switch motif, recruits the tyrosine phosphatase SHP-2 and to a lesser extent SHP-1 to the PD-1 cytoplasmic domain (3, 4). Recruitment of these phosphatases leads to dephosphorylation of TCR proximal signaling molecules including ZAP70, PKCθ, and CD3ζ, leading to attenuation of the TCR/CD28 signal. PD-1 signaling prevents CD28-mediated activation of phosphatidylinositol 3-kinase, resulting in reduced Akt phosphorylation and glucose metabolism.
Expression of PD-1 ligands
The PD-1 ligands have distinct patterns of expression. PD-L2 (B7-DC; CD273) is inducibly expressed only on dendritic cells and macrophages (for review see references 1, 2, 5, 6), whereas PD-L1 (B7-H1; CD274) is broadly expressed on both professional and nonprofessional antigen-presenting cells (APCs) (1, 2, 5–7). PD-L1 is constitutively expressed on B cells, dendritic cells, macrophages, and T cells, and is further up-regulated upon activation. PD-L1 also is expressed on a wide variety of nonhematopoietic cell types, including vascular endothelial cells, kidney tubular epithelial cells, cardiac myocardium, pancreatic islet cells, glial cells in the brain, inflamed muscle, and keratinocytes. Interferons α, β, and γ are powerful up-regulators of PD-L1 expression on APCs, endothelial cells, and epithelial cells (8, 9). During proinflammatory immune responses, such as infection or transplant rejection, PD-L1 expression is intense and extensive (10). For example, PD-L1 is expressed on liver Kupffer cells and is up-regulated on hepatocytes after hepatitis B infection (9). PD-L1 is also expressed at sites of immune privilege such as the placenta and eye (5). PD-L1 expression is found on many solid tumors, and high PD-L1 expression is associated with poor prognosis (4, 5, 11, 12).
Viral exhaustion and the PD-1–PD-L pathway
Effective antiviral CD8 T cells possess several functional properties including cytokine production (e.g., IFN-γ, TNF-α, IL-2), cytotoxic potential (e.g., perforin/granzyme granule exocytosis), high proliferative potential, low apoptosis, and, for memory T cells, the ability to self-renew via homeostatic turnover (13). One of the key features of a memory CD8 T cell is the ability to rapidly reactivate multiple effector functions and undergo vigorous proliferation after reexposure to antigen. In contrast to the high functional capacity of effector and memory CD8 T cells generated after acute infection or vaccination, CD8 T cell function is often impaired or exhausted during chronic infections. Exhaustion was originally described during chronic LCMV infection as the persistence of virus-specific CD8 T cells that lacked effector functions (14). CD8 T cell exhaustion appears to be a prominent feature not only of experimental chronic infections in mice but also during chronic infections in primates and humans (for review see references 13, 15). For example, nonfunctional antigen-specific CD8 T cells have been observed during SIV infection of primates and human infection with HIV, hepatitis B, hepatitis C, and human T lymphotropic virus-1.
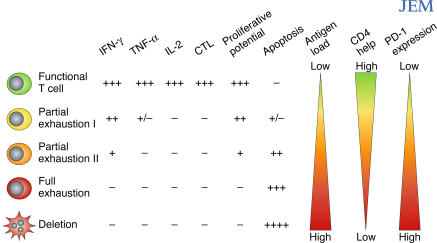
Studies both in mouse models and human chronic infections have demonstrated that exhaustion comprises a range of dysfunctions from relatively mild to extreme (Fig. 1) (13). Impaired proliferative potential is a key feature of exhaustion that often occurs when other functions, such as cytokine production, are largely intact. Indeed, high proliferative potential correlates with nonprogression during HIV infection (16) and may be a critical property of those T cells that can respond to therapeutic intervention (17). There appears to be a distinct hierarchy of exhaustion with certain functional properties, such as IL-2 production and proliferative potential, which are lost first, and other functions, such as IFN-γ production, which are more resistant to inactivation (Fig. 1). The duration of infection, level of antigen exposure, and the availability of CD4 T cell help are all critical factors that affect the level of exhaustion during any given chronic infection.
Figure 1.
T cell exhaustion during chronic viral infections. Virus-specific CD8 T cells posses multiple functions including production of IFN-γ, TNF-α, IL-2, cytotoxicity, antigen-driven proliferation, and resistance to apoptosis. During chronic infections, functions can be exhausted. Exhaustion represents a spectrum from mild (Partial exhaustion I: little IL-2 and poor TNF-α and cytotoxicity) to moderate (Partial exhaustion II: modestly defective IFN-γ, cytotoxicity, and little IL-2 or TNF-α) to severe (Full exhaustion: lack of IFN-γ, TNF-α, IL-2, and cytotoxicity). Finally, physical deletion (apoptosis) of T cells occurs. Proliferative potential decreases concomitantly with the loss of other functions while apoptosis increases. Antigen and CD4 help strongly influence exhaustion; as antigen increases and/or CD4 help decreases, virus-specific T cells become more exhausted. Recent studies now identify the PD-1–PD-L pathway as a key regulator of exhaustion. Increased expression of PD-1 by virus-specific T cells, and PD-L1 by APCs, leads to more severe exhaustion during chronic viral infection.
APC type and quality may also change dramatically during chronic infections as, for example, professional APCs are killed and inflammatory signals change (18–20). It is likely that this altered APC repertoire and the associated changes in costimulation will influence T cell exhaustion during chronic infections. During HIV infection, surface expression of PD-L1 on APCs is increased and that of CD86 is decreased, tipping the balance between inhibitory and stimulatory signals delivered to T cells toward inhibition (21). In addition, the inhibitory signals delivered by PD-L1 on epithelial and endothelial cells may take on added significance when professional APCs are reduced during chronic infection.
Recent studies have shown that PD-1 is highly expressed by CD8 T cells during chronic LCMV infection and that the PD-1–PD-L pathway plays a major role in regulating T cell exhaustion during this infection (22). When antibodies were used to block the PD-1–PD-L pathway in vivo during chronic LCMV infection, virus-specific CD8 T cell responses were potently enhanced. Not only was the number of LCMV-specific CD8 T cells increased dramatically, but their function was also improved. After in vivo PD-1–PD-L blockade, virus-specific CD8 T cells produced more IFN-γ and TNF-α on a per cell basis. The consequence of this reversal of virus-specific CD8 T cell exhaustion was a considerable reduction in viral load. This study set the stage for investigations into the expression of PD-1, and the possible control of T cell exhaustion by the PD-1– PD-L pathway, during human chronic viral infections.
The PD-1–PD-L pathway in HIV infection
Several new studies suggest a role for the PD-1–PD-L pathway in exhaustion of virus-specific CD8 T cells during HIV infection. The study by Petrovas, et al. (on p. 2281; [23]), in this issue, and studies by Day et al. (24) and Trautmann et al. (25), published recently in Nature and Nature Medicine, respectively, show that PD-1 expression is elevated on HIV-specific CD8 T cells and that blocking the PD-1–PD-L pathway leads to increased T cell proliferation and effector cytokine production (illustrated in Fig. 2). Previous work has shown that PD-L1 is up-regulated in HIV infection (21). Collectively, these observations suggest that the PD-1– PD-L pathway may indeed be operating during chronic HIV infection.
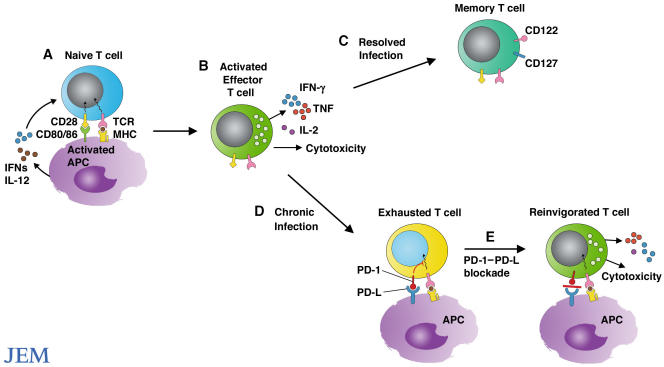
Figure 2.
Reinvigorating exhausted T cells. (A) Microbial products and cytokines produced in response to microbes activate APCs and stimulate expression of CD80 and CD86. Engagement of CD28 by CD80/CD86 stimulates the expansion and differentiation of naive T cells into effector T cells. (B) Effector T cells eliminate the invading pathogens by secreting cytokines and killing infected cells. (C) Upon resolution of infection, effector T cells give rise to long-lived protective memory T cells. (D) However, during chronic infection, T cells lose function and the ability to proliferate and become functionally exhausted. Exhausted T cells express high levels of PD-1. (E) Blockade of interactions between PD-1 and its ligands can reinvigorate T cells to expand and regain effector functions, including cytokine production and cytolysis.
A large percentage of HIV-specific CD8 T cells expressed PD-1, and the expression of this receptor was elevated on a per cell basis. A large proportion of HIV-specific CD8 T cells also expressed CD27 and CD45RO, indicating previous activation. These CD8 T cells had lost expression of the costimulatory receptor CD28 and perforin and expressed only low levels of CCR7 and CD127 (IL-7 receptor α), which are important molecules for the maintenance of memory T cells. This phenotype suggests that the T cells are poorly functional, are not transiting into memory cells, and are particularly receptive to inhibitory signals.
CD8 T cells in individuals infected with HIV have previously been shown to be dysfunctional with reduced proliferative capacity and effector function (13, 15, 16, 26). Day et al. show that disease severity, as judged by viral load and declining CD4 counts, correlated with both the level of PD-1 expression on HIV-specific CD8 T cells and the percentage of cells expressing PD-1, providing a marker on CD8 T cells that correlates with disease severity (24). The level of PD-1 expression was also associated with decreased CD8 T cell proliferation in response to in vitro stimulation with HIV antigen. Collectively, these findings show that the level of PD-1 correlates with the extent of T cell exhaustion. Day et al. (24) and Trautmann et al. (25) further demonstrated that PD-1 expression on HIV-specific CD8 T cells was reduced in patients undergoing effective highly active antiretroviral therapy, consistent with the notion that high antigen levels drive PD-1 expression and functional exhaustion. According to Trautmann et al., the CD8 dysfunction did not result from changes in TCR expression or stimulation with altered peptide ligands (25).
There were striking differences in the levels of PD-1 expression on virus-specific T cells in chronic versus resolved infection. T cells specific for vaccinia virus, which causes a self-limiting acute infection, expressed little PD-1 compared with high levels of PD-1 expressed by HIV-specific T cells. In contrast, T cells specific for the chronic viruses, CMV and Epstein Barr virus, expressed moderate to high levels of PD-1, respectively. This suggests that sustained viremia and antigen presentation maintain the high levels of PD-1 expression. It will be important to determine if up-regulation of PD-1 and PD-L is a consequence of the antiviral interferon response, an indirect effect of T cell activation and inflammatory cytokine production, or whether HIV proteins directly up-regulate their expression.
All three papers demonstrate functional significance of PD-1 expression on HIV-specific T cells. Blocking PD-L1 with a monoclonal antibody led to increased T cell proliferation and production of TNF-α, IFN-γ, and granzyme B, indicating an overall increase in effector function. Although short-term blockade of the PD-1–PD-L pathway in a 6-h cytokine assay had little effect on HIV-specific CD8 T cell function, blockade of this pathway during a 6 d proliferation assay enhanced the proliferation of both HIV-specific CD8 and CD4 T cells and resulted in more functional T cells at the end of the culture. Whether proliferation is a prerequisite for the recovery of other T cell functions remains to be determined. Cytolytic activity, for example, was not examined and will be of great interest. It is also unclear whether the recovery of proliferative potential observed in the LCMV and HIV systems reflects greater cell division, less death, or both.
Petrovas et al. used a plate-bound, PD-1-specific polyclonal antibody to show that engagement of PD-1 reduces HIV-antigen specific T cell proliferation (23). They examined apoptosis in the PD-1+ HIV-specific CD8 T cells and found increases in both spontaneous and Fas-mediated apoptosis, suggesting that cross-talk may occur between PD-1 and Fas receptors. The interpretation of these findings, however, is complicated by the finding that PD-1− HIV-specific CD8 T cells also have increased susceptibility to apoptosis. It is possible that other factors such as the level of T cell activation are involved. Cells expressing very high levels of PD-1 were much more susceptible to death signals, suggesting that PD-1 expression leads to a survival defect in vivo.
Because of the link between CD4 help and CD8 function, Day et al. also examined the effects of PD-1 ligand blockade on CD4 T cell expansion (24). Remarkably, in five of six patients with undetectable CD4 proliferative responses to HIV p24 antigen, blocking PD-L1 restored vigorous CD4 T cell expansion, suggesting that HIV-specific CD4 T cells may be present but so functionally impaired that they are undetectable in standard assays.
Infections that exploit the PD-1–PD-L pathway
In addition to HIV and LCMV, a variety of microbial pathogens appear to have usurped the PD-1–PD-L pathway to inhibit antimicrobial immunity and immune-mediated host tissue damage. During chronic viral and parasite infections, up-regulation of PD-1 and its ligands may attenuate immune responses and facilitate persistence of the pathogens. For example, the expression of PD-1 ligands is required for the suppressive function of macrophages and the induction of T cell anergy during infections with the helminths Schistomosa mansoni and Taenia crassiceps (27). Up-regulation of PD-1 and its ligands might also be used to limit potentially detrimental inflammatory consequences of antimicrobial effector responses. Such a protective role for PD-1 is suggested by studies of adenovirus-infected PD-1−/− mice, which more rapidly clear the virus but develop more severe hepatocellular injury than wild-type mice (1).
Concerns and unresolved issues
The PD-1–PD-L pathway has a critical role in regulating the balance between T cell activation and tolerance (1, 28). Blocking or eliminating PD-1 or its ligands can accelerate and exacerbate autoimmune disease. Single nucleotide polymorphisms in PD-1 have been linked with several autoimmune diseases, although how PD-1 affects susceptibility to human autoimmune diseases is not yet clear. It will be important to learn how to modulate this pathway to reactivate antiviral T cells while minimizing the risk of autoimmunity and immunopathology.
How does PD-1 exert its inhibitory effects? Many reports have shown that PD-1 signaling inhibits T cell activation. Early reports found an effect on cell cycle arrest rather than cell death (4), but recent studies, including the study by Petrovas et al., emphasize the role of PD-1 in promoting T cell death (9, 12, 23). PD-1 might directly engage a death pathway or more likely indirectly influence cell death by down-regulating survival signals and growth factors or synergizing with death pathways. For example, PD-1 ligation inhibits expression of the cell survival gene bcl-xl and several growth factors (2–5). The effects of PD-1 ligation on expression of death receptors and death pathways need further study.
The outcome of PD-1–PD-L interactions on T cell expansion and survival might also depend on the microenvironment and the type of APC that interacts with the T cell. When macrophages are used as APCs, anti–PD-L1 or anti–PD-1 monoclonal antibody increases IFN-γ and IL-2 production by T cells but, paradoxically, inhibits their proliferation. This inhibition was caused by the IFN-γ–dependent induction of nitric oxide production by macrophages, which inhibits T cell proliferation (29). Increased IFN-γ production after PD-L1 blockade is seen in HIV-specific T cells and is a common result reported in many experimental systems. Thus, combining inducible nitric oxide synthase blockade with PD-1–PD-L blockade may have therapeutic potential as a means to augment immune responses and protect against the potential proinflammatory effects of nitric oxide.
To optimally manipulate this pathway during chronic viral infection, further work is needed to understand the functions of PD-L1 versus PD-L2, and the role of PD-1 on B cells and macrophages and on T cells. Since PD-1 is expressed on B cells and macrophages and PD-L1 is expressed on T cells, there might also be bidirectional signaling. Some reports describe reverse signaling of PD-L1 and PD-L2 into cells that express them (6). There are also data that support stimulatory functions of PD-L1 or PD-L2, perhaps mediated by an as yet unidentified second receptor. The contributions of each of these interactions to the therapeutic efficacy of pathway manipulations need to be evaluated.
Concluding remarks
The reports by Petrovas et al. (23), Day et al. (24), and Trautmann et al. (25) suggest that blocking PD-1–PD-L interactions may be a therapeutic strategy for HIV infection that could allow the reactivation of anti-HIV T cell responses. Because this pathway has an important role in regulating the balance between T cell activation and tolerance, it will be important to identify the optimal timing and frequency of therapy to minimize the risk of immunopathology or autoimmunity. The effects of blocking only PD-1, PD-L1, or PD-L2 or a combination thereof will need to be evaluated for efficacy and safety. It might also be beneficial to combine PD-1 blockade with antiviral therapy or therapeutic vaccination. In particular, since the therapeutic effects of interferons may be limited by the induction of PD-1 ligands, there may be synergistic benefits of PD-1 blockade in conjunction with interferon therapy. Further studies are needed to investigate to what extent, and for how long, PD-1–PD-L blockade can restore different effector functions, particularly cytolysis.
Acknowledgments
This work was supported by grants from the National Institutes of Health AI56299 and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative. Because of space restrictions, we were able to cite only a fraction of the relevant literature and apologize to colleagues whose contributions may not be appropriately acknowledged.
G.J.F. is at Department of Medical Oncology, Dana-Farber Cancer Institute, Department of Medicine, Harvard Medical School, Boston, MA 02115.
E.J.W. is at Immunology Program, The Wistar Institute, Philadelphia, PA 19104.
R.A. is at Emory Vaccine Center and Department of Microbiology and Immunology, Emory University School of Medicine, Atlanta, GA 30322.
A.H.S. is at Department of Pathology, Harvard Medical School and Brigham and Women's Hospital, Boston, MA 02115.
References
- 1.Okazaki, T., and T. Honjo. 2006. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 27:195–201. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald, R.J., G.J. Freeman, and A.H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548. [DOI] [PubMed] [Google Scholar]
- 3.Parry, R.V., J.M. Chemnitz, K.A. Frauwirth, A.R. Lanfranco, I. Braunstein, S.V. Kobayashi, P.S. Linsley, C.B. Thompson, and J.L. Riley. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latchman, Y., C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A.J. Long, J.A. Brown, R. Nunes, et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J.A., D.M. Dorfman, F.R. Ma, E.L. Sullivan, O. Munoz, C.R. Wood, E.A. Greenfield, and G.J. Freeman. 2003. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170:1257–1266. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L. 2004. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 4:336–347. [DOI] [PubMed] [Google Scholar]
- 7.Freeman, G.J., A.J. Long, Y. Iwai, K. Bourque, T. Chernova, H. Nishimura, L.J. Fitz, N. Malenkovich, T. Okazaki, M.C. Byrne, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eppihimer, M.J., J. Gunn, G.J. Freeman, E.A. Greenfield, T. Chernova, J. Erickson, and J.P. Leonard. 2002. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 9:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlbauer, M., M. Fleck, C. Schutz, T. Weiss, M. Froh, C. Blank, J. Scholmerich, and C. Hellerbrand. 2006. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 45:520–528. [DOI] [PubMed] [Google Scholar]
- 10.Koga, N., J. Suzuki, H. Kosuge, G. Haraguchi, Y. Onai, H. Futamatsu, Y. Maejima, R. Gotoh, H. Saiki, F. Tsushima, et al. 2004. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler. Thromb. Vasc. Biol. 24:2057–2062. [DOI] [PubMed] [Google Scholar]
- 11.Thompson, R.H., S.M. Kuntz, B.C. Leibovich, H. Dong, C.M. Lohse, W.S. Webster, S. Sengupta, I. Frank, A.S. Parker, H. Zincke, et al. 2006. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66:3381–3385. [DOI] [PubMed] [Google Scholar]
- 12.Dong, H., S.E. Strome, D.R. Salomao, H. Tamura, F. Hirano, D.B. Flies, P.C. Roche, J. Lu, G. Zhu, K. Tamada, et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. [DOI] [PubMed] [Google Scholar]
- 13.Wherry, E.J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zajac, A.J., J.N. Blattman, K. Murali-Krishna, D.J. Sourdive, M. Suresh, J.D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879. [DOI] [PubMed] [Google Scholar]
- 16.Migueles, S.A., A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 17.Wherry, E.J., J.N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevilla, N., D.B. McGavern, C. Teng, S. Kunz, and M.B. Oldstone. 2004. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 113:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 101:4505–4511. [DOI] [PubMed] [Google Scholar]
- 20.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 98:2574–2576. [DOI] [PubMed] [Google Scholar]
- 21.Trabattoni, D., M. Saresella, M. Biasin, A. Boasso, L. Piacentini, P. Ferrante, H. Dong, R. Maserati, G.M. Shearer, L. Chen, and M. Clerici. 2003. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 101:2514–2520. [DOI] [PubMed] [Google Scholar]
- 22.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 23.Petrovas, C., J.P. Casazza, J.M. Brenchley, D.A. Price, E. Gostick, W.C. Adams, M.L. Precopio, T. Schacker, M. Roederer, D.C. Douek, and R.A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day, C.L., D.E. Kaufmann, P. Kiepiela, J.A. Brown, E.S. Moodley, S. Reddy, E.W. Mackey, J.D. Miller, A.J. Leslie, C. DePierres, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T cell exhaustion and disease progression. 443:350–354. [DOI] [PubMed] [Google Scholar]
- 25.Trautmann, L., L. Janbazian, N. Chomont, E.A. Said, G. Wang, S. Gimmig, B. Bessette, M.R. Boulassel, E. Delwart, H. Sepulveda, et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+T cells leads to reversible immune dysfunction. Nat. Med. In press. [DOI] [PubMed]
- 26.Zhang, D., P. Shanker, Z. Xu, B. Harnisch, G. Chen, C. Lange, S.J. Lee, H. Valdez, M.M. Lederman, and J. Lieberman. 2003. Most antiviral CD8T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 101:226–235. [DOI] [PubMed] [Google Scholar]
- 27.Smith, P., C.M. Walsh, N.E. Mangan, R.E. Fallon, J.R. Sayers, A.N. McKenzie, and P.G. Fallon. 2004. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J. Immunol. 173:1240–1248. [DOI] [PubMed] [Google Scholar]
- 28.Keir, M.E., S.C. Liang, I. Guleria, Y.E. Latchman, A. Qipo, L.A. Albacker, M. Koulmanda, G.J. Freeman, M.H. Sayegh, and A.H. Sharpe. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki, T., H. Akiba, A. Koyanagi, M. Azuma, H. Yagita, and K. Okumura. 2005. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J. Immunol. 175:1586–1592. [DOI] [PubMed] [Google Scholar]