Abstract
Follicular lymphoma is one of the most common adult lymphoma, and remains virtually incurable despite its relatively indolent nature. t(14;18)(q32;q21) translocation, the genetic hallmark and early initiating event of follicular lymphoma (FL) pathogenesis, is also present at low frequency in the peripheral blood of healthy individuals. It has long been assumed that in healthy individuals t(14;18) is carried by circulating quiescent naive B cells, where its oncogenic potential would be restrained. Here, we question this current view and demonstrate that in healthy individuals, t(14;18) is actually carried by an expanding population of atypical B cells issued from germinal centers, displaying genotypic and phenotypic features of FL, and prone to constitute potent premalignant FL niches. These findings strongly impact both on the current understanding of disease progression and on the proper handling of t(14;18) frequency in blood as a potential early biomarker for lymphoma.
The t(14;18)(q32;q21) translocation results from a mistake of V(D)J recombination and juxtaposes the BCL2 protooncogene with the nonexpressed immunoglobulin heavy chain (IGH) allele (1). As a consequence, the BCL2 gene comes under the control of the IGH enhancer, causing deregulated expression of the antiapoptotic BCL2 protein. Although t(14;18) and ectopic BCL2 expression constitute the initial events of follicular lymphoma (FL) pathogenesis, further B cell differentiation and additional events are clearly required for malignant transformation (2–4). However, early steps of disease progression are still unclear. The presence of the translocation in peripheral blood from healthy individuals (HI) at an average rate of 0,1–10 cell every million (5–7) led to the hypothesis that t(14;18) is carried by resting naive B cells. In such cells, BCL2 oncogenic potential would be restrained, partly because of the fact that they express BCL2 physiologically. Intriguingly, we recently reported that in absence of manifest clinical lymphoma, clonotypic t(14;18)+ cells can persist over a 3-yr period in the peripheral blood from HI (8). As peripheral blood contains a mixture of naive and memory B cells, this unexpected finding led us to question the current model of FL pathogenesis and characterize further such long-lived t(14;18)+ clones.
RESULTS AND DISCUSSION
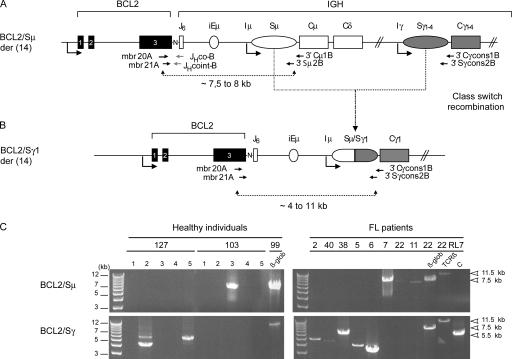
As a first approach, we sought to determine if t(14;18)+ cells in HI are indeed naive B cells by assessing their class-switch recombination (CSR) status. CSR is a hallmark of the germinal center (GC) reaction and is absent in naive cells. To characterize rare t(14;18)+ cells out of the bulk of normal cells, we took advantage of their unique BCL2/IGH translocation signature. Indeed, the BCL2/JH fusion does not prevent CSR occurring on the downstream constant region of the IGH locus (9, 10), and frequency above 10−5 (Table I., BCL2/JH) were selected from our previous cohort of HI (8), and two separate LR-PCR reactions were conducted: one designed to amplify the unswitched BCL2/Sμ region, and the other to amplify switched BCL2/Sγ regions (Fig.1). Results show that only two out of six individuals were found positive for BCL2/Sμ, whereas five out of six were positive for BCL2/Sγ (Fig. 1 C, left, and Table I). Accordingly, individual and cumulative ratios of amplicons were significantly higher for BCL2/Sγ (9:50) than for BCL2/Sμ (2:55) (P = 0.02). In addition, identical BCL2/JH junction signatures were found between BCL2/JH and BCL2/Sγ amplicons, but not with BCL2/Sμ amplicons (Table I, asterisks), indicating that most translocations previously detected in the blood of HI (5–8) are actually switched. Furthermore, clonal filiations between switched and unswitched cells were found in one sample (#102), supporting the presence of an active CSR process in the t(14;18)+ clones. Altogether, and contrary to previous assumptions, these data clearly show that most peripheral t(14;18)+ cells already underwent CSR, and therefore such cells are not naive B cells.
Table I.
Analysis of CSR on the t(14;18)+ translocated allele in peripheral blood from HI
| Sample | BCL2/JH
a
|
BCL2/Sμ
|
BCL2/Sγ
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detection | Ratio | Frequency | Detection | Ratio | Associated JH | Detection | Ratio | Associated JH | Isotype | |
| 99 | yes | 11:30 | 1.1 × 10−5 | no | 0:10 | - | yes | 1:5 | JH6* | γ1 |
| 102 | yes | 23:30 | 3.6 × 10−5 | yes | 1:5 | JH5 | yes | 1:5 | JH5 | γ1 |
| 103 | yes | 14:30 | 1.6 × 10−5 | yes | 1:10 | JH6b | no | 0:10 | - | - |
| 109 | yes | 12:30 | 1.3 × 10−5 | no | 0:10 | - | yes | 1:10 | JH? | γ2 |
| 120 | yes | 11:30 | 1.1 × 10−5 | no | 0:10 | - | yes | 2:10 | JH4* | γ1 |
| 127 | yes | 23:30 | 3.6 × 10−5 | no | 0:10 | - | yes | 4:10 | JH6* | γ2 |
| JH6* | γ1 | |||||||||
| Mean | 2.1 × 10−5 | |||||||||
| Total | 6:6 | 2:6 | 2:55b | 5:6 | 9:50b | |||||
Previously described in reference 8 Ratio, positive replicates:total replicates.
Cumulative occurrence of BCL2/Sμ junctions, as determined by the Pearson's χ2 test, was significantly lower than BCL2/Sγ junctions (P = 0.02).
BCL2/JH junction identical to the one previously found with the BCL2/JH PCR assay (8).
Figure 1.
Most t(14;18) translocations in HI are associated with CSR. The translocation region (here with a mbr/JH6 breakpoint) on the der(14) chromosome is depicted with either an unswitched (A) or a switched (B) configuration. PCR primers are indicated. Reverse 3′C/Sγ primers are consensus for the 4 γ isotypic subclass regions (Cγ1-4/S γ1-4). The sizes of the BCL2/Sμ amplicons are expected to range from 7.5 to 8 kb, depending on BCL2 breakpoints. As CSR is imprecise and can occur anywhere in the ∼3 kb Sμ and Sγ recombination sites (29), BCL2/Sγ amplicon sizes are expected to range from 4 to 11 kb. N, N nucleotides present in the BCL2/JH junction; ovals, switch regions upstream of each constant region (except Cδ). (C) BCL2/Sμ (top) and BCL2/Sγ (bottom) LR-PCR from two of the six HI (#127, #103; left) and from 8 of the 30 FL patients (right) are shown. 23 out of 30 FL samples underwent CSR to Cγ (not depicted). Given the low t(14;18) frequency level in HI, a two-step fluctuation nested LR-PCR assay was used, consisting of at least five parallel replicates per sample (left, lanes 1–5). In the fluctuation range, at most one target molecule is present per PCR replicate, and if so will give rise to a detectable amplicon. The ratio of positive versus negative replicates allows the estimation of the frequency of the target. A standard single round LR-PCR was used for the FL samples (right). The t(14;18)+ RL7 cell line harbors a Sγ1-associated BCL2/JH6 der(14), and was used as positive control for the BCL2/Sγ amplification (C+) and as negative control for the BCL2/Sμ (C−); PCR amplifications of the β-globin gene (β-glob, 7.5 kb) and of a Dβ1-Jβ2.7 germline fragment of the human TCR-β locus (TCR-β, 11.5 kb) were performed on each sample as controls for LR-PCR. In case #127, BCL2/Sγ amplification gave rise to two different sizes of amplicons. Cloning/sequencing revealed that the two amplicons corresponded to two distinct translocations, indicating the presence of oligo/polyclonality in the switched population. In FL, the differences in PCR intensities are related to the individual t(14;18) frequency, the origin (lymph node, bone marrow, PBMCs), and the conditioning (e.g., paraffin) of the particular samples (28). All positive PCR products were confirmed by cloning/sequencing (unpublished data).
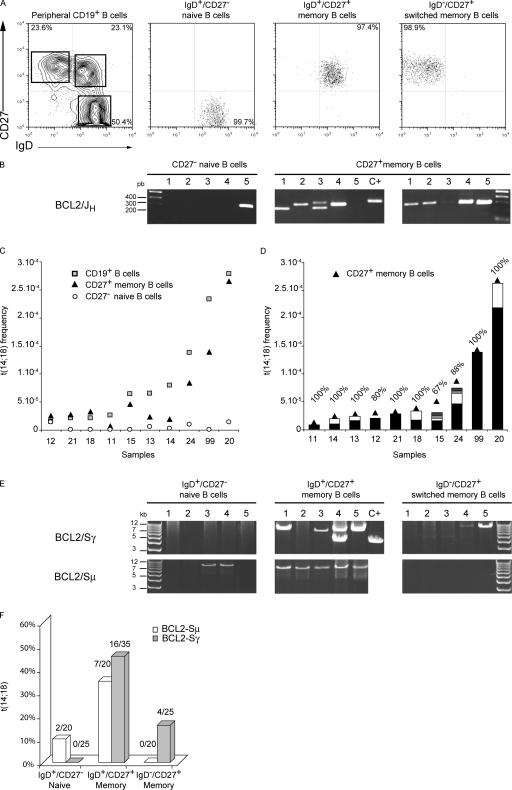
We next assessed if these long-lived t(14;18)-bearing B cells in HI already transited through the GCs. Circulating naive and memory B cells can be distinguished on the basis of the CD27 cell surface marker and sIg isotypes (11–13). Fresh PBMCs from 10 healthy donors were collected, and IgD+/CD27− naive, IgD+/CD27+ memory, and IgD−/CD27+ switched memory B cell subsets isolated by cell sorting (Fig. 2 A). The occurrence of t(14;18) was then assessed in total PBMCs and in each fraction by the short-range BCL2/JH PCR assay (Fig. 2 B and Table II). As a first approach, we pooled data from the two CD27+ memory subsets and examined the overall contribution of naive (CD27−) and memory (CD27+) B cells to the total t(14;18)+ frequency calculated as a fraction of CD19+ B cells (Fig. 2 C). t(14;18) frequencies of CD19+ B cells ranged from <1/105 to the unexpectedly high rate of ∼1/3.500 B cells in some individuals (Fig. 2 C, squares). Strikingly, although the level of naive t(14;18)+ cells constantly remained at baseline (Fig. 2 C, circles), CD27+ B cells accounted in a large part for the amplitude of t(14;18) frequencies (Fig. 2 C, triangles). This clearly indicates that circulating t(14;18)+ clones in HI are indeed predominantly B cells which transited through the GCs. To determine if the presence of high levels of t(14;18) in some individuals was caused by a higher incidence of distinct translocations or to the clonal expansion of a given t(14;18)+ B cell, we cloned and sequenced 55 out of 61 BCL2/JH fragments from the CD27+ subset. One major BCL2/JH junction was observed in most individuals (Fig. 2 D, black bars), indicating that only one clone mainly accounted for t(14;18) frequencies. Remarkably, this data demonstrate that the wide modulation of t(14;18) frequency in HI is not caused by the accumulation of clonally unrelated t(14;18) naive B cells in some individuals, but rather to the clonal expansion in the GCs of t(14;18)+ B cells.
Figure 2.
t(14;18)+ B cells in the peripheral blood of HI are not naive B cells and constitute an expanding population of FL-like clones. (A) Human PBMCs from 10 consecutive blood samples were collected from anonymous healthy donors, and IgD+/CD27− naive, IgD+/CD27+ memory, and IgD−/CD27+ switched memory B cell populations were isolated by cell sorting. Results from a representative cell-sorting experiment are shown. (B) Genomic DNA from the three purified subsets was tested for the presence of t(14;18) using the short-range BCL2/JH PCR assay (mbr20A/21A and JHcoB/cointB nested primers; see Fig. 1 A). A two-step “fluctuation” nested PCR assay was used, consisting of at least five parallel replicates performed for each sample (lanes 1–5). Results from sample #20 using 100 ng per replicate is depicted. (C) The contribution of naive (CD27−, white circles) and memory (CD27+, black triangles) B cells to the total t(14;18)+ frequency (CD19+, gray squares) in the healthy donors is shown. The translocation frequency in each subset was plotted for each donor (Table II). The 10 samples are ordered according to increasing CD19+ total B cell translocation frequencies (gray squares). (D) PCR fragments from CD27+ memory B cells were cloned and sequenced, and clonality was identified on the basis of the BCL2/JH junction, which is unique for a given translocation. The frequency of each independent BCL2/JH clone was calculated, and the contribution of each clone (vertical bars, each color represents a given clone in a given sample) was superimposed on the t(14;18) frequency from the CD27+ subset (black triangles). All positive amplicons were sequenced except one in sample #12, three in #15, and two in #24 (percentage on top of histograms corresponds to the number of sequenced amplicons on the total number of positive replicates; Table II). For #99, which required DNA dilution for the calculation of the frequency, 100% corresponds to the sequencing of 10 out of 10 clones obtained from amplifications performed on diluted DNA (1:5; Table II) and undiluted DNA (9:9; not depicted). (E) BCL2-Sγ (top) and BCL2/Sμ LR-PCR (bottom) were performed in the 3 IgD+/CD27− naive, IgD+/CD27+ memory, and IgD−/CD27+ switched memory B cell subsets for four HI (#15, #20, #21, and #24). Results from sample #24 are shown. β-globin/TCR-β controls were performed as control for long-range amplifications (unpublished data). Most positive amplicons were confirmed by cloning/sequencing and alignment to germline BCL2, Sμ, and Sγ loci (not depicted). Samples presenting multiple size bands revealed oligo/polyclonality. (F) The overall distribution of switched (gray histograms) versus unswitched (white histograms) translocated alleles in the three B cell subsets from samples #15, #20, #21, and #24 is represented. The ratio of positive replicates is indicated at the top of the bars.
Table II.
Analysis of BCL2/JH translocations in total PBMCs and FACS-sorted B cell subsets from healthy donors
| Sample | PBMCs
|
IgD+/CD27− naive B cells
|
IgD+/CD27+ memory B cells
|
IgD−/CD27+ switched memory B cells
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 10 | Detection | Ratio | Frequency | % CD19+ a | Detection | Ratio | Frequency | % | Detection | Ratio | Frequency | % | Detection | Ratio | Frequency | % |
| 11 | yes | 1:30 | 2.1 × 10−6 | 7.9 | no | 0:10 | <1.1 × 10−6 | 73 | yes | 1:10 | 6.6 × 10−6 | 16 | yes | 0:10 | <1.1 × 10−6 | 7.5 |
| 12 | no | 0:30 | <1.1 × 10−6 | 6.7 | yes | 3:15 | 1.4 × 10−5 | 63.5 | yes | 3:13 | 1.6 × 10−5 | 17 | yes | 2:15 | 8.9 × 10−6 | 15 |
| 13 | yes | 3:60 | 3.2 × 10−6 | 4.9 | yes | 1:10 | 6.6 × 10−6 | 57 | yes | 3:10 | 2.2 × 10−5 | 28.5 | no | 0:10 | <1.1 × 10−6 | 10.5 |
| 14 | yes | 2:30 | 4.3 × 10−6 | 5.4 | yes | 1:20 | 3.2 × 10−6 | 61 | yes | 5:20 | 1.8 × 10−5 | 15 | no | 0:20 | <1.1 × 10−6 | 21 |
| 15 | yes | 2:30 | 4.3 × 10−6 | 6.6 | no | 0:15 | <1.1 × 10−6 | 50 | yes | 6:15 | 3.2 × 10−5 | 23 | yes | 3:15 | 1.4 × 10−5 | 23 |
| 18 | yes | 1:30 | 2.1 × 10−6 | 10 | no | 0:5 | <1.1 × 10−6 | 60 | yes | 2:5 | 3.2 × 10−5 | 10 | no | 0:5 | <1.1 × 10−6 | 20 |
| 20 | yes | 6:30 | 1.4 × 10−5 | 5 | yes | 1:5 | 1.4 × 10−5 | 72 | yes | 2:5b | 6.4 × 10−5 | 10.6 | yes | 4:5b | 2 × 10−4 | 15.5 |
| 21 | no | 0:30 | <1.1 × 10−6 | 5 | no | 0:5 | <1.1 × 10−6 | 60 | yes | 1:5 | 1.4 × 10−5 | 11 | yes | 1:5 | 1.4 × 10−5 | 25 |
| 24 | yes | 6:30 | 1.4 × 10−5 | 10.1 | yes | 3:20 | 1.0 × 10−5 | 46.5 | yes | 13:20 | 6.6 × 10−5 | 19 | yes | 5:20 | 1.8 × 10−5 | 39 |
| 99c | yes | 11:30 | 1.1 × 10−5 | 4.9 | no | 0:10 | 1.1 × 10−6 | 59 | yes | 1:5d | 1.4 × 10−4 | 37 | - | - | - | - |
| Mean | 5.1 × 10−6 | 6.6 | 5.3 × 10−6 | 60.3 | 3.0 × 10−5 | 17.8 | 2.9 × 10−5 | 18.8 | ||||||||
| Total | 8:10 | 5:10 | 9:105 | 9:9 | 36:103 | 6:9 | 15:105 | |||||||||
| [%] | [80%] | [50%] | [8.6%]e | [100%] | [35%]e | [67%] | [14%]e | |||||||||
Determined by FACS.
Positive replicates were out of the fluctuation range in the IgD+/CD27+ (4:5) and IgD−/CD27+ (5:5) fractions. PCR were performed on 50 ng DNA, and frequencies were calculated accordingly.
B cell subsets were sorted only as IgD+/CD27− and CD19+/CD27+. #99 was excluded from the calculations of the mean t(14;18) frequency.
Positive replicates were out of the fluctuation range (9:9) in the CD27+ subset. PCR were performed on 10 ng DNA, and frequencies were calculated accordingly.
P values were determined by Pearson's χ2 test. Distributions of BCL2/JH translocations in IgD+/CD27+ memory B cells were statistically different from naive and switched memory B cells both in terms of detection rate in single individuals (P = 0.05) and of cumulative ratio of BCL2/JH-positive replicates (P < 10−4).
To define further the t(14;18)+ cells, we next examined the repartition of the translocation in the two memory B cell subsets: the IgD−/CD27+ switched memory and the IgD+/CD27+ so-called “IgM memory” (Table II) (11–14). Unexpectedly, the IgD+/CD27+ subset contained significantly higher rates of translocation, both in terms of prevalence (nine out of nine samples versus six out of nine, P = 0.05) and frequency (35 versus 14%, P = 0.001). This predominance of a surface IgD/M and not IgG in t(14;18)+ cells was in apparent contradiction with our previous LR-PCR results, in which switch to Cγ was prevalent on the translocated allele (Table I). To investigate reasons underlying this paradox, we performed the BCL2/Sμ and BCL2/Sγ LR-PCR assays on the fractionated cells from four healthy donor samples (Fig. 2 E). As expected, CSR happened on both alleles in the IgD−/CD27+ subset (Fig. 2 F). More surprisingly, CSR also frequently occurred on the translocated allele in the predominant IgD+/CD27+ subset. This is in sharp contrast with the features of the peripheral IgD+CD27+ IgM memory B cell subset, which is entirely devoid of CSR both on the productive and the nonproductive alleles (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061292/DC1) (12, 13). It is therefore very likely that the t(14;18)+ cells are distinct from this subset, albeit superimposed on the basis of the CD27 and IgD markers. Most importantly, the presence of CSR on the nonfunctional, but not on the productive allele is atypical among normal circulating memory B cells. However, this “allelic paradox” stands as a hallmark of FL (9, 10). FL derives from follicle center B cells that encountered antigen and are undergoing somatic hypermutation and CSR (1, 15). In FL, CSR occurs as frequently on both alleles, but “downstream switch” (e.g., Sγ-to-Sα) happens at unusually high frequency on the productive allele, specifically sparing the Cμ region from deletion and allowing sIgM/D expression (9). Consequently, despite CSR occurring on both alleles in >80% of cases, most FL cases still express a sIgM and only a minority expresses sIgG, sIgA, or no sIg (Fig. 1 C) (9, 10, 16). This allelic paradox indicates the presence of a selective pressure in favor of sIgM expression on a B cell population that is at the same time permanently driven to switch (9). Together with the presence of sIgM+ B cell follicular hyperplasia in BCL2 transgenic mice (2, 4), this atypical feature of t(14;18)+ cells both in FL and HI suggests a direct role of BCL2 ectopic expression in the GCs for this selection. Data from BCL2-Tg mice indicate that long-term survival of follicular B lymphocytes in the GC is of key importance for the acquisition of further genetic changes, which in turn favor further cell transformation and progression to disease (17). It is likely that in HI most t(14;18)+ cells were similarly rescued by BCL2 from apoptosis and “frozen” at a differentiation stage in which constitutive activation-induced cytidine deaminase expression drives continuous somatic hypermutation and CSR activity (18), two mechanisms conferring a high propensity for further oncogenic aberrations in the context of accumulating genomic instability (e.g., BCL6/p53) (1, 19, 20). In this scenario, t(14;18)+ cells in HI would constitute much more advanced precursors of the FL pathogenesis pathway than previously thought.
Based on our data, we propose a new model in which long-lived t(14;18)+ cells in peripheral blood of HI are mainly constituted by an atypical population of BCL2-rescued and incompletely maturated FL-like B cells released from the GCs (Fig. S2, http://www.jem.org/cgi/content/full/jem.20061292/DC1). Interestingly, this incomplete maturation phenotype does not prevent FL to exit from the GC nor its spreading to other secondary organs, including lymph nodes and bone marrow (16, 21). Contrary to normal GC-derived B cells, FL clones are prone to intense trafficking between follicles (3, 22). Do the similar features of FL-like cells in HI confer the same migration properties, and similarly to BCL2-Tg mice (17), allow the seeding of premalignant niches? The persistence and clonal expansion of t(14;18)+ follicular-like B cells over years in blood from HI (6, 8) goes along with the existence of niches, where follicular microenvironment might provide support for maintenance/proliferation in the context of polyclonal/chronic antigenic stimulation (1, 15, 23, 24). In support of this, hepatitus C virus patients, who display increased prevalence of t(14;18), show parallel regression of viral load and t(14;18) frequency in blood after antiviral therapy (25).
It remains fundamental to know if the rate of expanded FL-like cells in the peripheral blood of HI translates their activation status. Epidemiological studies have reported an increased prevalence of the t(14;18) associated with environmental exposures that might contribute to lymphomagenesis (26,27). Further studies on the origin and status of the circulating FL-like B cells will thus be of prime importance, both to gain insights into disease progression and for the proper handling of t(14;18) as an early biomarker of lymphoma.
MATERIALS AND METHODS
Patients and HI samples.
Human samples were derived from three origins: 6 DNA samples from a cohort of HI (8), for which the presence of t(14;18) translocation had been previously assessed on total PBMCs (7, 8); 10 consecutive anonymous blood samples (200–300 ml) from the local blood bank; 30 FL DNA samples from two independent sources previously described (28). Samples were collected after informed consent and approval by the local ethical committee (CCPPRB# 99–07, France).
Antibodies.
The following antibodies were used: IgD biotinylated, CD19-APC (BD PharMingen), CD27-PE, CD20-FITC (Immunotech), and IgD-FITC (Caltag Laboratories). Primary biotinylated antibody was revealed with Streptavidin–PerCP-Cy5.5 conjugate (BD PharMingen).
Cell separation and flow cytometry.
PBMCs were isolated from peripheral blood by Ficoll-Hypaque density centrifugation (BD Biosciences); B cells were enriched to >95% using the B cell negative isolation kit (Dynal). B-enriched cells were preincubated for 15 min with PBS-BSA-1%–Azide-5% normal mouse serum and stained with either IgD biotinylated or IgD-FITC, CD27-PE, and CD19-APC for 20 min at +4°C. After washing with PBS-1% BSA-Azide and 15-min incubation with Streptavidin–PercP-Cy5.5, cells were analyzed on a FACScalibur (BD Biosciences). Cell sorting of (a) IgD+/CD27− naive B cells, (b) IgD+/CD27+ IgM memory B cells, and (c) IgD−/CD27+ switched memory B cell subsets (11) was performed on a FACSAria (BD Biosciences). For all individuals, IgD+CD27− and IgD−CD27+ B cell subsets were sorted to >98% purity, and the IgD+CD27+ B cell subset was sorted to >95% purity. Isolated cells were then cryopreserved at −80°C until DNA extraction. IgM- and IgD-only cells that represent only minor components (1–3%) of the memory B cell pool were not sorted as single cells (11, 12).
Short-range PCR amplification of BCL2-IGH junctions.
Total genomic DNA was prepared from PBMCs and/or sorted B cell subpopulations by the Qiagen DNA Blood Mini kit according to the manufacturer's instructions including RNase treatment (Qiagen). A two-step double-nested fluctuation PCR assay (8) was used to amplify BCL2/JH junctions (Fig. 1 A and Table S1, available at http://www.jem.org/cgi/content/full/jem.20061292/DC1. , for mbr20A/21A and JHCo-B/Coint-B primers). As the frequency of t(14;18) junctions is low in PBMCs from HI, 5–10 reactions were performed in parallel using 100 ng of DNA (per reaction) from each isolated B cell subpopulation. In the fluctuation range, at most one target molecule is present per PCR replicate, and if so will give rise to a detectable amplicon. The detection threshold of the assay is the function of the number of replicates performed with a constant amount of DNA per replicate (here, 2 × 10−6 for 100 ng). The frequency of the event can then be calculated from the number of positive BCL2/JH amplicons using a Poisson's assumption (7). The primary PCR conditions were as follows: 30 s at 94°C, 30 s at 62°C, and 1 min at 72°C, 40 cycles. 1 μl of each primary PCR reaction was used in the nested secondary PCR in the same conditions, except for 20 cycles.
LR-PCR amplification of BCL2/Cμ and BCL2/Cγ regions.
For the amplification of BCL2/Cμ, the mbr20A/21A pair of nested forward primers was used in combination with a pair of nested reverse primers located in the Cμ and Sμ regions, respectively (3′Cμ-1B/3′Sμ-2B). For the amplification of BCL2/Cγ, the same forward primers were used in combination with a pair of reverse nested consensus primers designed to hybridize to the 3′ flanking sequences of all Cγ and Sγ1-4 regions (3′CγCons-1B/3′SγCons-2B). The same fluctuation PCR approach as for the short-range PCR assay was used (100 ng/replicate). Given the clonal nature of the tumor cells, a one-step standard LR-PCR approach was performed for FL patients. DNA extracted from all samples was controlled to be suitable for LR-PCR by performing the amplification of a 7.5-kb fragment β-globin gene and an 11-kb germline fragment of the TCR-β locus. The following PCR conditions were used: 3 min at 95°C, 35 cycles (1 min at 95°C, 12 min at 68°C) and 10 min at 68°C. 1 μl of the primary LR-PCR was used in the double-nested LR-PCR performed in the same conditions, except for 20 cycles.
Analysis of BCL2/Sμ and BCL2/Sγ clones.
PCR fragments were purified and cloned into the pGEM-T Easy Vector (Promega). Blunt-ended fragments generated by LR-PCR were purified and submitted to the A-tailing procedure before cloning. The resulting clones were sequenced and analyzed using the BLASTn shareware (http://www.ncbi.nlm.nih.gov) and Vector NTI 9.0.0 sequence analysis software (Informax).
Statistical analysis.
p values for t(14;18)-associated switch junctions analysis and analysis of t(14;18)+ cell distributions in peripheral B cell compartments were calculated by Fisher's exact test or chi-square test using STATA software (STATA corporation release 7.0). p values for t(14;18) frequency were calculated by two-tailed Student's t test.
Online supplemental material.
Fig.S1 illustrates the set of LR-PCR data showing absence of CSR on the nonfunctional allele of the peripheral blood sIgM/D+CD27+ B cell subset. Fig. S2 illustrates a reappraisal of the current model of FL pathogenesis. Table S1 describes the sequence of the PCR primers. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061292/DC1.
Supplemental Material
Acknowledgments
We are grateful to J. Ewbank, U. Jäger, and R. Marculescu for helpful discussions. We are grateful to the Division of Hematology, Medical University of Vienna (Vienna, Austria) for providing the FL DNA samples. We thank C. Beziers-Guigue for help with illustrations. We also thank N. Brun, M. Barad, M. Briand, and Y. Lecluse for technical assistance.
This work was supported by grant no. INE2003114116 from la Fondation pour la Recherche Médicale, grant nos. 7877 and 3618 from the Association pour la Recherche sur le Cancer (ARC), a grant from Le Conseil Général des Bouches du Rhône, and institutional grants from INSERM and CNRS. This study was also supported by grants from la Ligue Nationale contre le Cancer (comité de la Manche), Conseil Général du Calvados, and Université de Caen Basse-Normandie. Work in the lab of B. Nadel is supported by the AVENIR2003 grant from INSERM. S. Roulland is a recipient of the Young Investigators Program from INSERM. B. Montpellier is a recipient of a fellowship from la Ligue Nationale Contre le Cancer. B. Nadel is a recipient of a Contrat d'Interface INSERM/Assistance Publique-Hôpitaux de Marseille.
The authors have no conflicting financial interests.
Abbreviations used in this paper: CSR, class-switch recombination; FL, follicular lymphoma; GC, germinal center; HI, healthy individuals; IGH, immunoglobulin heavy chain; LR-PCR, long-range PCR; SHM, somatic hypermutation.
References
- 1.Kuppers, R. 2005. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer. 5:251–262. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell, T.J., N. Deane, F.M. Platt, G. Nunez, U. Jaeger, J.P. McKearn, and S.J. Korsmeyer. 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 57:79–88. [DOI] [PubMed] [Google Scholar]
- 3.Cong, P., M. Raffeld, J. Teruya-Feldstein, L. Sorbara, S. Pittaluga, and E.S. Jaffe. 2002. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 99:3376–3382. [DOI] [PubMed] [Google Scholar]
- 4.Egle, A., A.W. Harris, M.L. Bath, L. O'Reilly, and S. Cory. 2004. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 103:2276–2283. [DOI] [PubMed] [Google Scholar]
- 5.Limpens, J., R. Stad, C. Vos, C. de Vlaam, D. De Jong, G.J. van Ommen, E. chuuring, and P.M. Kluin. 1995. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 85:2528–2536. [PubMed] [Google Scholar]
- 6.Dolken, G., G. Illerhaus, C. Hirt, and R. Mertelsmann. 1996. BCL-2/JH rearrangements in circulating B cells of healthy blood donors and patients with nonmalignant diseases. J. Clin. Oncol. 14:1333–1344. [DOI] [PubMed] [Google Scholar]
- 7.Roulland, S., P. Lebailly, G. Roussel, M. Briand, D. Cappellen, D. Pottier, A. Hardouin, X. Troussard, C. Bastard, M. Henry-Amar, and P. Gauduchon. 2003. BCL-2/JH translocation in peripheral blood lymphocytes of unexposed individuals: lack of seasonal variations in frequency and molecular features. Int. J. Cancer. 104:695–698. [DOI] [PubMed] [Google Scholar]
- 8.Roulland, S., P. Lebailly, Y. Lecluse, N. Heutte, B. Nadel, and P. Gauduchon. 2006. Long-term clonal persistence and evolution of t(14;18)-bearing B cells in healthy individuals. Leukemia. 20:158–162. [DOI] [PubMed] [Google Scholar]
- 9.Vaandrager, J.W., E. Schuuring, H.C. Kluin-Nelemans, M.J. Dyer, A.K. Raap, and P.M. Kluin. 1998. DNA fiber fluorescence in situ hybridization analysis of immunoglobulin class switching in B-cell neoplasia: aberrant CH gene rearrangements in follicle center-cell lymphoma. Blood. 92:2871–2878. [PubMed] [Google Scholar]
- 10.Akasaka, T., H. Akasaka, N. Yonetani, H. Ohno, H. Yamabe, S. Fukuhara, and M. Okuma. 1998. Refinement of the BCL2/immunoglobulin heavy chain fusion gene in t(14;18)(q32;q21) by polymerase chain reaction amplification for long targets. Genes Chromosomes Cancer. 21:17–29. [DOI] [PubMed] [Google Scholar]
- 11.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller, S., M.C. Braun, B.K. Tan, A. Rosenwald, C. Cordier, M.E. Conley, A. Plebani, D.S. Kumararatne, D. Bonnet, O. Tournilhac, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruetzmann, S., M.M. Rosado, H. Weber, U. Germing, O. Tournilhac, H.H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, et al. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma, C.S., S. Pittaluga, D.T. Avery, N.J. Hare, I. Maric, A.D. Klion, K.E. Nichols, and S.G. Tangye. 2006. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J. Clin. Invest. 116:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelenetz, A.D., T.T. Chen, and R. Levy. 1992. Clonal expansion in follicular lymphoma occurs subsequent to antigenic selection. J. Exp. Med. 176:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, N.L., E.S. Jaffe, H. Stein, P.M. Banks, J.K. Chan, M.L. Cleary, G. Delsol, C. Wolf-Peeters, B. Falini, and K.C. Gatter. 1994. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 84:1361–1392. [PubMed] [Google Scholar]
- 17.McDonnell, T.J., and S.J. Korsmeyer. 1991. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 349:254–256. [DOI] [PubMed] [Google Scholar]
- 18.Greeve, J., A. Philipsen, K. Krause, W. Klapper, K. Heidorn, B.E. Castle, J. Janda, K.B. Marcu, and R. Parwaresch. 2003. Expression of activation-induced cytidine deaminase in human B-cell non-Hodgkin lymphomas. Blood. 101:3574–3580. [DOI] [PubMed] [Google Scholar]
- 19.Ramiro, A.R., M. Jankovic, T. Eisenreich, S. Difilippantonio, S. Chen-Kiang, M. Muramatsu, T. Honjo, A. Nussenzweig, and M.C. Nussenzweig. 2004. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 118:431–438. [DOI] [PubMed] [Google Scholar]
- 20.Ramiro, A.R., M. Jankovic, E. Callen, S. Difilippantonio, H.T. Chen, K.M. McBride, T.R. Eisenreich, J. Chen, R.A. Dickins, S.W. Lowe, et al. 2006. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 440:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bognar, A., B. Csernus, C. Bodor, L. Reiniger, A. Szepesi, E. Toth, L. Kopper, and A. Matolcsy. 2005. Clonal selection in the bone marrow involvement of follicular lymphoma. Leukemia. 19:1656–1662. [DOI] [PubMed] [Google Scholar]
- 22.Oeschger, S., A. Brauninger, R. Kuppers, and M.L. Hansmann. 2002. Tumor cell dissemination in follicular lymphoma. Blood. 99:2192–2198. [DOI] [PubMed] [Google Scholar]
- 23.Vinuesa, C.G., S.G. Tangye, B. Moser, and C.R. Mackay. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5:853–865. [DOI] [PubMed] [Google Scholar]
- 24.Bernasconi, N.L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 298:2199–2202. [DOI] [PubMed] [Google Scholar]
- 25.Giannelli, F., S. Moscarella, C. Giannini, P. Caini, M. Monti, L. Gragnani, R.G. Romanelli, V. Solazzo, G. Laffi, G. La Villa, et al. 2003. Effect of antiviral treatment in patients with chronic HCV infection and t(14;18) translocation. Blood. 102:1196–1201. [DOI] [PubMed] [Google Scholar]
- 26.Roulland, S., P. Lebailly, Y. Lecluse, M. Briand, D. Pottier, and P. Gauduchon. 2004. Characterization of the t(14;18) BCL2-IGH translocation in farmers occupationally exposed to pesticides. Cancer Res. 64:2264–2269. [DOI] [PubMed] [Google Scholar]
- 27.Chiu, B.C., B.J. Dave, A. Blair, S.M. Gapstur, S.H. Zahm, and D.D. Weisenburger. 2006. Agricultural pesticide use and risk of t(14;18)-defined subtypes of non-Hodgkin lymphoma. Blood. 108:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jager, U., S. Bocskor, T. Le, G. Mitterbauer, I. Bolz, A. Chott, M. Kneba, C. Mannhalter, and B. Nadel. 2000. Follicular lymphomas' BCL-2/IgH junctions contain templated nucleotide insertions: novel insights into the mechanism of t(14;18) translocation. Blood. 95:3520–3529. [PubMed] [Google Scholar]
- 29.Chaudhuri, J., and F.W. Alt. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 4:541–552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.