Abstract
Numerous microbes establish persistent infections, accompanied by antigen-specific CD8 T cell activation. Pathogen-specific T cells in chronically infected hosts are often phenotypically and functionally variable, as well as distinct from T cells responding to nonpersistent infections; this phenotypic heterogeneity has been attributed to an ongoing reencounter with antigen. Paradoxically, maintenance of memory CD8 T cells to acutely resolved infections is antigen independent, whereas there is a dependence on antigen for T cell survival in chronically infected hosts. Using two chronic viral infections, we demonstrate that new naive antigen-specific CD8 T cells are primed after the acute phase of infection. These newly recruited T cells are phenotypically distinct from those primed earlier. Long-lived antiviral CD8 T cells are defective in self-renewal, and lack of thymic output results in the decline of virus-specific CD8 T cells, indicating that newly generated T cells preserve antiviral CD8 T cell populations during chronic infection. These findings reveal a novel role for antigen in maintaining virus-specific CD8 T cells during persistent infection and provide insight toward understanding T cell differentiation in chronic infection.
Many viral infections persist indefinitely after acute infection and may give rise to considerable clinical problems. Chronic viral infection is established despite expansion of antigen-specific CD8 T cells, which often control viral replication during acute infection (1, 2). Although exhaustion of antigen-specific CD8 T cells has been invoked as one reason for pathogen persistence (3), viruses also persist in situations in which CD8 T cell functions are not completely compromised (4–7). Current evidence suggests that maintenance of the CD8 T cell population responding to chronic infection is dependent on antigen (8–10). This requirement fits with evidence that virus-specific CD8 T cell responses decay in hepatitis C virus– and HIV-infected patients responding to antiviral therapy (10–12). In marked contrast, memory T cells generated to an acutely resolved infection survive in the absence of antigen (13, 14). The reason for this discrepancy is unknown.
Various models have been constructed to understand antigen-driven differentiation of naive T cells (15). These models are based on phenotypic changes within a T cell population, and they rely on the assumption that individual cells analyzed in the end are the progeny of cells sampled in the beginning. Any input of primed cells into the population after the initial readout complicates the delineation of differentiation pathways.
We sought to gain a clearer understanding of the dichotomy of antigen dependence for antiviral CD8 T cell survival during chronic infection by examining the impact of persistent antigen on T cell priming, phenotype, and survival. In this paper, we demonstrate that after initial CD8 T cell priming, which occurs early after viral exposure, the presence of persistent antigen recruits new, naive antigen-specific CD8 T cells. Because these T cells are phenotypically distinct from those primed earlier, they contribute to the diversity seen within populations of chronic viral antigen-specific CD8 T cells. Collectively, our data that antiviral CD8 T cells in chronic infection are defective in homeostatic proliferation, and that thymectomy results in a loss of virus-specific CD8 T cells, indicate a novel role for antigen in maintaining CD8 T cells in persistent infection.
RESULTS AND DISCUSSION
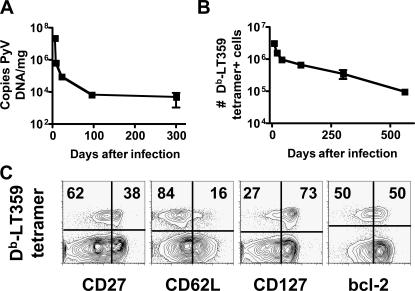
Polyoma virus (PyV) establishes a systemic persistent infection in mice (Fig. 1 A) (7). CD8 T cell responses to PyV infection are detected via binding of Db tetramers complexed to an immunodominant epitope encoded by aa 359–368 of the viral large T protein (LT359) (Fig. 1, B and C). After the acute phase of infection, LT359-specific CD8 T cells are detected as late as 560 d after infection (Fig. 1 B). At 246 d after infection, this population of antigen-specific cells is heterogeneous for expression of CD27, CD62L, CD127, and bcl-2 (Fig. 1 C). In addition, viral DNA is detectable in the spleen and other organs for at least that length of time (Fig. 1 A and not depicted) (7). We asked whether this phenotypic heterogeneity represented differentiation of cells primed during the acute phase of infection or resulted from an amalgam with cells newly generated during persistent infection.
Figure 1.
Visualizing PyV infection. (A) Splenic PyV DNA levels ± SEM over time. (B) Number of splenic Db-LT359 tetramer+ CD8 T cells ± SD over time. (C) Splenic CD8 T cell responses 246 d after infection. Plots are gated on CD8 T cells, and values reflect the percentage of tetramer+ cells in each quadrant (n = 3–9 mice).
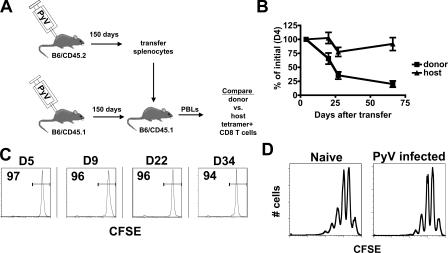
We assessed the capacity of antiviral CD8 T cells to survive during persistent infection. T cells from mice infected by PyV 150 d earlier were transferred into infection-matched congenic hosts (Fig. 2 A). LT359-specific CD8 T cells of both host and donor origin were monitored over time. Whereas host CD8 T cells were maintained at a constant level, donor cells decreased over time (Fig. 2 B); the most rapid decline occurred over the first 3 wk, followed by a slower rate of attrition. This finding was unexpected because of the slower kinetics of virus-specific CD8 T cell loss during persistent infection in unmanipulated mice (Fig. 1 B). The transferred cells did not divide over 34 d (Fig. 2 C), although PyV-infected mice support homeostatic division of conventional memory CD8 T cells (Fig. 2 D), indicating that this cell loss is a cell-intrinsic phenomenon. Similarly, CD8 T cells from mice persistently infected by lymphocytic choriomeningitis virus (LCMV) fail to undergo homeostatic proliferation when transferred to naive mice (9). Because the PyV-specific host and donor CD8 T cells were elicited and maintained under identical conditions in this transfer experiment and coexist in the same environment, this data is inconsistent with the concept that antigen maintains the survival of antiviral CD8 T cells during chronic infection. To reconcile this discrepancy, we reasoned that host cells, but not donor cells, can be resupplied through thymic output, and perhaps new, naive PyV-specific CD8 T cells were being generated and subsequently primed during persistent infection.
Figure 2.
Loss of PyV-specific CD8 T cells in an antigen-bearing host. (A) Experimental setup. (B) Host mice were bled at day 4 after transfer to establish a base level of Db-LT359 tetramer+ cells of donor and host origin. PBLs were monitored over time, and values indicate the percentage of tetramer+ cells ± SEM of either host or donor origin normalized for input at day 4 after transfer (n = 4 mice). Two independent experiments were performed. The donor tetramer+ cell frequencies varied from 0.3–0.6% of CD8 T cells at day 4, and host tetramer+ frequencies varied from 1.3-3.8% of CD8 T cells. (C) Transferred PyV-specific cells were monitored over time for CFSE fluorescence. Plots are gated on donor tetramer+ cells. Values indicate the percentage of cells within the marked regions. (D) CFSE- labeled memory P14 cells were transferred to naive congenic mice (n = 3) or congenic mice infected by PyV 200 d earlier (n = 4). Spleens of secondary hosts were analyzed 36 d later. Plots are gated on P14 cells.
To test this hypothesis, we induced partial hematopoietic chimerism in persistently infected mice using congenic bone marrow (Fig. 3 A). Such animals now have stem cells that give rise to a population of naive CD8 T cells distinguished either by host or donor allelic differences (Fig. 3 B). This protocol permits stem cell engraftment without irradiation, thereby limiting the perturbance of viral load or established T cell populations, as preexisting PyV-specific T cells are not affected by busulfan treatment alone (unpublished data) (7). Moreover, the emergence of donor naive T cells beginning at ∼1 mo after busulfan conditioning is thymus dependent (16). Additionally, this protocol does not replace all bone marrow stem cells, and the population of newly developing T cells will be a mix of donor and host cells. 50 d after infusion of congenic bone marrow in busulfan-conditioned mice infected by PyV 115 d previously, animals were analyzed for PyV-specific CD8 T cells. As shown in Fig. 3 C, host-derived tetramer+ cells were detected in chimeric animals. Interestingly, a population of PyV-specific CD8 T cells originating from the donor population was also detected. This was not caused by contaminating naive or memory CD8 T cells in the donor cell inoculum, because transfer of the same number of bone marrow cells or splenocytes without busulfan did not generate a detectable donor cell population (Fig. 3 B). It has been estimated that thymic education occurs over the course of 3–4 wk (17). This is consistent with the inability to detect donor antigen-specific T cells earlier than 30 d after busulfan conditioning (unpublished data). Recruitment of recent thymic emigrants during the persistent stage of infection was not limited to PyV infection. We performed the same chimeric induction protocol in mice infected 120 d earlier by LCMV clone 13; at this time point, LCMV is only detected in the kidneys and brain (3). It is important to mention that this LCMV–mouse model is different from other models of persistent LCMV infection in which virus is detectable in the thymus (e.g., congenitally infected carrier mice, CD4-depleted LCMV clone 13–infected adult mice, and LCMV clone 13–infected adult mice approximately day 30 after infection) (18, 19), but it more closely approximates the low-level viral replication seen in persistent PyV infection and many persistent viral infections in humans. Fig. 3 D depicts the presence of donor and host LCMV-specific CD8 T cells in chimeric animals.
Figure 3.
Priming of new, naive antiviral CD8 T cells during persistent infection. (A) Partial bone marrow chimerism protocol. (B) Mice infected by PyV 84 d earlier were either injected with splenocytes or bone marrow cells or treated with busulfan and injected with bone marrow cells the next day (n = 3–8 mice). Spleens were analyzed 42 d later and assessed for chimerism. Each plot contains 105 events, and values represent the percentage of lymphocytes within the indicated regions. (C) Mice infected by PyV 115 d earlier were treated with busulfan and injected with bone marrow cells as in A. Organs were analyzed for Db-LT359 tetramer+ CD8 T cells 51 d after cell transfer. Plots are gated on donor or host CD8 T cells, and values indicate the percentage of tetramer+ cells. 10 and 8% of tetramer+ cells are of donor origin in the spleen and lung, respectively. (D) Mice infected by LCMV clone 13 120 d earlier were treated with busulfan and received bone marrow cells as in A. Organs were analyzed for Db-gp276 tetramer+ CD8 T cells 48 d after cell transfer (n = 6). Plots are gated on host or donor CD8 T cells, and values represent the percentage of CD8 T cells that are tetramer+. 7.5 and 9.4% of tetramer+ cells are of donor origin in the liver and lung, respectively. (E) Naive mice were thymectomized (thymx; n = 8) or sham operated (sham; n = 6). 45 d after PyV infection, spleens were analyzed for tetramer+ cells. The experiment was repeated twice with a combined p-value of 0.04.
To assess the contribution of newly primed T cells to the long-term pool of antiviral CD8 T cells, we thymectomized naive mice and determined the number of PyV-specific CD8 T cells after infection. Thymectomy significantly reduced the number of tetramer+ CD8 T cells (Fig. 3 E). This is consistent with our interpretation that continuous priming of new thymic emigrants is required for the maintenance of the population of virus-specific CD8 T cells. Our data also support the conclusions of a recent study exploring the contribution of thymic emigrants to the CD4 T cell response, where prolonged antigen presentation occurs, despite the lack of infectious virus (20).
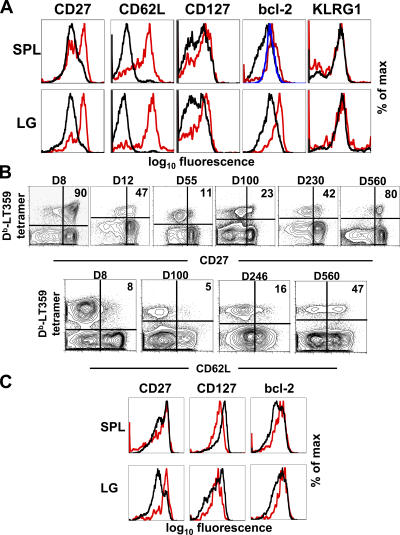
Because the T cell priming environment may vary between acute and persistent phases of infection and differentially affect T cell programming, we also analyzed PyV-specific CD8 T cells for the expression of markers of differentiation. As shown in Fig. 4 A, there are variations in expression of CD27, CD62L, CD127, and bcl-2 between the cell populations primed at different times. When the expression of either CD27 or CD62L is monitored over time on the bulk population of PyV-specific T cells, an interesting pattern emerges (Fig. 4 B). At the peak of CD8 T cell expansion, the majority of cells are CD27hi. These cells quickly down-regulate surface expression of this molecule, but the proportion of CD27hi cells progressively increases over time. By 560 d after infection, the majority of PyV-specific CD8 T cells are CD27hi. A similar pattern is observed with CD62L. This is consistent with the concept that newly primed T cells, being CD27hi and CD62Lhi (Fig. 4 A), are productively contributing to the pool of antigen-specific CD8 T cells and modify the phenotype of the entire virus-specific CD8 T cell population. Current evidence suggests that CD27lo T cells do not regain expression of CD27 and preferentially die (21). The data in Fig. 4 (A and B) are in line with the interpretation that the CD27lo PyV-specific CD8 T cell population is undergoing progressive attrition. Because PyV antigen is present up to 300 d after infection (Fig. 1 A), it is likely that antigen modifies the population of tetramer+ cells in at least two ways: antigen is required to prime recent thymic emigrants, which have a CD27hi CD62Lhi phenotype, but antigen can also induce down-regulation of CD27 and CD62L. Antigen-independent events may modulate the expression of these molecules as well.
Figure 4.
Dynamic phenotype of PyV-specific CD8 T cells. Mice infected by PyV 35 (A) or 95 (C) d earlier received busulfan and bone marrow cells as in Fig. 3 A. Organs were analyzed for tetramer+ CD8 T cells 45 (A) or 140 (C) d after cell transfer. Plots are gated on tetramer+ cells. Host cells (black), donor cells (red), and host CD44lo CD8 T cells (blue) are shown. In A, 6–7% of tetramer+ cells are of donor origin; in C, 5–6% of tetramer+ cells are of donor origin (n = 3–8 mice). SPL, spleen; LG, lung. (B) CD27 and CD62L expression on splenic tetramer+ cells over time. Plots are gated on CD8 T cells, and values in plots indicate the percentage of tetramer+ cells that express high levels of either CD27 or CD62L. The plot for CD62L at day 246 is the same as in Fig. 1 C (n = 3–12 mice per time point).
Newly recruited antiviral CD8 T cells may differ qualitatively from their older counterparts. We have found that the number of newly primed cells making IFN-γ closely corresponds to the number of Db-LT359 tetramer+ cells (unpublished data). A comprehensive comparison of the functional attributes of new and old antiviral CD8 T cells should prove particularly informative in elucidating the contribution of each cell population to viral control.
Analysis of chimeric animals at late time points (Fig. 4 C) reveals a narrowing in the phenotype of virus-specific CD8 T cells primed over the course of persistent infection (compare with Fig. 4 A). Although some differences are still apparent, such as bcl-2 levels, by 140 d after induction of chimerism, splenic CD27 levels by host and donor PyV-specific T cells are similar. Because chimerism induction does not displace the original host stem cells (Fig. 3 B), new naive T cells can originate from either donor or recipient precursors. The priming environment is also dynamic over time in terms of antigen load and virus-associated inflammation, with these changes affecting the strength and frequency of T cell activation and, therefore, longevity. Jelley-Gibbs et al. recently demonstrated that influenza virus–specific CD4 T cells primed late in the response survive better than those recruited earlier, suggesting that early and protracted antigen encounter is detrimental to T cell survival (20). Collectively, these findings imply that many of the virus-specific CD8 T cells late in persistent infection may be derived from more recently primed cells.
Recent studies indicate a dependence on antigen for maintenance of antiviral CD8 T cells in chronic infections, although there are some exceptions (8–10, 22). Our data demonstrate that new, naive virus-specific CD8 T cells are primed to viral antigens long after initial infection. Priming of newly emerging CD8 T cells to persisting antigens has been suggested before, and a recent report shows de novo CD4 T cell responses to chronic infections in baboons after autologous bone marrow transplant (23–25). In a chronic LCMV infection model, continuous thymic output does not appear to be required to maintain CD8 T cell responses, although a T cell deficit was observed at day 30 after infection (26). In contrast, thymectomy is associated with loss of CTL activity in cats persistently infected by a feline lentivirus (27). Our data are consistent with reports describing antigen dependence for chronic pathogen-specific T cell maintenance, especially as newly primed cells may be important for preserving the population of virus-specific T cells (Fig. 3 E) (8–12). Moreover, the new, naive antigen-specific CD8 T cells are able to survive thymic education, despite persisting antigen, and exit to the periphery.
It has been suggested that memory-like CD8 T cells in chronic infections go through an abortive differentiation pathway, as dissected through phenotypic analysis of CD8 T cells in infected individuals (4, 15, 28). The lineage relationship between these stages is comprehensible only if the end cell stems from the beginning cell or its progeny. Our data indicate that new naive cells are continuously being recruited during the chronic phase of infection and that the time point of priming influences the phenotype of CD8 T cells. These observations affect our understanding of T cell differentiation pathways during chronic infections, as the T cell population analyzed will potentially contain cells primed at any time after infection.
In summary, we propose that ongoing selection and priming of naive CD8 T cells during persistent infection contributes to the maintenance of chronic memory-like cells. Phenotypic heterogeneity indicates programming differences in cells primed more recently, and their continual entry into the virus-specific T cell reservoir complicates interpretation of current models of lineage differentiation. In other situations in which antigen persists, such as cancer, autoimmunity, and organ transplantation, it will be important to address whether naive T cells continue to be recruited. Our findings may offer new avenues for therapeutic vaccination, as newly recruited cells may be more amenable to boosting strategies.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) female mice were purchased from the National Cancer Institute. B6/CD45.1 female mice were purchased from Taconic or bred in our facility. P14 transgenic mice bearing the TCR specific for the gp33-41 epitope of LCMV were bred on a B6/CD90.1 background in our facility. All mice were used between 4 and 10 wk of age. Thoracic thymi were removed from anesthetized mice, which were rested for at least 2 wk before infection. All protocols for animal studies were approved by the Institutional Animal Care and Use Committee of Emory University.
Viral DNA detection.
DNA was extracted from frozen tissue using a DNA mini kit (QIAamp; QIAGEN). PyV genomic copy numbers were determined by quantitative PCR (TaqMan; Glen Research Corporation), as described previously (7). PyV DNA quantity is expressed in genome copies per milligram of tissue and is calculated based on a standard curve of known PyV genome copy numbers versus threshold cycle of detection. The detection limit with this assay is 10 copies of genomic viral DNA.
Viruses and cell transfers.
Mice were infected with 2 × 106 PFU of PyV (strain A2) s.c.; 2 × 106 PFU LCMV clone 13 was administered i.v. Spleens from PyV-infected mice were harvested at the indicated time points and depleted of IgG+ cells by panning on plates coated with anti-IgG antibodies (The Jackson Laboratory). Cells were labeled with 5 μM CFSE (Invitrogen) for 10 min at 37°C. 50 × 106 CFSE-labeled cells were transferred to infection-matched congenic hosts. Naive B6/CD90.2 mice received 5 × 105 naive P14 cells i.v. 1 d before i.p. infection by 2 × 105 PFU of LCMV-Armstrong. 120 d after infection, memory P14 cells were isolated from spleens and labeled with CFSE, and 20 × 106 cells were transferred to naive B6/CD90.2 mice or B6/CD90.2 mice infected by PyV 200 d earlier.
Cell isolation and flow cytometry.
Femurs and tibias were crushed to isolate bone marrow cells. Single cell suspensions were made of RBC-lysed spleens. Animals were perfused before lung and liver isolation. Lung pieces were treated with EDTA and digested with collagenase with subsequent density centrifugation to isolate lymphocytes, as previously described (29). Single-cell suspensions were made from liver by passing tissue through nylon mesh, followed by density centrifugation to isolate lymphocytes (29). Antibodies to CD8, CD90.1, and bcl-2 were purchased from Becton Dickinson and used according to the manufacturer's specifications. Antibodies to CD27, CD45.1, CD45.2, CD62L, and CD127 were purchased from eBioscience. Antibodies to KLRG1 were purchased from Southern Biotechnology Associates, Inc. Tetramers were constructed as previously described (7). All samples were collected on a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc.).
Bone marrow chimerism induction.
Mice persistently infected either by PyV or LCMV clone 13 at least 35 d earlier received 600 μg busulfan i.p. (Busulfex; Orphan Medical). The next day, 25 × 106 cells isolated from the bone marrow of congenic mice were injected i.v.
Statistical analysis.
Statistical significance for individual experiments was determined using the unpaired Student's t test. The Mack-Skillings test was used to determine statistical significance of multiple experiments. A p-value ≤0.05 was considered statistically significant.
Acknowledgments
We wish to thank Barry Rouse for his comments and Andrew Hill for statistical help.
This work was supported by grants from the National Institutes of Health (F32CA106090 to V. Vezys; RO1AI040519 and PO1AI44644 to C.P. Larsen; R37AI30048 to R. Ahmed; and RO1CA71971 and RO1CA100644 to A.E. Lukacher) and a postdoctoral fellowship from the Cancer Research Institute (to D. Masopust).
The authors have no conflicting financial interests.
References
- 1.Shoukry, N.H., A. Grakoui, M. Houghton, D.Y. Chien, J. Ghrayeb, K.A. Reimann, and C.M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz, J.E., M.J. Kuroda, S. Santra, V.G. Sasseville, M.A. Simon, M.A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B.J. Scallon, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 283:857–860. [DOI] [PubMed] [Google Scholar]
- 3.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hislop, A.D., N.E. Annels, N.H. Gudgeon, A.M. Leese, and A.B. Rickinson. 2002. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreansky, S., H. Liu, H. Adler, U.H. Koszinowski, S. Efstathiou, and P.C. Doherty. 2004. The limits of protection by “memory” T cells in Ig−/− mice persistently infected with a γ−herpesvirus. Proc. Natl. Acad. Sci. USA. 101:2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierro, S., R. Rothkopf, and P. Klenerman. 2005. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 35:1113–1123. [DOI] [PubMed] [Google Scholar]
- 7.Kemball, C.C., E.D. Lee, V. Vezys, T.C. Pearson, C.P. Larsen, and A.E. Lukacher. 2005. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J. Immunol. 174:7950–7960. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 9.Wherry, E.J., D.L. Barber, S.M. Kaech, J.N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 101:16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman, F., T. Heller, Y. Sobao, E. Mizukoshi, M. Nascimbeni, H. Alter, S. Herrine, J. Hoofnagle, T.J. Liang, and B. Rehermann. 2004. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 40:87–97. [DOI] [PubMed] [Google Scholar]
- 11.Oxenius, A., D.A. Price, P.J. Easterbrook, C.A. O'Callaghan, A.D. Kelleher, J.A. Whelan, G. Sontag, A.K. Sewell, and R.E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 97:3382–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casazza, J.P., M.R. Betts, L.J. Picker, and R.A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 14.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 15.Appay, V., and S.L. Rowland-Jones. 2004. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin. Immunol. 16:205–212. [DOI] [PubMed] [Google Scholar]
- 16.Williams, M.A., A.B. Adams, M.B. Walsh, N. Shirasugi, T.M. Onami, T.C. Pearson, R. Ahmed, and C.P. Larsen. 2003. Primary and secondary immunocompetence in mixed allogeneic chimeras. J. Immunol. 170:2382–2389. [DOI] [PubMed] [Google Scholar]
- 17.Porritt, H.E., K. Gordon, and H.T. Petrie. 2003. Kinetics of steady-state differentiation and mapping of intrathymic signaling environments by stem cell transplantation in nonirradiated mice. J. Exp. Med. 198:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson, B.D., T. Somasundaram, and R. Ahmed. 1991. Abrogation of tolerance to a chronic viral infection. J. Immunol. 147:3521–3529. [PubMed] [Google Scholar]
- 19.Zajac, A.J., J.N. Blattman, K. Murali-Krishna, D.J. Sourdive, M. Suresh, J.D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jelley-Gibbs, D.M., D.M. Brown, J.P. Dibble, L. Haynes, S.M. Eaton, and S.L. Swain. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsenbein, A.F., S.R. Riddell, M. Brown, L. Corey, G.M. Baerlocher, P.M. Lansdorp, and P.D. Greenberg. 2004. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J. Exp. Med. 200:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaph, C., J. Uzonna, S.M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 10:1104–1110. [DOI] [PubMed] [Google Scholar]
- 23.Muraro, P.A., D.C. Douek, A. Packer, K. Chung, F.J. Guenaga, R. Cassiani-Ingoni, C. Campbell, S. Memon, J.W. Nagle, F.T. Hakim, et al. 2005. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lurquin, C., B. Lethe, E. De Plaen, V. Corbiere, I. Theate, N. van Baren, P.G. Coulie, and T. Boon. 2005. Contrasting frequencies of antitumor and antivaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J. Exp. Med. 201:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalina, T., H. Lu, Z. Zhao, E. Blewett, D.P. Dittmer, J. Randolph-Habecker, D.G. Maloney, R.G. Andrews, H.P. Kiem, and J. Storek. 2005. De novo generation of CD4 T cells against viruses present in the host during immune reconstitution. Blood. 105:2410–2414. [DOI] [PubMed] [Google Scholar]
- 26.Miller, N.E., J.R. Bonczyk, Y. Nakayama, and M. Suresh. 2005. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J. Virol. 79:9419–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes, K.A., S. Koksoy, A.J. Phipps, W.R. Buck, G.J. Kociba, and L.E. Mathes. 2005. Lentivirus-specific cytotoxic T-lymphocyte responses are rapidly lost in thymectomized cats infected with feline immunodeficiency virus. J. Virol. 79:8237–8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champagne, P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]
- 29.Masopust, D., V. Vezys, E.J. Usherwood, L.S. Cauley, S. Olson, A.L. Marzo, R.L. Ward, D.L. Woodland, and L. Lefrancois. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875–4882. [DOI] [PubMed] [Google Scholar]