Abstract
Interleukin (IL)-15 is expressed in a variety of inflammatory diseases. However, the contribution of dendritic cell (DC)–derived IL-15 to the development of diseases is uncertain. Using established models of Propionibacterium acnes (P. acnes)– and zymosan-induced liver inflammation, we observed granuloma formation in the livers of wild-type (WT) and RAG-2−/− mice but not in those of IL-15−/− mice. We demonstrate that this is likely caused by an impaired sequential induction of IL-12, IFN-γ, and chemokines necessary for monocyte migration. Likewise, lethal endotoxin shock was not induced in P. acnes– and zymosan-primed IL-15−/− mice or in WT mice treated with a new IL-15–neutralizing antibody. In both systems, proinflammatory cytokine production was impaired. Surprisingly, neither granuloma formation, lethal endotoxin shock, nor IL-15 production was induced in mice deficient for DCs, and adoptive transfer of WT but not IL-15−/− DCs restored the disease development in IL-15−/− mice. Collectively, these data indicate the importance of DC-derived IL-15 as a mediator of inflammatory responses in vivo.
Proper activation followed by inactivation of immune responses is essential for the maintenance of immunological homeostasis in vivo. Prolonged or aberrant activation of immune responses cause a variety of immunopathological disorders, which often result in tissue destruction mediated by effector cells and cytokines.
We and others have found that IL-15 is a pivotal cytokine for the development and function of innate immune cells, including NK cells, NKT cells, TCRγδ+ intestinal intraepithelial lymphocytes, and DCs (1–9). IL-15 also affects the acquired immune system because the proliferation and survival of CD8+ T cells with naive and memory phenotypes, as well as antigen-specific memory CD8+ T cells, are impaired in both IL-15Rα−/− and IL-15−/− mice (4, 6, 10–12). These studies showed a beneficial effect of IL-15 on the establishment and maintenance of the immune system against pathogen infections as well as malignancies.
In contrast, circumstantial evidence suggests that IL-15 is also involved in the development of immunopathological disorders. IL-15 mRNA and protein are expressed in synovial membranes in rheumatoid arthritis (RA) (13, 14). It has been suggested that IL-15 precedes TNF-α production in cytokine cascade to recruit T cells into the synovial membranes, possibly contributing to the pathogenesis of RA (13, 14). Supporting this notion, treatment of DBA/1 mice with the soluble IL-15Rα and IL-15 mutant/Fcγ2a fusion protein, which bind the IL-15R with high affinity but do not trigger signaling events, prevents the mice from developing collagen-induced arthritis (15, 16). The elevated numbers of IL-15–expressing cells and/or elevated levels of IL-15 production correlate with the activities of inflammatory bowel disease (17), type C chronic liver disease (18), sarcoidosis (19), and multiple sclerosis (20). These observations suggest a harmful effect of IL-15 in various immunopathological and inflammatory diseases. However, as IL-15 is produced by many cell types, it remains unknown whether development of these diseases attributes to DC-derived IL-15.
In this study we generated new mAbs to detect and block mouse IL-15 activity and established an ELISA system that enabled us to examine the amounts of IL-15 at protein level. Using the mAb and ELISA system combined with gene-targeted mice, we investigated the role of IL-15 in well-characterized inflammatory disease models in mice. We demonstrate that IL-15 is essential for P. acnes– and zymosan-induced granuloma formation and subsequent LPS-induced endotoxin shock, as well as liver damage, through induction of proinflammatory cytokines and chemokines. Of interest, the development of liver diseases and endotoxin shock is unaffected in the absence of T, B, and NK cells, whereas it was severely affected in mice lacking DC-derived IL-15. These experiments identify an irreplaceable function of DC-derived IL-15 in bacterial product–mediated inflammatory responses.
RESULTS
Impaired granuloma formation in IL-15−/− mice
P. acnes is suspected to be a causative bacteria for human sarcoidosis, and its cell wall components show strong immunoadjuvant activities, which induce monocyte migration into the liver and granuloma formation (21–23).
To examine the role of IL-15 in the granuloma formation in vivo, control WT and IL-15−/− mice were injected with heat-killed P. acnes. Consistent with previous reports (21–23), granuloma formation was evident in the liver of WT mice on day 6 after injection, whereas it was hardly seen in that of IL-15−/− mice (Fig. 1 A, top left and top middle). Importantly, the granuloma formation was substantially restored by IL-15 injection into IL-15−/− mice (Fig. 1 A, top right). It has been reported that on P. acnes infection circulating DC precursors migrate into the liver and, in cooperation with Kupffer cells, monocytes, and T cells, participate in the granulomatous reaction (24). Indeed, P. acnes–induced granulomas contained CD11c+ DCs (Fig. 1 B). The granuloma formation was observed in the liver of RAG-2−/− mice and NK cell–depleted RAG-2−/− mice but was absent in that of IL-15−/−RAG-2−/− mice (Fig. 1 A, bottom; and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061297/DC1), indicating that both T, B, and NK cells are dispensable in the process of granuloma formation. Because chemokine production is critical for monocyte migration into the liver, we next examined the chemokine production, in particular MCP-1 (CCL2) and MIP-1α/β (CCL3/4), which are critical chemoattractants for monocytes. On day 3 after P. acnes injection, considerable levels of these chemokines were detected in the sera of WT, RAG-2−/−, and NK cell–depleted RAG-2−/− mice (Fig. 1 C and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061297/DC1). In contrast, these chemokines were produced only marginally in IL-15−/− and IL-15−/−RAG-2−/− mice (Fig. 1 C). Again, the chemokine production was restored by IL-15 injection into IL-15−/− mice. To further examine whether IL-15 directly controls the chemokine production, we analyzed IFN-γ−/− mice. As reported previously (23), P. acnes–induced granuloma formation was not seen in the liver of IFN-γ−/− mice (Fig. 1 E). In addition, we found that P. acnes–induced IL-12p70 and IFN-γ production in IL-15−/− and CCL2 production in IFN-γ−/− mice was impaired (Fig. 1 G), indicating that IL-15 indirectly induces chemokine production by regulating the IL-12–IFN-γ axis in vivo.
Figure 1.
IL-15−/− mice fail to develop P. acnes– and zymosan-induced granulomas. (A) Granuloma formation in the liver of WT, IL-15−/−, RAG-2−/−, and IL-15−/−RAG-2−/− mice. Livers were taken on day 6 after a 0.5-mg heat-killed P. acnes injection, and sections were stained with H&E. Bar, 100 μm. (B) The liver sections of P. acnes–injected WT and IL-15−/− mice were stained with biotinylated anti-CD11c mAb and streptavidin-HRP and further visualized with DAB. Slides were counterstained with Mayer's hematoxylin. Bar, 100 μm. (C) Chemokine levels in the sera of WT, IL-15−/−, RAG-2−/−, and IL-15−/−RAG-2−/− mice were assessed by ELISA at 72 h after a 0.5-mg heat-killed P. acnes injection. Values represent SD (n = 3 mice/group). Data are representative of two to four experiments. (D) Granuloma formation in the liver of zymosan-injected WT and IL-15−/− mice. Livers were taken on day 6 after a 1 mg zymosan injection, and sections were stained with biotinylated anti-CD11c mAb and streptavidin-HRP and further visualized with DAB. Slides were counterstained with Mayer's hematoxylin. Bar, 100 μm. (E) The liver sections of P. acnes–injected WT and IFN-γ−/− mice were stained as described in B. Bar, 100 μm. (F) 3 d after a 1-mg zymosan injection, CCL2 in the sera of WT and IL-15−/− mice was measured by ELISA. Data are representative of four experiments. (G) 3 d after a 0.5-mg P. acnes injection, IL-12p70, and IFN-γ in the sera of WT and IL-15−/− mice and CCL2 in the sera of WT and IFN-γ−/− mice were measured by ELISA. Values represent SD (n = 3 mice/group). Data are representative of three experiments.
Zymosan is a yeast cell wall particle containing β-glucan and mannan as major components. As P. acnes does, zymosan can activate and recruit monocytes, macrophages, and leukocytes (25–27), resulting in the secretion of inflammatory cytokines, hydrogen peroxide, and arachidonic acid (28–30). We also used zymosan to examine the role for IL-15 in the granuloma formation. Consistent with previous experiments (31), zymosan recruited monocytes and DCs and induced granuloma formation in the liver of WT mice. Again, the granulomas were not seen in the liver of IL-15−/− mice (Fig. 1 D), likely because of the lack of chemokine production, such as CCL2 (Fig. 1 F) (31). Our results collectively indicate that IL-15 controls P. acnes– and zymosan-induced granuloma formation, likely through the regulation of chemokine production in vivo.
IL-15 regulates LPS-induced lethal endotoxin shock
LPS injection into P. acnes–primed mice stimulates DCs and macrophages to produce large amounts of proinflammatory cytokines such as IL-12, IFN-γ, and TNF-α, which cause lethal endotoxin shock in vivo (32–34). Likewise, LPS injection into zymosan-primed mice induces shock and tissue injury (35). We next examined the role of IL-15 in LPS-induced endotoxin shock. On day 6 after a 0.5-mg heat-killed P. acnes injection, 1 μg LPS was injected into WT and IL-15−/− mice to induce lethal endotoxin shock. As reported (32–34), P. acnes–primed WT mice were sensitive to LPS-induced endotoxin shock, and all mice died within a day. In contrast, IL-15−/− mice were strongly resistant and survived (Fig. 2 A). In addition, NK cell–depleted WT mice, RAG-2−/−, mice and NK cell–depleted RAG-2−/− mice died just as control WT mice did, indicating that proinflammatory cytokines produced by T, B, and NK cells are dispensable for the endotoxin shock induction (Fig. 2 A).
Figure 2.
IL-15−/− mice are resistant to LPS-induced lethal endotoxin shock. (A) On day 6 after a 0.5-mg heat-killed P. acnes injection, 1 μg LPS were further injected into the indicated mice to induce lethal endotoxin shock. To deplete NK cells, mice were intraperitoneally injected with 300 μg of anti-asialo GM1 polyclonal antibody on the day before and day 3 after P. acnes injection. (B) Serum levels of IL-12p70, IFN-γ, and TNF-α were measured by ELISA in P. acnes–primed mice at 2 h after LPS injection. Values represent SD (n = 3 WT and 2 IL-15−/− mice/group). Data are representative of three experiments. (C) Serum GOT and GPT levels were assessed in P. acnes–primed mice at 2 h after LPS injection. Values represent SD (n = 3 mice/group). Data are representative of two to four experiments. (D) On day 6 after a 1-mg zymosan injection, 10 μg LPS was injected into the indicated mice, and the survival of the mice was monitored. (E) Serum levels of IL-12p70, IFN-γ, and TNF-α were measured by ELISA in zymosan-primed mice at 2 h after LPS injection. Values represent SD (n = 3 mice/group). Data are representative of three experiments.
IL-12, IFN-γ, and TNF-α are known to play important roles in induction of liver injury and/or endotoxin shock (23, 34, 36–39). We thus examined the production of proinflammatory cytokines, in particular IL-12p70, IFN-γ, and TNF-α, in control WT and IL-15−/− mice (Fig. 2 B). Shortly after LPS injection (2 h), these cytokines were detected in the sera of control WT mice, whereas only small amounts of these cytokines were produced in the sera of IL-15−/− mice (Fig. 2 B). Because these cytokines cause liver injury, the level of serum glutamic-pyruvic transaminase (GPT) and glutamic-oxaloacetic transaminase (GOT), an index for hepatocyte damage, was also measured. As expected from the minimal proinflammatory cytokine production, considerable reduction of GPT and GOT release was observed in the sera of IL-15−/− mice, as compared with those in WT mice (Fig. 2 C). Similar to these observations, zymosan-primed IL-15−/− mice exhibited strong resistance to LPS-induced lethality, which was consistent with the reduced production of IL-12, IFN-γ, and TNF-α (Fig. 2, D and E).
Unaffected DC survival in IL-15−/− mice
As recently reported that IL-15 regulates survival of DCs (40), impaired inflammatory responses observed in IL-15−/− mice might be caused by the impaired DC survival. To examine this possibility, we examined the number of splenic DCs between WT and IL-15−/− mice before and after P. acnes injection. The numbers of DCs in untreated WT and IL-15−/− mice were 0.83 × 106 ± 0.15 (n = 5) and 0.82 × 106 ± 0.13 (n = 5), respectively, and those in P. acnes–injected WT and IL-15−/− mice (6 d after injection) were 1.39 × 106 ± 0.28 (n = 4) and 1.54 × 106 ± 0.16 (n = 4), respectively. Using annexin V and propidium iodide staining, we further analyzed the number of apoptotic DCs before and after P. acnes injection and found no important difference between WT and IL-15−/− DCs (Fig. 3). These results suggested that impaired inflammatory responses observed in IL-15−/− mice are unlikely caused by the impaired DC survival in vivo.
Figure 3.
Unaffected apoptosis in IL-15−/− DCs. Splenic DCs were obtained from NK cell–depleted WT and IL-15−/− mice before (untreated) and 1 d (P. acnes, d1) and 6 d (P. acnes, d6) after heat-killed P. acnes injection. (A and B) The proportions of annexin V+ DCs of WT and IL-15−/− mice are shown. (C and D) The proportions of propidium iodide–positive DCs of WT and IL-15−/− mice are shown. Values represent SD (n = 2 mice/group). Data are representative of three experiments.
mAb generation to detect and block mouse IL-15 activity
As IL-15 was found to be a critical mediator for granuloma formation and endotoxin shock induction in vivo, it is important to quantitate the amount of IL-15 produced as a soluble protein in mice during the induction phase of granuloma formation and the eliciting phase of endotoxin shock. For this purpose, we generated rat mAbs specific for mouse IL-15 (for details see Materials and methods). Among 108 clones, 2 clones named AIO2 and AIO3 produced mAbs against mouse IL-15, which were suitable as coating antibodies for an ELISA system. Importantly, the newly developed ELISA system is specific for mouse IL-15 and does not cross react with other cytokines tested, which include IL-2, IL-4, IL-12, TNF-α, IFN-α, IFN-β, IFN-γ, and GM-CSF (Fig. 4 A). Using the ELISA system, we found that substantial amounts of IL-15 were produced in the sera of P. acnes–injected WT mice, and the IL-15 levels were dramatically enhanced immediately after LPS injection into P. acnes–primed WT mice (Fig. 4 B), which was consistent with the observation that the lack of IL-15 resulted in the impaired endotoxin shock.
Figure 4.
Quantification of IL-15 protein levels by ELISA and treatment of endotoxin shock by IL-15 neutralizing antibody. (A) Newly generated ELISA was specific for mouse IL-15. Specificity of one anti–IL-15 mAb (AIO3) was tested by using the panel of cytokines indicated in the figure. (B) Detection of IL-15 protein in the sera of P. acnes– and LPS-injected WT mice. Serum samples were collected from WT and IL-15−/− mice on days 2 and 5 after P. acnes injection and at 1.5 h after a subsequent LPS injection on day 6, and levels of IL-15 were assessed with our IL-15 ELISA system. Values represent SD (n = 3 mice/group). Data are representative of two to four experiments. (C) To test the capacity of AIO2 and AIO3 to block IL-15 activities, 5 × 104 CTLL-2 cells were stimulated with 10 ng/ml IL-15 or IL-2 in the presence or absence of 10 μg/ml AIO2 or AIO3 for 24 h and pulsed with [3H]thymidine for an additional 8 h. Values represent SD of triplicate cultures. Data are representative of two to four experiments. (D) To examine whether AIO2 can block IL-15 activities in vivo, WT mice were injected with 0.5 mg AIO2 either on day 0, 3, or 6 (x3 AIO2) or only on day 6 (x1 AIO2) after P. acnes injection. On day 6, 1 h after AIO2 injection, 1 μg LPS was further injected, and survival of these mice was monitored.
The results shown in Fig. 2 (A and D) and Fig. 4 B collectively raised a possibility of treatment of inflammatory diseases by blocking IL-15 activity in vivo. To this end, we examined whether anti–IL-15 mAbs were capable of blocking IL-15 activity (Fig. 4 C). It is well known that both IL-2 and IL-15 efficiently induce proliferation of a T cell line, CTLL-2. As shown in Fig. 4 C, IL-15–dependent proliferation of CTLL-2 was markedly reduced (>95% reduction) in the presence of AIO2 or AIO3, whereas IL-2–dependent proliferation of CTLL-2 was not affected at all by these mAbs, demonstrating that the mAbs efficiently and selectively block IL-15 activity in vitro. We further examined the effect of AIO2 in vivo (Fig. 4 D). WT mice that had been injected with AIO2 on days 0, 3, and 6 after P. acnes injection (x3 AIO2) and only on day 6 (1 h before LPS injection, x1 AIO2) were strongly resistant to LPS-induced lethality and survived. The results clearly show that antibody capable of neutralizing IL-15 activity is effective in blocking endotoxin shock in vivo.
Essential roles for DC-derived IL-15 in inflammatory response induction
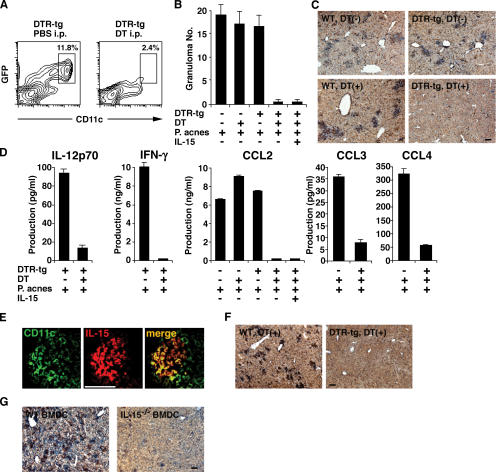
As DCs are present in granuloma regions (Fig. 1, B, D, and E), we further examined the role of DCs in the granuloma formation using CD11c–diphtheria toxin receptor (DTR)–GFP transgenic (DTR tg) mice (41). Because the mice carry a transgene encoding DTR-GFP fusion protein under the control of a mouse CD11c promoter, DT injection induces selective depletion of DCs in vivo (Fig. 5 A) (41). P. acnes– and zymosan-induced (Fig. 5, B and C; and Fig. 5 F, respectively) granulomas were observed in the livers of untreated WT, DT-injected WT, and untreated DTR tg mice, as expected. In contrast, neither CD11c+ DCs nor granulomas themselves were observed in the DT-injected DTR tg mice (Fig. 5, B, C, and F). Consistent with this observation, production of IL-12p70, IFN-γ, CCL2, and CCL3/4 was impaired in the DT-injected DTR tg mice, as observed in IL-15−/− mice (Fig. 5 D). Importantly, immunohistochemical analysis revealed that many IL-15–producing cells in granuloma regions were CD11c+ DCs (Fig. 5 E). Contrary to the case of IL-15−/− mice, however, IL-15 administration restored neither the P. acnes–induced granulomatous liver disease nor CCL2 production in DC-depleted DTR tg mice (Fig. 5, B and D), implying that direct action of DC-derived IL-15 on DCs is necessary for the chemokine-mediated granuloma formation in vivo. To formally demonstrate the importance of DC-derived IL-15 in the granuloma formation, BM-derived DCs (BMDCs) of WT and IL-15−/− mice were adoptively transferred into IL-15−/− mice. Importantly, the granuloma formation was substantially restored by the injection of WT but not IL-15−/− BMDCs into IL-15−/− mice (Fig. 5 G), demonstrating that DC-derived IL-15 is essential for the granuloma formation.
Figure 5.
DC-derived IL-15 controls granuloma formation. (A) Spleen cells were obtained from DTR tg mice 24 h after injection of 100 ng DT or PBS and stained with anti-CD11c–PE. The percentages indicate the proportions of DCs in a DC-enriched fraction; i.e., the cells at the interface after a dense BSA gradient centrifugation (see DC preparation… in Materials and methods). (B) Granuloma formation in the liver of P. acnes–primed DT-injected DTR tg mice. On day 3 after a 0.5-mg heat-killed P. acnes injection, livers were taken from WT and DTR tg mice that had been injected with either PBS or DT on the day before P. acnes injection, and sections were stained with H &E. Numbers of granulomas were counted in five different fields under a microscope. Values represent SD (n = 3 mice/group). Data are representative of three experiments. (C) To test whether DCs were present in the granuloma regions, the liver sections were stained with biotinylated anti-CD11c mAb and streptavidin-HRP and further visualized with DAB. Slides were counterstained with Mayer's hematoxylin. Bar, 100 μm. (D) IL-12p70, IFN-γ, and chemokine levels in the sera of DT-injected DTR tg mice were assessed by ELISA at 72 h after a 0.5-mg heat-killed P. acnes injection. Values represent SD (n = 3 mice/group). Data are representative of three experiments. (E) Immunofluoresence staining for the identification of IL-15–producing cells. Acetone-fixed frozen tissue sections were incubated with FITC-conjugated anti-CD11c (clone N418) and biotinylated anti–IL-15 antibodies and further developed with streptavidin-PE. Bar, 50 μm. (F) Granuloma formation in the liver of zymosan-primed DT-injected DTR tg mice. As described in B and C, livers were taken from the indicated mice on day 3 after a 1-mg zymosan injection, and sections were stained for CD11c. Slides were counterstained with Mayer's hematoxylin. Bar, 100 μm. (G) Granuloma formation in the liver of BMDC-injected IL-15−/− mice. IL-15−/− mice were injected with 1 × 106 WT BMDCs or IL-15−/− BMDCs. After 12 h, mice were injected with 0.5 mg P. acnes, and granuloma formation was analyzed 6 d later as described in B, C, and F. Bar, 100 μm.
Intracellular redox status affects the pattern of cytokine production by DCs and macrophages (42–45). For example, reductive DCs (and macrophages) with elevated intracellular glutathione (GSH) preferentially produce IL-12 and are involved in Th1 responses, whereas oxidative DCs with reduced GSH produce IL-10 and PGE2, which lead to Th2 cell induction. As P. acnes priming efficiently induces reductive status in DCs (44), we next examined the role for IL-15 in determining redox status in DCs (Fig. S3, available at http://jem.org.cgi/content/full/jem.20061297/DC1). As previously shown (44), P. acnes injection clearly induced a reductive condition with elevated intracellular GSH levels in WT DCs. In contrast, such a reductive condition was not induced, if any, in IL-15−/− DCs on P. acnes stimulation. These data indicate that IL-15 is one of the critical cytokines in reductive DC differentiation.
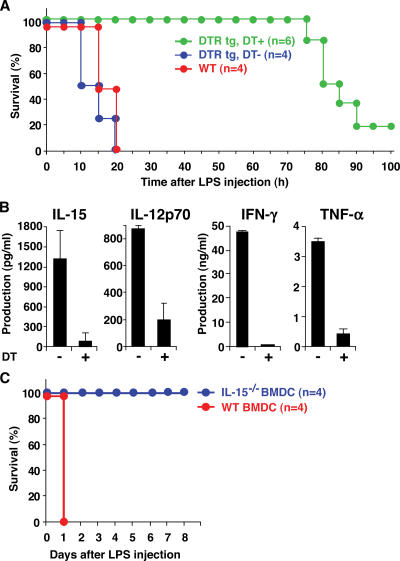
We also examined the role of DCs in endotoxin shock. 1 d after DT injection, control WT and DTR tg mice were primed with 0.5 mg P. acnes. On day 3 after P. acnes injection, these mice were injected with 1 μg LPS, and survival rate was monitored. On LPS injection, all P. acnes–primed WT and DT-untreated DTR tg mice died of endotoxin shock within 24 h (Fig. 6 A). However, P. acnes–primed and DT-injected DTR tg mice survived much longer than control mice, indicating that DCs play a pivotal role in endotoxin shock as well. Of note, DT-injected DTR tg mice survived relatively shorter than IL-15−/− mice (Fig. 2 A), which was likely because of the gradual recovery of DCs in DT-injected DTR tg mice as reported previously (41). P. acnes–primed DT-injected DTR tg mice showed impaired production of IL-15 and IL-15–regulated proinflammatory cytokines, IL-12p70, IFN-γ, and TNF-α on LPS stimulation (Fig. 6 B). In addition, WT BMDC-transferred IL-15−/− mice became sensitive to the endotoxin shock, and all mice died within a day, whereas IL-15−/− BMDC–injected IL-15−/− mice remained resistant (Fig. 6 C). Collectively, these results indicate that DC-derived IL-15 is critical for endotoxin shock induction in vivo.
Figure 6.
DC-derived IL-15 regulates endotoxin shock. (A) 1 d after DT injection, control WT and DTR tg mice were primed with 0.5 mg P. acnes. On day 3 after P. acnes injection, these mice were further injected with 1 μg LPS, and survival rate was monitored. (B) Serum levels of IL-15, IL-12p70, IFN-γ, and TNF-α were assessed in DT-injected and P. acnes–primed DTR tg mice at 2 h after LPS injection. Values represent SD (n = 3 mice/group). Data are representative of three experiments. (C) IL-15−/− mice were injected with 1 × 106 WT or IL-15−/− BMDCs. After 12 h, mice were injected with 0.5 mg P. acnes. On day 6 after P. acnes injection, these mice were further injected with 5 μg LPS, and survival rate was monitored.
DISCUSSION
DC-derived IL-15
Prolonged or aberrant activation of immune responses cause a variety of immunopathological disorders that are often mediated by effector cells and cytokines. We previously found that DC-derived IL-15 is required for the functional maturation of DCs, such as IL-12 production in response to LPS and agonistic anti-CD40 mAb combined with IL-4 stimulation in vitro (8), and have recently shown that DC-derived IL-15 is essential for CpG-induced protective immune activation against pathogen infections in vivo (9). In contrast to the beneficial effect of DC-derived IL-15, we maintain in this study that DC-derived IL-15 has harmful aspects and causes inflammatory diseases, such as granuloma formation and endotoxin shock in vivo.
IL-15 is involved in a variety of inflammatory and autoimmune diseases (13, 14, 17-20). To address whether development of these diseases attributes to IL-15, analyses using IL-15−/− mice, anti–IL-15 neutralizing antibody, soluble IL-15Rα, and IL-15 mutant/Fcγ2a fusion protein are in progress in both human and mouse models (15, 16). As IL-15 is produced by multiple cell types, which include DCs, macrophages, monocytes, and endothelial cells (5), an advanced and unresolved question has been whether DC-derived IL-15 is exclusively required for the development of certain diseases in vivo. Numerous previous in vitro studies indicated that DC-derived IL-15 is capable of inducing activation of Th1 cells, CTL, NK cells, monocyte differentiation into DCs, and antigen-processing machinery in DCs (5, 46), but these studies did not directly prove the irreplaceable role of DC-derived IL-15 in disease development in vivo, where multiple IL-15 producers are present. To address this issue, it is important to show that the development of certain diseases is impaired in IL-15−/− or WT mice treated with reagents to block IL-15 activity, and to further restore the disease development by adoptively transferring WT but not IL-15−/− DCs into these mice. In this respect, only one paper has shown that IL-15−/− or WT mice treated with soluble IL-15Rα were impaired in CD8+ T cell–dependent delayed-type hypersensitivity response, and the delayed-type hypersensitivity response was restored by injecting antigen-labeled WT DCs in vivo (47). It is unclear, however, whether IL-15 is important for cytokine production or antigen presentation in this study (47). Other groups have shown that IL-15Rα and IL-15 expression by hematopoietic cells is critical for the maintenance of antigen-specific memory CD8+ T cells and bystander CD8+ T cell proliferation through a “transpresentation” pathway (48–53), and implied DCs as the major source of IL-15 but never proved it. Accordingly, it remains unknown whether DC-derived IL-15 is essential in the maintenance of innate and acquired immune responses, and whether it causes inflammatory disease development in vivo. It is thus important to prove the in vivo role of DC-derived IL-15 for the development of antiinflammatory drugs that selectively block the DC-derived IL-15 activity.
Mechanisms of how IL-15 controls cytokine production are unknown. As shown in this paper, IL-15 increased the intracellular GSH levels in DCs. Intracellular GSH levels in DCs and macrophages play an important role in determining the profiles of proinflammatory cytokines (42–45). We have previously demonstrated that reductive DCs with high intracellular GSH levels preferentially produce IFN-γ, which in turn augment GSH levels in the cells (44). As shown in this paper, IL-15 is also one of the positive regulators of intracellular GSH status in DCs, augmenting the production of proinflammatory cytokines. In future studies, it should be determined how IL-15 controls the amounts of intracellular GSH in DCs.
Granuloma formation, liver injury, and IL-15
The number of P. acnes–induced granulomas and LPS-induced hepatic necrosis after priming with P. acnes are substantially reduced in IFN-γ−/− mice (Fig. 1 E) (23). In addition, P. acnes does not induce granuloma formation in TNF-RI−/− mice and mice treated with soluble TNF-RI (21). These studies clearly demonstrate the importance of IFN-γ and TNF-α in P. acnes–induced liver diseases in vivo. In contrast, it has been shown that CCL3 attracts DC precursors in the blood into the sinusoidal granuloma and lets them participate in inflammatory responses in P. acnes–primed mice (24). In addition, CCL2 has also been reported as an important monocyte chemoattractant for granuloma models induced by zymosan, P. acnes, and Mycobacterium tuberculosis (54–56). These studies show that the chemokines play a critical role in granuloma models, though the importance of IFN-γ and TNF-α in chemokine production remains unclear. Notably, we determined that an IL-15–IL-12–IFN-γ–chemokine (CCL2/3/4) axis in innate immune system is essential, whereas T and B cells are dispensable for the development of granulomatous disease and/or liver injury. Immunohistochemical analysis revealed that DCs preferentially expressed IL-15 in the granuloma regions, and the granuloma formation was impaired in mice lacking DC and DC-derived IL-15 production, clearly demonstrating that DC-derived IL-15 is an initiator for the development of liver diseases.
Of note, IL-15 injection restored the granuloma formation in the liver of IL-15−/− mice but not DC-depleted DTR tg mice. These results suggest that a critical first step for the granuloma formation is to stimulate DCs with DC-derived IL-15 in an autocrine manner. Although Kupffer cells are critically involved as initial antigen-presenting (P. acnes and zymosan) cells (24), Kupffer cells isolated from P. acnes–primed mice were unable to produce IL-15 on LPS stimulation in vitro (unpublished data).
Endotoxin shock and IL-15
LPS-induced liver injury is closely coupled to endotoxin shock. Indeed, mice deficient in IL-12, IFN-γ, TNF-α, or the receptors for these cytokines displayed resistance to LPS-induced endotoxin shock (34, 36, 38, 57). We showed in this paper that DCs are essential for endotoxin shock, and DC-derived IL-15 controls endotoxin shock induction by controlling the production of IL-12, IFN-γ, and TNF-α, as DC-depleted mice produced these cytokines at much reduced levels, and WT DC-injected IL-15−/− mice became sensitive to endotoxin shock. Contrary to IL-15−/− mice, P. acnes–primed IL-18−/− mice showed much higher TNF-α production and susceptibility to LPS-induced endotoxin shock (58). Together with our results, IL-15 and IL-18 function apparently through distinct pathways in terms of TNF-α production, and IL-15 induces, whereas IL-18 suppresses, TNF-α production.
Collectively, we propose here that DC-derived IL-15 is a master regulator of inflammatory responses in granulomatous liver diseases and related endotoxin shock. Indeed, DC-derived IL-15 regulates the production of IL-12p70, IFN-γ, TNF-α, and downstream CCL2/3/4. Given that elevated IL-15 production and IL-15–expressing cells are evident in RA (13, 14), inflammatory bowel disease (17), type C chronic liver disease (18), sarcoidosis (19), multiple sclerosis (20), and celiac disease (59), it is important to further investigate the roles of IL-15 in these inflammatory diseases and search for the propriety of IL-15 as a target for the development of antiinflammatory drugs.
MATERIALS AND METHODS
Mice.
B6–IL-15−/− (IL-15−/−) mice (6) were purchased from Taconic, and B6-RAG2−/− (RAG2−/−) mice were provided by Taconic and Central Laboratories for Experimental Animals. B6–IFN-γ−/− mice were purchased from The Jackson Laboratory. To obtain B6–IL-15−/−RAG2−/− mice (IL-15−/− × RAG2−/−), F1 mice were backcrossed with RAG2−/− mice, and the obtained IL-15+/−RAG2−/− mice were intercrossed. The offspring were genotyped for IL-15, and IL-15−/−RAG2−/− mice were used for experiments. B6-CD11c-DTR-GFP mice (41) were provided by Steffen Jung, Dan R. Littman, and Richard A. Lang (New York University School of Medicine, New York, NY). All mice were maintained in our specific pathogen-free animal facility, and experiments were performed between 6–12 wk of age in accordance with the guidelines of the Institutional Animal Care Committee of Akita University and Keio University School of Medicine.
Histologic and immunohistochemical analysis.
Frozen livers embedded in OCT compound (Sakura Finetek) were sliced into 5-μm-thick sections and fixed with 1% paraformaldehyde and stained with Mayer's hematoxylin and eosin (H&E). Sections of livers were observed with a microscope (DM4500 B; Leica). For CD11c immunostaining, acetone-fixed 5-μm fresh-frozen tissue sections were incubated with biotinylated anti-CD11c mAb (clone, N418; eBioscience) overnight at 4°C and with streptavidin-conjugated horseradish peroxidase (HRP; PerkinElmer). Sections were immunostained using 3,3′-diaminobenzidine (DAB) substrate liquid (DakoCytomation). Slides were counterstained with Mayer's hematoxylin. For CD11c and IL-15 double immunofluorescence staining, acetone-fixed 5-μm fresh-frozen tissue sections were incubated with FITC-conjugated anti-CD11c (clone, N418; eBioscience) and biotinylated goat anti–mouse IL-15 polyclonal antibody (R&D Systems) for 1 h at room temperature. Sections were further stained with streptavidin-conjugated PE (eBioscience) for 30 min at room temperature and observed by fluorescence microscopy.
Measurement of serum chemokines, cytokines, GPT, and GOT.
Levels of IL-12p70, IFN-γ, TNF-α, and CCL2/3/4 in the sera were measured by ELISA kits (IL-12p70, IFN-γ, and TNF-α were obtained from BD Biosciences; CCL2/3/4 was obtained from R&D Systems), according to the manufacturer's instructions. The concentrations of cytokines were determined using a data analysis program (Softmax PRO; Molecular Devices). Serum GPT and GOT levels were determined with Fuji Dri-Chem 5500V (Fuji Medical System), according to the manufacturer's instructions.
Generation of anti–mouse IL-15 mAb and ELISA for mouse IL-15.
To detect mouse IL-15 protein, mAbs specific for mouse IL-15 were generated by immunizing mouse IL-15 into Lewis rat. Using conventional methods, spleen cells isolated from the immunized rat were fused with X63-Ag8.653 myeloma cells, and limiting dilution for hybridoma cells was performed. Positive clones producing anti–IL-15 mAb were screened based on the binding capacity to coated mouse IL-15. Among the mAbs, AIO2 and AIO3 clones were further selected as neutralizing mAbs based on the inhibition of IL-15–dependent CTLL-2 cell proliferation. In brief, 5 × 104 CTLL-2 cells were cultured with 10 ng/ml IL-15 or IL-2 in the presence or absence of 10 μg/ml AIO2 and AIO3 for 24 h and pulsed with [3H]thymidine for an additional 8 h. For mouse IL-15 sandwich ELISA, microwells were coated with AIO3 overnight at 4°C and incubated with Block Ace (Dainippon Pharmaceutical) for 90 min. The diluted serum samples were incubated for 2 h, then for 60 min with biotinylated goat anti–mouse IL-15 antibody (R&D Systems) and for 60 min with avidin-HRP (Sigma-Aldrich). The absorbance of substance released from the substrate was measured at 450 nm. The IL-15 concentrations in samples were determined using Softmax PRO, based on a standard curve of recombinant mouse IL-15.
Reagents and in vivo treatment.
Mice were injected with either 0.5 mg of heat-killed P. acnes (American Type Culture Collection) or 1 mg of zymosan (Sigma-Aldrich). 6 d later, the amounts of LPS indicated in the figures (Escherichia coli O55:B5; Sigma-Aldrich) were further injected into P. acnes– and zymosan-primed mice, respectively, to induce endotoxin shock. To deplete NK cells, 300 μg/200 μl of polyclonal anti-asialo GM1 (Wako) was injected. To neutralize IL-15 in vivo, 0.5 mg AIO2 was injected. For systemic DC depletion in vivo, CD11c-DTR-GFP mice were injected intraperitoneally with 100 ng/body of diphtheria toxin (Sigma-Aldrich).
DC preparation, detection of apoptosis, and adoptive transfer.
DCs were prepared from spleens as previously described (8). In brief, collagenase-digested spleen cells were suspended in a 28% BSA solution in 1.08 g/ml PBS, overlaid with 1 ml FCS-free RPMI 1640 medium (Sigma-Aldrich), and centrifuged at 9,500 g for 20 min at 4°C. The cells at the interface were collected, washed, and resuspended. DCs were further purified using anti-CD11c (clone N418) microbeads with an autoMACS separation system (Miltenyi Biotec). To detect apoptotic DCs, 5 × 105 DCs were cultured in vitro for 6 h and incubated for 15 min at room temperature in 500 μl annexin V binding buffer with 150 ng/ml annexin V (R&D Systems) or for 10 min at 4°C in 500 μl PBS with 2 μg/ml propidium idodide (Sigma-Aldrich), respectively. For generation of BMDCs, WT and IL-15−/− BM cells were cultured at 1.5 × 106 cells/ml in 10% FCS RPMI 1640 medium in the presence of 10 ng/ml GM-CSF (RDI Division of Fitzgerald Industries). After 3 d of culture, half of the medium was exchanged with a fresh one. After 6 d of culture, BMDCs were purified using anti-CD11c microbeads with an autoMACS separation system. For adoptive transfer experiments, 1 × 106 BMDCs were intravenously injected into IL-15−/− mice.
Qualitative determination of intracellular GSH with ACAS.
The procedures have previously been described (44). In brief, 300 μl of a suspension of splenic DCs, adjusted to a density of 3 × 105 cells/ml in an RPMI 1640 (phenol red free) medium, were applied into a chamber slide (Lab-Tek; Nunc) and incubated for 3 h. After washing, 300 μl of 10 μM monochlorobiman was added, and the reaction was conducted for 30 min. The fluorescence intensity was monitored by argon-ion laser cytometry with a workstation (ACAS 570; Meridian Instruments). Intracellular GSH levels were detected with an excitation wavelength of 350 nm and an emission wavelength of 460 nm.
Online supplemental material.
Fig. S1 shows granuloma formation in the liver of NK cell–depleted RAG-2−/− mice. Fig. S2 shows the level of serum CCL2 in NK cell–depleted RAG-2−/− mice. Fig. S3 shows reductive status in P. acnes–stimulated IL-15−/− DCs.
Supplemental Material
Acknowledgments
We thank M. Shibata, N. Kakizaki, and M. Motouchi for animal care; M. Kondo and K. Maekawa for screening of hybridoma cells and mAb purification; K. Yamashita and Y. Abe for experimental support; and T. Yoshimoto and K. Nakanishi for Kupffer cell isolation and critical reading of the manuscript.
This work was supported in part by the Toray Science Foundation (T.Ohteki); the Uehara Memorial Foundation (T.Ohteki); the Takeda Science Foundation (T. Ohteki); the Novartis Foundation for the Promotion of Science (T. Ohteki); the Sankyo Foundation for Life Science (T. Ohteki); grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (14021110 to S. Koyasu; 16017212 and 16043204 to T. Ohteki); the 21st Century Center of Excellence Program; a Keio University special grant-in-aid for Innovative Collaborative Research Projects; and the Uehara Memorial Foundation special project research grant (to S. Koyasu). H. Tada is supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists.
The authors have no conflicting financial interests.
Abbreviations used: BMDC, BM-derived DC; DAB, 3,3′-diaminobenzidine; DTR, diphtheria toxin receptor; DTR tg, CD11c-DTR-GFP transgenic; GOT and GPT, glutamic-oxaloacetic and -pyruvic transaminase, respectively; GSH, glutathione; H&E, hematoxylin and eosin; HRP, horseradish peroxidase; RA, rheumatoid arthritis.
T. Ohteki and H. Tada contributed equally to this work.
References
- 1.Ohteki, T., S. Ho, H. Suzuki, T.W. Mak, and P.S. Ohashi. 1997. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 159:5931–5935. [PubMed] [Google Scholar]
- 2.Ogasawara, K., S. Hida, N. Azimi, Y. Tagaya, T. Sato, T. Yokochi-Fukada, T.A. Waldmann, T. Taniguchi, and S. Taki. 1998. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 391:700–703. [DOI] [PubMed] [Google Scholar]
- 3.Ohteki, T., H. Yoshida, T. Matsuyama, G.S. Duncan, T.W. Mak, and P.S. Ohashi. 1998. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann, T.A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19–49. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattei, F., G. Schiavoni, F. Belardelli, and D.F. Tough. 2001. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167:1179–1187. [DOI] [PubMed] [Google Scholar]
- 8.Ohteki, T., K. Suzue, C. Maki, T. Ota, and S. Koyasu. 2001. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2:1138–1143. [DOI] [PubMed] [Google Scholar]
- 9.Kuwajima, S., T. Sato, K. Ishida, H. Tada, H. Tezuka, and T. Ohteki. 2006. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat. Immunol. 7:740–746. [DOI] [PubMed] [Google Scholar]
- 10.Becker, T.C., E.J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldrath, A.W., P.V. Sivalumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluns, K.S., K. Williams, A. Ma, X.X. Zheng, and L. Lefrançois. 2002. Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831. [DOI] [PubMed] [Google Scholar]
- 13.McInnes, I.B., J. Al-Mughales, M. Field, B.P. Leung, F.-P. Huang, R. Dixon, R.D. Sturrock, P.C. Wilkinson, and F.Y. Liew. 1996. The role of interleukin 15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 2:175–182. [DOI] [PubMed] [Google Scholar]
- 14.McInnes, I.B., B.P. Leung, R.D. Sturrock, M. Field, and F.Y. Liew. 1997. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 3:189–195. [DOI] [PubMed] [Google Scholar]
- 15.Ruchatz, H., B.P. Leung, X. Wei, I.B. McInnes, and F.Y. Liew. 1998. Soluble IL-15 receptor α-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 160:5654–5660. [PubMed] [Google Scholar]
- 16.Ferrari-Lacraz, S., E. Zanelli, M. Neuberg, E. Donskoy, Y.S. Kim, X.X. Zheng, W.W. Hancock, W. Maslinski, X.C. Li, T.B. Strom, and T. Moll. 2004. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J. Immunol. 173:5818–5826. [DOI] [PubMed] [Google Scholar]
- 17.Kirman, I., and O.H. Nielsen. 1996. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am. J. Gastroenterol. 91:1789–1794. [PubMed] [Google Scholar]
- 18.Kakumu, S., A. Okumura, T. Ishikawa, M. Yano, A. Enomoto, H. Nishimura, K. Yoshioka, and Y. Yoshikai. 1997. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-α) receptors in type C chronic liver disease. Clin. Exp. Immunol. 109:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agostini, C., L. Trentin, M. Facco, R. Sancetta, A. Cerutti, C. Tassinari, L. Cimarosto, F. Adami, A. Cipriani, R. Zambello, and G. Semenzato. 1996. Role of IL-15, IL-2 and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J. Immunol. 157:910–918. [PubMed] [Google Scholar]
- 20.Kivisäkk, P., D. Matusevicius, B. He, M. Söderström, S. Fredrikson, and H. Link. 1998. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS). Clin. Exp. Immunol. 111:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senaldi, G., S. Yin, C.L. Shaklee, P.-F. Piguet, T.W. Mak, and T.R. Ulich. 1996. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guérin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 157:5022–5026. [PubMed] [Google Scholar]
- 22.Marino, M.W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, et al. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA. 94:8093–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji, H., N. Mukaida, A. Harada, S. Kaneko, E. Matsushita, Y. Nakamura, H. Tsutsui, H. Okamura, K. Nakanishi, Y. Tagawa, et al. 1999. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-γ-deficient mice by a concomitant reduction of TNF-α, IL-12, and IL-18 production. J. Immunol. 162:1049–1055. [PubMed] [Google Scholar]
- 24.Yoneyama, H., K. Matsuno, Y. Zhang, M. Murai, M. Itakura, S. Ishikawa, G. Hasegawa, M. Naito, H. Asakura, and K. Matsushima. 2001. Regulation by chemokines of circulating dendritic cell precursors and the formation of portal tract–associated lymphoid tissue in a granulomatous liver disease. J. Exp. Med. 193:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanguedolce, M.V., C. Capo, P. Bongrand, and J.-L. Mege. 1992. Zymosan-stimulated tumor necrosis factor-α production by human monocytes: down-modulation by phorbol ester. J. Immunol. 148:2229–2236. [PubMed] [Google Scholar]
- 26.Underhill, D.M., A. Ozinsky, A. Hajjar, A. Stevens, C.B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 401:811–815. [DOI] [PubMed] [Google Scholar]
- 27.Young, S.-H., J. Ye, D.G. Frazer, X. Shi, and V. Castranova. 2001. Molecular mechanism of tumor necrosis-α production in 1,3-β-glucan (zymosan)-activated macrophages. J. Biol. Chem. 276:20781–20787. [DOI] [PubMed] [Google Scholar]
- 28.Daum, T., and M.S. Rohrbach. 1992. Zymosan induces selective release of arachidonic acid from rabbit alveolar macrophages via stimulation of a β-glucan receptor. FEBS Lett. 309:119–122. [DOI] [PubMed] [Google Scholar]
- 29.Nobel, P.W., P.M. Henson, C. Lucas, M. Mora-Worms, P.C. Carré, and D.W.H. Riches. 1993. Transforming growth factor-β primes macrophages to express inflammatory gene products in response to particulate stimuli by an autocrine/paracrine mechanism. J. Immunol. 151:979–989. [PubMed] [Google Scholar]
- 30.Okazaki, M., N. Chiba, Y. Adachi, N. Ohno, and T. Yadomae. 1996. Signal transduction pathway on β-glucans-triggered hydrogen peroxide production by murine peritoneal macrophages in vitro. Biol. Pharm. Bull. 19:18–23. [DOI] [PubMed] [Google Scholar]
- 31.Jinnouchi, K., Y. Terasaki, S. Fujiyama, K. Tomita, W.A. Kuziel, N. Maeda, K. Takahashi, and M. Takeya. 2003. Impaired hepatic granuloma formation in mice deficient in C-C chemokine receptor 2. J. Pathol. 200:406–416. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto, T., K. Nakanishi, S. Hirose, K. Hiroishi, H. Okamura, Y. Takemoto, A. Kanamaru, T. Hada, T. Tamura, E. Kakishita, and K. Higashino. 1992. High serum IL-6 level reflects susceptible status of the host to endotoxin and IL-1/tumor necrosis factor. J. Immunol. 148:3596–3603. [PubMed] [Google Scholar]
- 33.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M.A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, et al. 1997. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 275:206–209. [DOI] [PubMed] [Google Scholar]
- 34.Merlin, T., A. Sing, P.J. Nielsen, C. Galanos, and M.A. Freudenberg. 2001. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the LPS (d) C57BL/10ScCr mouse. J. Immunol. 166:566–573. [DOI] [PubMed] [Google Scholar]
- 35.Sun, X.M., W. Hsueh, and G. Torre-Amione. 1990. Effects of in vivo “priming” on endotoxin-induced hypotension and tissue injury. The role of PAF and tumor necrosis factor. Am. J. Pathol. 136:949–956. [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeffer, K., T. Matsuyama, T.M. Kündig, A. Wakeham, K. Wiegmann, P.S. Ohashi, M. Krönke, and T.W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73:457–467. [DOI] [PubMed] [Google Scholar]
- 37.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 364:798–802. [DOI] [PubMed] [Google Scholar]
- 38.Car, B.D., V.M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Galley, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon γ receptor–deficient mice are resistant to endotoxic shock. J. Exp. Med. 179:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNFα-deficient mice:a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois, S.P., T.A. Waldmann, and J.R. Muller. 2005. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. USA. 102:8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, et al. 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson, J.D., L.A. Herzenberg, K. Vasquez, and C. Waltenbaugh. 1998. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA. 95:3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata, Y., M. Amao, J. Yoneda, and J. Hamuro. 2002. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol. Immunol. 38:747–757. [DOI] [PubMed] [Google Scholar]
- 44.Murata, Y., T. Ohteki, S. Koyasu, and J. Hamuro. 2002. IFN-γ and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur. J. Immunol. 32:2866–2873. [DOI] [PubMed] [Google Scholar]
- 45.Murata, Y., T. Shimamura, and J. Hamuro. 2002. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 14:201–212. [DOI] [PubMed] [Google Scholar]
- 46.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-5, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657–679. [DOI] [PubMed] [Google Scholar]
- 47.Rückert, R., K. Brandt, E. Bulanova, F. Mirghomizadeh, R. Paus, and S. Bulfone-Paus. 2003. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 33:3493–3503. [DOI] [PubMed] [Google Scholar]
- 48.Lodolce, J.P., P.R. Burkett, D.L. Boone, M. Chien, and A. Ma. 2001. T cell–independent interleukin-15α signals are required for bystander proliferation. J. Exp. Med. 194:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Judge, A.D., X. Zhang, H. Fujii, C.D. Surh, and J. Sprent. 2002. Interleukin-15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkett, P.R., R. Koka, M. Chien, S. Chai, F. Chan, A. Ma, and D.L. Boone. 2003. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 100:4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burkett, P.R., R. Koka, M. Chien, S. Chai, D.L. Boone, and A. Ma. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schluns, K.S., K.D. Klonowski, and L. Lefrancois. 2004. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood. 103:988–994. [DOI] [PubMed] [Google Scholar]
- 53.Schluns, K.S., E.C. Nowak, A. Cabrera-Hernandez, L. Puddington, L. Lefrancois, and H.L. Aguila. 2004. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor α expression. Proc. Natl. Acad. Sci. USA. 101:5616–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuziel, W.A., S.J. Morgan, T.C. Dawson, S. Griffin, O. Smithies, K. Ley, and N. Maeda. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA. 94:12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichiyasu, H., M. Suga, A. Matsukawa, K. Iyonaga, T. Mizobe, T. Takahashi, and M. Ando. 1999. Functional roles of MCP-1 in Propionibacterium acnes-induced, T cell-mediated pulmonary granulomatosis in rabbits. J. Leukoc. Biol. 65:482–491. [DOI] [PubMed] [Google Scholar]
- 56.Peters, W., H.M. Scott, H.F. Chambers, J.L. Flynn, I.F. Charo, and J.D. Ernst. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 98:7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamijo, R., J. Le, D. Shapiro, E.A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakao, Y., K. Takeda, H. Tsutsui, T. Kaisho, F. Nomura, H. Okamura, K. Nakanishi, and S. Akira. 1999. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 11:471–480. [DOI] [PubMed] [Google Scholar]
- 59.Hüe, S., J.-J. Mention, R.C. Monteiro, S. Zhang, C. Cellier, J. Schmitz, V. Verkarre, N. Fodil, S. Bahram, N. Cerf-Bensussan, and S.C. Caillat-Zucman. 2004. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 21:367–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.