Abstract
CD40 was initially identified as a receptor expressed by B cells that is crucial for inducing an effective adaptive immune response. CD40 was subsequently shown to be expressed by endothelial cells and to promote angiogenesis. New data now show that in tumor-prone transgenic mice, CD40-mediated neovascularization is essential for early stage tumorigenicity. This suggests, at least in this mouse model, that CD40 has an important role in the angiogenic process that is coupled to carcinogenesis, a finding that could lead to novel therapeutic opportunities.
The development of cancer is a complex process (1); both the tumor cells and the tumor environment must fulfill several requirements to enable a transformed cell to grow into a primary tumor and, eventually, to metastasize to distant organs. The fate of a tumor is determined both by the immune response against the tumor and the vasculature of the host. The immune system can attack and destroy transformed cells, whereas newly formed vessels in the tumor unwittingly support the survival and growth of tumor cells by supplying them with oxygen and nutrients. CD40 is a cell surface protein that is involved in both immune responses to tumors and in angiogenesis and vascularization. The article by Chiodoni et al. (on p. 2441 in this issue [2]) shows that the overriding role of CD40 in a tumor-prone transgenic mouse model of breast cancer is to promote neovascularization.
The two faces of CD40
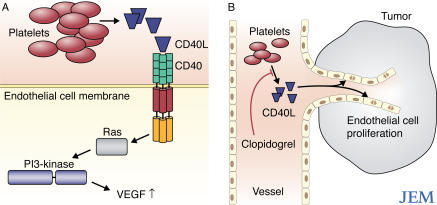
CD40 is expressed by all mature B cells, as well as by dendritic cells, macrophages, fibroblasts, epithelial cells, and endothelial cells (3, 4). CD40 is crucial for the induction of effective adaptive immune and inflammatory responses (5). The interaction between CD40 and CD40 ligand (CD40L; also known as CD154), which is expressed by activated T cells, activated B cells, and activated platelets, promotes both humoral and cell-mediated immune responses. Indeed, studies using CD40-deficient mice show that CD40 has an essential role in T cell–dependent immunoglobulin class switching, memory B cell development, and germinal center formation (6, 7). Engagement of CD40 by CD40L on the surface of human endothelial cells induces the activation of Ras and phosphatidylinositol 3–kinase (PI3; Fig. 1 A). Activation of this signaling pathway leads to the expression of several angiogenic factors—such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2)—and promotes angiogenesis in xenografts in vivo (5, 8, 9).
Figure 1.
CD40 signaling regulates angiogenesis. (A) CD40-induced signaling pathway in endothelial cells. Interaction of CD40L with CD40 on the surface of endothelial cells results in activation of the Ras signaling pathway. Ras associates with PI3 to induce the expression of VEGF, which in turn stimulates angiogenesis. Platelets are likely to be a major, but not unique, source of CD40L (see The two faces of CD40). (B) Platelets induce neoangiogenesis. Activation of platelets causes them to release a soluble form of CD40L, which can bind CD40 on the surface of endothelial cells. Stimulation of CD40 on endothelial cells activates them and, in a tumor, leads to neovascularization of the tumor. The antiplatelet drug Clopidogrel blocks CD40L secretion by platelets and thereby inhibits neovascularization.
These two functions of CD40, in promoting both immune responses and angiogenesis, have contradictory effects on the growth of a tumor. Tumor-specific immune responses limit tumor growth, whereas increased angiogenesis supports tumor growth by providing nutrients to the tumor cells and providing them with a route to distant organs. However, although CD40 is expressed by blood vessels of renal cell carcinomas (10) and Kaposi's sarcoma (11), so far a requirement for CD40 in neovascularization of tumors has not been established experimentally. Determining whether CD40 has more of an effect on antitumor immune responses or tumor vascularization may have important implications for the design and implementation of therapeutics that target this molecule.
Which face of CD40 leads the way?
To test the role of CD40 in the development of mammary carcinomas, Chiodoni et al. crossed transgenic mice expressing the Her2/neu oncogene under the control of the mouse mammary tumor virus promoter with CD40-deficient mice, and compared tumor onset and progression in these mice with that in CD40-sufficient Her2/neu-transgenic mice (2). Remarkably, the analysis of tumors in the CD40-deficient transgenic mice showed increased latency, reduced numbers of tumors, and decreased total tumor size in comparison to the CD40-sufficient mice. These findings were surprising. Because CD40 is crucial in mounting an immune response, one would predict that mice lacking this protein would be more prone to tumors than their CD40-sufficient counterparts.
The reduced tumorigenicity was not a result of a functional defect in CD40-deficient bone marrow–derived cells in the tumor microenvironment. Rather, a lack of CD40 on endothelial cells impaired tumor angiogenesis, leading to a block in tumor growth. Indeed, in Matrigel implantation assays, the authors found that the interaction between CD40L (or an antibody specific for CD40) and CD40 on mouse endothelial cells triggered vessel formation in vivo. When the formation of tumor-associated blood vessels was analyzed in the mice, striking defects in the size, number, and organization of these vessels were observed in the absence of CD40 (2).
These data suggest, at least in this model, that CD40 is required to establish a blood supply for the tumor. The authors next investigated CD40L expression in the tumor tissue. It is well known that CD40L is expressed by activated CD4+ T cells (12, 13). More recently, CD40L has also been detected on human platelets, which release the soluble form of CD40L upon activation (14, 15). Because CD4+ T cells are absent from the tumors in Her2/neu-transgenic mice, the authors focused on platelets. Treatment of CD40-sufficient Her2/neu-transgenic mice with Clopidogrel, a drug that blocks platelet aggregation and the release of soluble CD40L (16, 17), decreased both the tumor size and number. These data led the authors to suggest that the effect of Clopidogrel on tumor growth is probably caused by the inhibition of platelet activation and, therefore, the inhibition of CD40L release (Fig. 1 B).
The tumor environment and tumorigenesis
The tumor microenvironment has a major impact on tumor growth, invasion into the bloodstream, and metastasis. Different cell populations present in the microenvironment affect these processes in distinct ways. For example, recent studies (18, 19) have shown that myeloid cells within tumors are not passive structural elements. Rather, these cells can take up tumor antigens and, upon maturation and activation, migrate to draining lymph nodes, where they can elicit a tumor-specific immune response. In contrast, activation of bone marrow–derived cells in the tumor can induce an inflammatory reaction, which either promotes or inhibits tumorigenesis. Furthermore, bone marrow–derived progenitors in the tumor can generate endothelial cells and hematopoietic cells that support tumor growth and metastasis to different organs through angiogenesis (20).
Soluble factors in the tumor micro-environment also influence tumor growth and metastasis. Neovascularization, for example, is regulated by factors that either promote or inhibit angiogenesis. VEGF, FGF1, and FGF2 are known to trigger angiogenesis by binding to tyrosine kinase receptors on endothelial cells, inducing proliferation of the vasculature (21, 22). A prototypical angiogenesis inhibitor is thrombospondin-1, which binds CD36, a transmembrane receptor expressed by endothelial cells that is coupled to intracellular SRC-like tyrosine kinases (23). This signaling pathway leads to the expression of Fas ligand (also known as CD95L) by endothelial cells and their subsequent apoptosis (23).
In general, the relative amounts of the different activators and inhibitors dictate whether an endothelial cell will be in a quiescent or proangiogenic state. During tumor development, neovascularization is initiated by an “angiogenic switch,” which alters the appropriate balance between the activating and inhibitory signals received by an endothelial cell and causes the endothelial cells to proliferate (24).
Until recently, the predominant view on the vascularization of a tumor was “tumor centric,” whereby the tumor induces angiogenesis by either secretion of proangiogenic factors and/or by transcriptional down-regulation of the expression of angiogenic inhibitors. Now, the observations of Chiodoni et al. (2), along with other recent publications (25, 26), show that the induction of proangiogenic factors by the tumor alone is not sufficient. Rather, the tumor and its environment have a dynamic relationship that results in neovascularization and the promotion of tumor growth.
The importance of timing
The report by Chiodoni et al. (2) also demonstrates that the same protein can have different functions in distinct physiological compartments in the tumor environment and, thus, can either promote or inhibit tumorigenesis. In the breast cancer model used by Chiodoni et al., the proangiogenic role of CD40 is dominant over its role in generating an antitumor immune response, thus promoting tumor progression (2). The opposite was true, however, when the authors transplanted fast-growing tumor cell lines derived from Her2/neu-transgenic mice into CD40-deficient or CD40-sufficient mice. Under these conditions, more aggressive tumors developed in CD40-deficient mice than in CD40-sufficient mice (2). In this transplant model of breast cancer, the transplanted tumor cells have already been selected for tumorigenic and malignant potential, and the immunological role of CD40 prevailed over the proangiogenic effect. Hence, it is not possible to focus only on the proangiogenic aspect of CD40 function and to neglect its role in immunosurveillance. This study seems to indicate that the involvement of CD40 in immune responses is more important during later stages of tumorigenesis.
The absence of CD40 might therefore have different outcomes on tumor growth depending on the stage of tumor development. In this breast cancer model, a lack of CD40 before tumors arise inhibited tumor growth, whereas a lack of CD40 at later stages of tumor development enhanced tumorigenicity and caused a more severe outcome. However, this conclusion is somewhat surprising, as it is generally assumed that advanced tumors are more dependent on neovascularization than early stage tumors. It is therefore essential to clearly define the tumor stage at which the switch from CD40-dependent vascularization to CD40-independent vascularization takes place and to understand what causes CD40 independence of late-stage tumors.
Based on the observations of Chiodoni et al. (2), it is conceivable that the activation of endothelial cells by CD40 is an early event in tumorigenesis, whereas multiple angiogenic stimuli become available later in tumor progression and render CD40 expression by endothelial cells dispensable. It is now necessary to identify these other angiogenic factors and to understand how they stimulate vascularization independently of CD40.
Experiments with antiangiogenesis factors in a mouse model of pancreatic islet carcinogenesis have shown that the efficacy of angiogenesis inhibitors depends on the stage of tumor development: some inhibitors of angiogenesis are successful for the treatment of early stage disease but do not cause regression of end-stage tumors, whereas others are effective at reducing the mass of end-stage tumors but have no effect on preventing progression during early stage disease (27). Therefore, the actual mechanism of tumor neovascularization seems to depend on the stage of tumor development, and specific pro- and antiangiogenic factors seem to be relevant at different stages of the disease. It is interesting to speculate that the responsiveness of tumor-associated endothelial cells to stimulation through CD40 and/or their level of expression of CD40 have a role in mediating the outcome of different antiangiogenesis therapies.
The complexities of targeted tumor therapy against cancer
Another important observation of the paper by Chiodoni et al. (2) is the possible identification of the cells that are responsible for CD40-triggered angiogenesis. Because treatment of CD40-sufficient Her2/neu-transgenic mice with the antiplatelet drug Clopidogrel reduced tumor growth, it seems probable that platelets are responsible for CD40-triggered angiogenesis in this model. However, cell types other than platelets also express CD40L, and these cells could activate CD40 if located in a tumor. The ability of such cells to reside in the tumor or be recruited to a tumor could differ depending on the type of tumor or the stage of tumor development. Indeed, although T cells were absent from the tumors analyzed by Chiodoni et al., T cells are often found in leukocyte infiltrates in human breast carcinoma (28). Therefore, in different breast cancer models T cells could have a more prominent role as CD40L-expressing cells in the tumor. Furthermore, as endothelial cells express CD40L (29), one could envision an “autocrine” CD40–CD40L stimulating circuit within the endothelium.
Tumor angiogenesis offers an attractive target for the treatment of individuals with cancer, and several antiangiogenic drugs are already in use, albeit with variable therapeutic success (30). A combinatorial approach to treatment that targets both cancer cells and the surrounding stroma might prove more promising than using single antiangiogenic agents.
Chiodoni et al. suggest a novel and intriguing approach to the treatment of cancer: the use of anticoagulants to prevent the interaction between platelets and endothelial cell progenitors in the tumor, thereby blocking the CD40–CD40L interaction that causes platelets to promote tumor angiogenesis. So far, therapies targeting CD40 have been designed to trigger CD40 signaling and thus boost the immune response against the tumor (31). But this approach might be a double-edged sword, because promoting CD40 expression might also promote angiogenesis.
Importantly, as in the previous paragraph, the effect of CD40 loss on tumorigenesis differs depending on the stage of tumor development, which therefore has implications for therapy. Although down-regulation of CD40 expression might be desirable during early tumor development, it could have catastrophic consequences at later stages, when a tumor is more aggressive, by inhibiting the tumor-specific immune response. It is also important to point out that this study focuses exclusively on mammary tumors. Although the authors show that CD40 is required for neovascularization and growth of carcinomas in a model of breast cancer, other tumor types, especially more aggressive and faster growing tumors, might not be dependent on CD40. For this reason, the use of a CD40-based therapy for cancer is premature at this stage. Nevertheless, this study clearly highlights the fact that a targeted therapy has to be tailored not only to the genetic makeup of the tumor but also to the particular stage of tumor progression and the intricate relationship between the tumor and its stromal environment.
S.B. and P.P.P. are at the Cancer Biology and Genetics Program, Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, NY 10021.
References
- 1.Hanahan, D., and R.A. Weinberg. 2000. The hallmarks of cancer. Cell. 100:57–70. [DOI] [PubMed] [Google Scholar]
- 2.Chiodoni, C., M. Iezzi, C. Guiducci, S. Sangaletti, I. Alessandrini, C. Ratti, F. Tiboni, P. Musiani, D.N. Granger, and M.P. Colombo. 2006. Triggering CD40 on endothelial cells contributes to tumor growth. J. Exp. Med. 203:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Kooten, C., and J. Banchereau. 2000. 1998. CD40-CD40 ligand. J. Leukoc. Biol. 67:2–17. [DOI] [PubMed] [Google Scholar]
- 4.Grewal, I.S., and R.A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135. [DOI] [PubMed] [Google Scholar]
- 5.Melter, M., M.E. Reinders, M. Sho, S. Pal, C. Geehan, M.D. Denton, D. Mukhopadhyay, and D.M. Briscoe. 2000. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 96:3801–3808. [PubMed] [Google Scholar]
- 6.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1:167–178. [DOI] [PubMed] [Google Scholar]
- 7.Castigli, E., F.W. Alt, L. Davidson, A. Bottaro, E. Mizoguchi, A.K. Bhan, and R.S. Geha. 1994. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc. Natl. Acad. Sci. USA. 91:12135–12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinders, M.E., M. Sho, S.W. Robertson, C.S. Geehan, and D.M. Briscoe. 2003. Proangiogenic function of CD40 ligand-CD40 interactions. J. Immunol. 171:1534–1541. [DOI] [PubMed] [Google Scholar]
- 9.Flaxenburg, J.A., M. Melter, P.H. Lapchak, D.M. Briscoe, and S. Pal. 2004. The CD40-induced signaling pathway in endothelial cells resulting in the overexpression of vascular endothelial growth factor involves Ras and phosphatidylinositol 3-kinase. J. Immunol. 172:7503–7509. [DOI] [PubMed] [Google Scholar]
- 10.Kluth, B., S. Hess, H. Engelmann, S. Schafnitzel, G. Riethmuller, and H.E. Feucht. 1997. Endothelial expression of CD40 in renal cell carcinoma. Cancer Res. 57:891–899. [PubMed] [Google Scholar]
- 11.Pammer, J., A. Plettenberg, W. Weninger, B. Diller, M. Mildner, A. Uthman, W. Issing, M. Sturzl, and E. Tschachler. 1996. CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi's sarcoma. Am. J. Pathol. 148:1387–1396. [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal, I.S., and R.A. Flavell. 1996. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol. Today. 17:410–414. [DOI] [PubMed] [Google Scholar]
- 13.Grewal, I.S., H.G. Foellmer, K.D. Grewal, J. Xu, F. Hardardottir, J.L. Baron, C.A. Janeway Jr., and R.A. Flavell. 1996. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 273:1864–1867. [DOI] [PubMed] [Google Scholar]
- 14.Henn, V., J.R. Slupsky, M. Grafe, I. Anagnostopoulos, R. Forster, G. Muller-Berghaus, and R.A. Kroczek. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 391:591–594. [DOI] [PubMed] [Google Scholar]
- 15.Buchner, K., V. Henn, M. Grafe, O.J. de Boer, A.E. Becker, and R.A. Kroczek. 2003. CD40 ligand is selectively expressed on CD4+ T cells and platelets: implications for CD40-CD40L signaling in atherosclerosis. J. Pathol. 201:288–295. [DOI] [PubMed] [Google Scholar]
- 16.Schafer, A.I. 1996. Antiplatelet therapy. Am. J. Med. 101:199–209. [DOI] [PubMed] [Google Scholar]
- 17.Hermann, A., B.H. Rauch, M. Braun, K. Schror, and A.A. Weber. 2001. Platelet CD40 ligand (CD40L)–subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 12:74–82. [DOI] [PubMed] [Google Scholar]
- 18.Condeelis, J., and J.W. Pollard. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263–266. [DOI] [PubMed] [Google Scholar]
- 19.Yuan, A., J.J. Chen, P.L. Yao, and P.C. Yang. 2005. The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. 10:853–865. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan, R.N., R.D. Riba, S. Zacharoulis, A.H. Bramley, L. Vincent, C. Costa, D.D. MacDonald, D.K. Jin, K. Shido, S.A. Kerns, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 438:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veikkola, T., and K. Alitalo. 1999. VEGFs, receptors and angiogenesis. Semin. Cancer Biol. 9:211–220. [DOI] [PubMed] [Google Scholar]
- 22.Roy, H., S. Bhardwaj, and S. Yla-Herttuala. 2006. Biology of vascular endothelial growth factors. FEBS Lett. 580:2879–2887. [DOI] [PubMed] [Google Scholar]
- 23.Ge, Y., and M.T. Elghetany. 2005. CD36: a multiligand molecule. Lab. Hematol. 11:31–37. [DOI] [PubMed] [Google Scholar]
- 24.Bergers, G., and L.E. Benjamin. 2003. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 3:401–410. [DOI] [PubMed] [Google Scholar]
- 25.De Raeve, H.R., and K. Vanderkerken. 2005. The role of the bone marrow microenvironment in multiple myeloma. Histol. Histopathol. 20:1227–1250. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Munoz, W., I. Kim, and R. Khokha. 2006. TIMP-3 deficiency in the host, but not in the tumor, enhances tumor growth and angiogenesis. Oncogene. 25:650–655. [DOI] [PubMed] [Google Scholar]
- 27.Bergers, G., K. Javaherian, K.M. Lo, J. Folkman, and D. Hanahan. 1999. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 284:808–812. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Baruch, A. 2003. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 5:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mach, F., U. Schonbeck, G.K. Sukhova, T. Bourcier, J.Y. Bonnefoy, J.S. Pober, and P. Libby. 1997. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA. 94:1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmeliet, P. 2005. Angiogenesis in life, disease and medicine. Nature. 438:932–936. [DOI] [PubMed] [Google Scholar]
- 31.Hill, S.C., S.J. Youde, S. Man, G.R. Teale, A.J. Baxendale, A. Hislop, C.C. Davies, D.M. Luesley, A.M. Blom, A.B. Rickinson, et al. 2005. Activation of CD40 in cervical carcinoma cells facilitates CTL responses and augments chemotherapy-induced apoptosis. J. Immunol. 174:41–50. [DOI] [PubMed] [Google Scholar]