Abstract
Inflammatory cells can either promote or inhibit tumor growth. Here we studied whether CD40, a key molecule for adaptive immune response, has any role in mammary carcinogenesis of BALB/NeuT transgenic tumor-prone mice. We transferred the HER2/neu oncogene into CD40-null background to obtain the CD40-KO/NeuT strain. CD40-KO/NeuT mice showed delayed tumor onset and reduced tumor multiplicity. BM (BM) transplantation experiments excluded a role of BM-derived cells in the reduced tumorigenicity associated with CD40 deficiency. Rather, CD40 expressed by endothelial cells (ECs) takes part to the angiogenic process. Accordingly, large vessels, well organized around the tumor lobular structures, characterize BALB/NeuT tumors, whereas tiny numerous vessels with scarce extracellular matrix are dispersed in the parenchyma of poorly organized CD40-KO/NeuT tumors.
Activated platelets, which may interact with and activate ECs, are a possible source of CD40L. Their localization within tumor vessels prompted the idea of treating BALB/NeuT and CD40-KO/NeuT mice chronically with the anti-platelet drug clopidogrel, known to inhibit platelet CD40L expression. Treatment of BALB/NeuT mice reduced tumor growth to a level similar to CD40-deficient mice, whereas CD40-KO/NeuT mice treated or not showed the same attenuated tumor outgrowth, indicating that activated platelets are the likely source of CD40L in this model. Collectively, these data point to a participation of CD40/CD40L in the angiogenic processes associated with mammary carcinogenesis of BALB/NeuT mice.
Increasing evidence suggests that cancer-associated inflammation fosters tumor growth and progression (1, 2). CD40, a key molecule for adaptive immune response (3), is expressed on dendritic cells (4), but also on B cells (5), macrophages (6), endothelial cells (ECs; reference7), fibroblasts, and epithelial cells (8). Thus, it may bridge the immune and the stromal compartments, a link highlighted also by the recent finding of B cells promoting inflammation in a mouse model of epithelial carcinogenesis (9).
Malignant transformation can be followed in mice transgenic for the expression of oncogenes under tissue-specific promoters. We used the BALB/NeuT transgenic mouse model carrying the mutated rat HER-2/neu (r-p185) oncogene under the control of mouse mammary tumor virus promoter and developing mammary tumors in all 10 mammary glands by 32–33 wk of age. These mice have been fully characterized during the steps of malignant transformation from initial lobular hyperplasia to invasive and metastatic lobular carcinoma (10), a progression that has been similarly described in human breast cancer. Although in BALB/NeuT mice the rat HER-2/neu oncogene is recognized as self-antigen, DNA vaccination using the extracellular and transmembrane domains of rat HER-2/neu partially hampers tumor progression by the induction of high titers of anti–r-p185 antibody (Ab), capable of down-modulating r-p185 on preneoplastic mammary cells (11).
To study a possible role of CD40 in cancer development and progression, we transferred the rat HER-2/neu oncogene into a CD40-null background and studied tumor development. Although CD40 deficiency might suggest weak “immunosurveillance” against developing tumors, and therefore increased morbidity, we rather found CD40-KO/NeuT mice developing fewer, smaller, and delayed tumors compared with CD40-sufficient BALB/NeuT counterpart. To explain this less severe phenotype we tested two alternative hypotheses: (a) reduced tolerance to self-antigens due to the lower number of CD4+CD25+ T regulatory (T reg) cells characterizing CD40-null mice, and (b) impaired tumor angiogenesis as a consequence of the lack of CD40 on ECs.
RESULTS
Reduced mammary carcinogenesis in CD40-KO/neuT mice
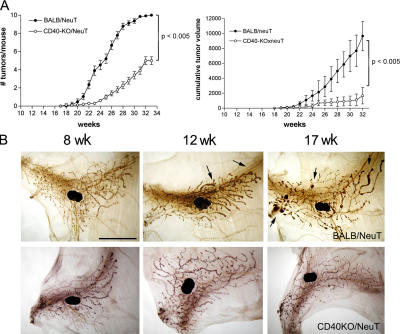
To test whether CD40 has any role in the development of mammary carcinomas, we have introduced the rat HER2/neu oncogene into the CD40-KO background and evaluated tumor onset and progression. The analysis of >50 CD40-KO/NeuT mice showed slower tumor onset, reduced multiplicity (Fig. 1 A, left), and decreased total tumor volume (Fig. 1 A, right) compared with BALB/NeuT mice. Whole mount analysis of mammary glands from BALB/NeuT and CD40-KO/NeuT mice from 6 wk, when atypical hyperplasia is evident, until 17 wk, when palpable invasive carcinomas appear, confirmed the delayed carcinogenesis in the KO strain (Fig. 1 B). Comparison of whole mount samples from wild-type and CD40-deficient mice at different time points excluded any macroscopic difference in normal mammary gland development between the two strains, and therefore the possibility that a slower mammary gland development could account for the reduced tumor growth in CD40-KO/NeuT mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20060844/DC1).
Figure 1.
Development of mammary carcinomas in BALB/NeuT and CD40-KO/NeuT mice. (A) Tumor growth curves showing delayed tumor onset and reduced tumor incidence (left) and tumor volume (right) in CD40-KO/NeuT (◯) versus BALB/NeuT mice (•). Tumor multiplicity is calculated as the cumulative number of incident tumors per total number of mice. Mean (± SE) of 52 mice for each group is shown. Single tumor volume is calculated as r2xR where r is the minor diameter and R the major one. The mean of cumulative tumor volumes (± SD) per mouse is shown. (B) Whole mount preparation of BALB/NeuT (top) and CD40-KO/NeuT (bottom) mammary glands at 8, 12, and 17 wk of age. The black spot in the middle is the inguinal lymph node. The dark areas surrounding the ducts correspond to hyperplastic areas. Black arrows point to solid tumor nodules. Bar, 5 mm.
CD40 expression on BM-derived cells is not involved in tumor development in BALB/neuT mice
To explain the reduced tumorigenicity of CD40-KO/NeuT mice, we tested two hypotheses: a weaker tolerance to tumor-associated antigens because of the reduced number of T reg cells characterizing CD40-KO mice (12), and an impaired tumor angiogenesis because of the lack of CD40 on ECs. A single experimental approach that allows discrimination between the two hypotheses is BM transplantation (BMT). CD40-null donors, transferred into BALB/NeuT recipients, were expected to reproduce the CD40-KO/NeuT tumor phenotype if cells of BM origin, including T reg cells, are actively involved in the process. Complementary results could be obtained by the transfer of BALB/c BM into CD40-KO/NeuT mice to restore the BALB/NeuT morbidity. 5–6-wk-old mice, which have completed mammary gland development, were transplanted. At this time point, no sign of carcinomas are detectable. An earlier time point has been avoided because of radiation-induced impairment of normal mammary tree development.
Although CD40-KO > BALB/NeuT chimeras showed a reduced number of T reg cells, similar to that of CD40-null and CD40-KO/NeuT mice (Fig. 2 A), tumor onset and multiplicity remained identical to that of BALB/NeuT mice (Fig. 2 B, left). Accordingly, BALB/c > CD40-KO/NeuT chimeras showed the same number of T reg cells present in the periphery of BALB/c and BALB/NeuT mice (Fig. 2 A), without any effect on tumor onset and multiplicity (Fig. 2 B, right). Because donor BM did not modify the host tumor phenotype, we excluded a preeminent immunological role of CD40 in this tumor model and therefore focused on the second hypothesis testing whether CD40 is involved in tumor angiogenesis.
Figure 2.
BMT experiments in BALB/NeuT and CD40-KO/NeuT mice. (A) Number of T reg cells in BALB/NeuT, CD40-KO/NeuT, CD40-KO > BALB/NeuT, and BALB/c > CD40-KO/NeuT chimeras. CD4+CD25+ T reg cell numbers in CD40-KO/NeuT mice (▴) is lower than in BALB/NeuT mice (▪). CD40-KO > BALB/NeuT chimeras (•) show several T reg cells comparable to that of CD40-KO/NeuT mice, whereas BALB/c > CD40-KO/NeuT chimeras (♦) have several T reg cells not different from those of BALB/NeuT animals. (B) Tumor incidence in CD40-KO > BALB/NeuT chimeras (▵, left) and in BALB/c > CD40-KO/NeuT chimeras (□, right). Tumor growth in BALB/NeuT mice (•) and CD40-KO/NeutT mice (◯) is shown for comparison. Mean ± SE is shown. (C) Mean of cumulative tumor volume in CD40-KO > BALB/NeuT chimeras (▵, left) and in BALB/c > CD40-KO/NeuT chimeras (□, right). Volumes for BALB/NeuT mice (•) and CD40-KO/NeutT mice (◯) are shown for comparison. Mean ± SD is shown. Mice number: 14–18 per group.
Despite the fact that the contribution of BM-derived endothelial progenitor cells to tumor endothelium is still debated, the most accepted theory is that tumor vessels originate mainly from host, rather than BM-derived, cells (13). Indeed, (FVBTieβ-galxBALB/NeuT)F1 double transgenic mice and (FVBTieβ-galxBALB/c)F1 > BALB/NeuT chimeras show all blood vessels, or only vessels of BM origin, respectively, stained in blue because the β-galactosidase gene is placed under the endothelial Tie2 promoter (14). Comparison of their tumor vasculature indicates that, in our tumor model, tumor-associated vessels derive mostly from host ECs rather than from donor BM-derived EC precursors (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20060844/DC1).
CD40 expression on mouse blood vessels and tumor-associated vasculature
We first assessed CD40 expression on mouse ECs in vitro using the 1G11 EC clone isolated from mouse lung tissue (15), which presents a typical endothelial phenotype, forms contact-inhibited monolayers on gelatin and capillary-like “tubes” in Matrigel, and can be kept in culture for several passages without loosing their endothelial characteristics (15). 1G11 cells express low level of CD40 (25–28% positive cells) that is up-regulated by TNF-α plus IFN-γ treatment (50–55% positive cells). Triggering of CD40 receptor on 1G11 cells by recombinant sCD40L is able to up-regulate VCAM-1 expression on their surface and induce the production of vascular endothelial growth factor (VEGF; Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20060844/DC1).
To verify the expression of CD40 on ECs in vivo, we have used a recently developed, very sensitive method, the dual radiolabeled mAb technique, which allows the quantification of EC-associated proteins with a precision and sensitivity not previously possible using immunohistochemical procedures (16, 17). Indeed, although immunohistochemistry failed to detect CD40 expression on mouse ECs, the dual radiolabeled mAb technique demonstrated the presence of CD40 on vessels of different organs, mainly the kidney, lung, pancreas, and in the tumor masses collected from BALB/NeuT mice (Table I). Although the abnormal, irregular, and leaky vessels characterizing tumor vasculature could allow a small fraction of CD40 Ab to extravasate and bind to some CD40-expressing tumor-infiltrating cells, such as macrophages, the short pulse with the Ab and the extensive washing minimize such contribution. Indeed, the ratio of CD40/platelet EC adhesion molecule (PECAM)-1 in tumor vasculature was one of the highest, confirming CD40 expression on tumor vessels as part of their activate/inflamed state. As expected, no CD40 expression was detected on either normal or tumor vessels from CD40-KO/NeuT mice (not depicted).
Table I.
CD40 and PECAM-1 expression on mouse EC in BALB/NeuT mice
| Tissue | CD40 (ng mAb/g tissue) |
PECAM-1 (ng mAb/g tissue) |
CD40/PECAM-1 ratio |
|---|---|---|---|
| Lung | 70.06 ± 22.56a | 3716.82 ± 1191.06 | 0.0188 |
| Kidneys | 68.52 ± 15.58 | 1061.06 ± 227.94 | 0.0646 |
| Heart | 4.94 ± 2.92 | 794.68 ± 234.58 | 0.0062 |
| Brain | 0.77 ± 0.86 | 46.60 ± 3.94 | 0.0165 |
| Muscles | 1.76 ± 2.16 | 167.92 ± 42.35 | 0.0105 |
| Pancreas | 4.47 ± 2.30 | 233.54 ± 74.85 | 0.0192 |
| Mesentery | 1.92 ± 1.49 | 350.14 ± 106.92 | 0.0055 |
| Small bowel | 5.99 ± 1.70 | 844.05 ± 83.09 | 0.0071 |
| Colon | 2.27 ± 2.07 | 293.43 ± 61.06 | 0.0077 |
| Tumors | 13.21 ± 6.78 | 426.44 ± 77.01 | 0.0310 |
Mean ± standard deviation.
Triggering of CD40 recruits ECs within Matrigel plug in vivo
To functionally test, in vivo, the effect of CD40 engagement on angiogenesis, we used the Matrigel implantation assay. Liquid Matrigel, containing agonist Ab to CD40, isotype-matched control Ab, sCD40L, bFGF (as positive control), or no additives, was injected s.c. in CD40-sufficient and -deficient mice. Semisolid plugs were recovered after 7–10 d and processed for light microscopy analysis or immunostaining.
sCD40L and Ab to CD40, but not isotype control Ab, are able to recruit ECs in Matrigel plugs implanted into CD40-sufficient, but not CD40-deficient, mice. On the contrary, bFGF recruited ECs into Matrigel plugs regardless of the CD40 genotype of recipient mice (Fig. 3 A). Immunostaining with Ab to CD31 confirmed the endothelial nature of some cells infiltrating the Matrigel plugs (Fig. 3 C). The number of CD40-recruited CD31+ cells was significantly different in Matrigel plugs collected from CD40-sufficient and -deficient mice (Fig. 3 B).
Figure 3.
Angiogenic effect of CD40 in vivo. Liquid growth factor–reduced Matrigel, containing Ab to CD40, isotype-matched control Ab, sCD40L plus enhancer, bFGF (as positive control), or without any additives, was injected s.c. in BALB/c or CD40-KO mice. After 7–10 d, semisolid plugs were recovered and included in paraffin or OCT. (A) Hematoxylin and eosin staining of Matrigel sections in BALB/c and CD40-KO mice. Bar, 500 μm. (B) Percentage of CD31+ cells over total number of cells in the Matrigel plugs in BALB/c and CD40-KO mice (mean of 10 fields/sample). (C) Immunostaining of Matrigel plug sections with Ab to CD31. Black arrows indicate the edge of the Matrigel plug. Bar, 300 μm; bar in inset, 300 μm.
Tumor vascularization in CD40-KO/neuT and BALB/NeuT mice
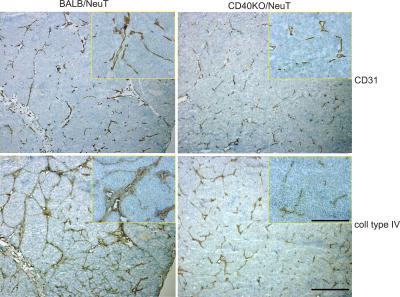
A role of CD40 in tumor vessel formation and/or organization was confirmed by immunohistochemical analysis of tumors collected from the two strains at different sizes and at different time points. Staining with Ab to CD31 showed a different organization of tumor-associated vessels (Fig. 4, top). Tumors from BALB/NeuT mice present large vessels surrounding the tumor lobular structures and a few tiny vessels inside the parenchyma, whereas those from CD40-KO/NeuT mice lack all large vessels while numerous tiny vessels are dispersed in the tumor parenchyma. Accordingly, tumors from BALB/NeuT mice show dense collagen type IV (Fig. 4, bottom) and laminin (not depicted) around the large vessels and in the stromal septa defining the tumor lobules. This feature is much less evident in CD40-KO/NeuT tumors, which are characterized by undefined lobules with thin stromal septa.
Figure 4.
Tumor vasculature in BALB/NeuT and CD40-KO/NeuT mice. Immunohistochemical analysis of tumor samples from BALB/NeuT and CD40-KO/NeuT mice. Tumors of similar size and from the same mammary gland are compared. Staining has been performed with Ab anti-CD31 and Ab anti–collagen type IV, as indicated. Bar, 1 mm; bar in inset, 500 μm.
This difference was confirmed by measuring the area occupied by blood vessels in tumors from the two strains (calculated as CD31 positive pixel number/tumoral area pixel number × 100 [4.09 ± 1 in BALB/NeuT tumors vs. 2.71 ± 0.58 in CD40-KO/NeuT tumors]; P < 0.05).
Possible involvement of platelets in BALB/NeuT mammary carcinogenesis
Aside from the well-known expression of CD40L on activated CD4+ T cells (18), this molecule has also recently been described on human platelets, which release its soluble form (sCD40L) upon activation (19, 20). Because of the paucity of CD4+ T cells infiltrating BALB/NeuT tumors (21), and their virtual absence in early lesions (not depicted), we tested whether platelet-released sCD40L may contribute to the development of BALB/NeuT tumors.
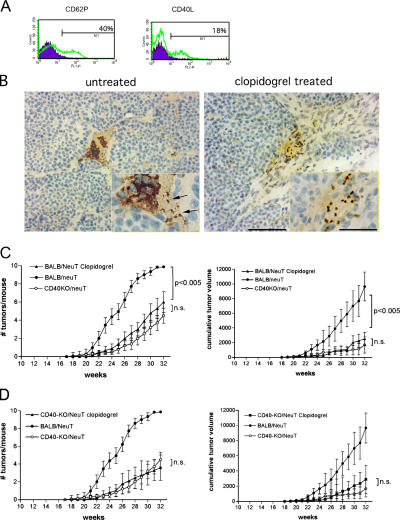
First, we confirmed the expression of CD40L on activated mouse platelets because this evidence is lacking in the current literature. Fig. 5 A shows the presence of CD40L on the surface of thrombin-activated, but not resting, platelets. The adenosine diphosphate (ADP) receptor antagonist, clopidogrel (22), is an anti-platelet drug with anti-aggregating properties. Among its actions, it inhibits ADP-induced CD40L expression on the platelet surface (23, 24). Therefore, to support the hypothesis that platelets might provide CD40L for interaction with CD40-expressing tumor ECs, we treated BALB/NeuT mice from an early age (4 wk) with clopidogrel, given chronically in the drinking water. The inhibitory activity of clopidogrel on platelet activation was confirmed by FACS analysis showing the inhibition of CD62P up-regulation on platelets stimulated in vitro with thrombin (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20060844/DC1). In addition, immunostaining of tumor vasculature from clopidogrel-treated and -untreated mice with Ab to CD41 shows dispersed or in blood clot–aggregated platelets, respectively (Fig. 5 B).
Figure 5.
Platelet involvement in tumor growth. (A) CD40L expression on mouse platelets by FACS analysis. Thrombin-activated platelets have been stained for CD62P, to assess their activation, and for CD40L. Filled histogram, resting platelets; open histogram, activated platelets. (B) Immunohistological staining for CD41, a platelet-specific marker, of tumor samples from untreated (left) and clopidogrel-treated (right) BALB/NeuT mice. In untreated mice, many platelets are clearly aggregated in a blood clot at the vessel bifurcation, and only a few of them are single or double (black arrows). In clopidogrel-treated mice, platelets are dispersed, not attached to the vessel wall, and no blood clots are present. Bar, 75 μm; bar in inset, 100 μm. (C and D) Clopidogrel effect on NeuT tumor growth. Tumor multiplicity and cumulative tumor volume per mouse of BALB/NeuT (C) and of CD40-KO/NeuT (D) mice treated chronically with clopidogrel (▴) compared with untreated CD40-KO/NeuT (◯) and BALB/NeuT mice (•). n = 15 for both BALB/NeuT and CD40-KO/NeuT mice.
Clopidogrel treatment effectively decreased tumor multiplicity and size in BALB/NeuT mice (Fig. 5, C and D) to the level of CD40-KO/NeuT mice. Because clopidogrel has additional effects other than inhibiting CD40L expression, we also treated CD40-KO/NeuT mice to evaluate any effects of clopidogrel other than inhibition of CD40L expression and therefore of CD40 triggering. Results show that clopidogrel had no significant effect on tumor growth in CD40-KO/NeuT mice (Fig. 5, E and F), indicating that, in our tumor system, its effect is mainly through the inhibition of platelet CD40L.
DISCUSSION
To study the role of CD40 in mammary carcinogenesis, we introduced the mutated rat HER-2/neu oncogene, driven by mouse mammary tumor virus promoter, into a CD40-null background. Because CD40 is crucial in mounting an immune response (6, 25), CD40-KO/NeuT mice might have been expected to be more tumor prone than their CD40-sufficient counterpart. On the contrary, CD40-KO/NeuT mice showed delayed tumor onset and reduced multiplicity compared with BALB/NeuT mice, data confirmed by whole mount analysis of mammary glands from the two strains.
Two hypotheses were formulated to explain such an unexpected phenotype: a weaker tolerance to tumor-associated antigens because of the reduced T reg cell number in CD40-KO mice (12), or an impaired tumor angiogenesis because of the lack of CD40 on ECs. Any possible direct role of CD40 on tumor cells was excluded because these tumors do not express it, neither in vitro nor in vivo (not depicted).
The spleen of CD40-KO mice has such a reduced number of CD4+CD25+ T reg cells to induce autoimmunity if transferred into nu/nu mice (12). Indeed, we found a 50–80% reduction of T reg cells in the peripheral blood, spleen, and thymus of CD40-KO mice compared with normal BALB/c mice of the same age. Although CD40-KO T reg cells were equally capable of suppressing CD4+CD25− cell proliferation as their wild-type counterpart (26), it might be possible that their low number might allow a response to tumor-associated antigen, otherwise ignored by the immune system, thus explaining the milder tumor phenotype of CD40-KO/NeuT mice.
Supporting the second hypothesis are several reports involving CD40 in EC activation and proliferation (7, 27–29), at least in humans. It has been shown that CD40 engagement on human ECs induces the expression of several angiogenic factors in vitro and promotes angiogenesis in vivo (7, 27). Interestingly, up-regulation of CD40 has been observed in tumor vessels of renal carcinomas and Kaposi's sarcoma (30). A Kaposi's sarcoma cell line engineered to release a soluble form of CD40, as decoy receptor, when injected s.c. into SCID mice develops significantly smaller tumors with reduced vascularization compared with the nontransduced counterpart (31).
Both hypotheses have been challenged at the same time using BMT experiments in which CD40-KO BM was transferred into BALB/NeuT recipients and BALB/c BM into CD40-KO/NeuT mice. If CD40 expressed on cells of BM origin was involved, BM replacement was expected to modify host tumor outgrowth. Despite the fact that BMT brought the number of recipient T reg cells to the donor level, mammary carcinogenesis remained unchanged, indicating that CD40 on BM cells did not have any relevant role in tumor development in BALB/NeuT mice. Rather, BMT data are consistent with the second hypothesis, suggesting that, in this experimental tumor model, the main role of CD40 is nonimmunological but likely associated to the angiogenic phenotype that fosters malignancy.
A large body of literature reports on the expression and function of CD40 on human ECs (7, 27–29), whereas no clear data are available in the mouse system, except for two reports that—although suggesting a functional role of CD40 in mouse angiogenesis—do not show CD40 expression on ECs (31, 32). Here, the dual radiolabeled mAb technique showed, in vivo, CD40 expression on normal and tumor blood vessels, and the functional activity of CD40 on mouse ECs has been demonstrated, both in vitro and in vivo, using a primary EC line and Matrigel implantation assay, respectively.
Accordingly, tumors from BALB/NeuT mice have large and well-structured vessels delimiting the tumor lobular structures and a few small vessels inside the parenchyma, whereas those from CD40-KO/NeuT mice have only numerous tiny vessels dispersed in the tumor parenchyma, while lacking all main large vessels. Thus, BMT experiments, Matrigel assay, in vitro and vivo detection of CD40 on both normal and tumor-associated ECs, and immunohistological analysis of the tumor vasculature concur to demonstrate a role of CD40 in tumor angiogenesis.
Such an effect of CD40 on tumor vasculature and, consequently, on tumor growth, is relevant in the BALB/NeuT tumor model, in which tumor development is a very slow process. On the other hand, in transplantable models of tumor cell lines derived from the same BALB/NeuT mice, such an effect is undetectable, and tumors, which develop very rapidly in 2–3 wk, are even more aggressive in CD40-deficient than in wild-type mice (not depicted). This evidence suggests that in very rapid transplantable models, probably, is the immunological, antitumoral role of CD40 that prevails over the pro-tumoral angiogenic effect we see in the slow and more “physiological” transgenic model. Moreover, considering the redundancy of the factors involved in tumor angiogenesis, it is very likely that EC activation by CD40 triggering is an early event, whereas subsequent angiogenic stimuli can render its effect dispensable. This view is consistent with the delayed onset but late progression of some tumors in the CD40-null background.
The search of the cells providing the ligand for CD40 triggering is quite restricted. CD40L is mainly expressed on activated CD4+ T cells (18) but is also present on human platelets, which upon activation release its soluble form (sCD40L; references 19 and 20). Human platelets up-regulate CD40L expression very rapidly after activation in vitro and during thrombus formation in vivo (33). Platelet-derived CD40L induces ECs to express tissue factor (34) and adhesion molecules, as well as the secretion of chemokines, all molecules responsible for the recruitment and extravasation of leukocytes at the site of injury. Moreover, an increased serum level of sCD40L has been detected in patients with lung cancers (35) and bone tumors (36).
There is increasing evidence that platelets may participate to tumor growth by contributing to the metastatic process (37, 38), protecting tumor cells from immune surveillance (39, 40) and regulating tumor cell invasion and angiogenesis (41–43). Platelet granules contain a variety of factors, such as VEGF, TGF-β, thrombin, and fibrinogen, which are secreted upon platelet activation. Many of these factors have been implicated in various steps of tumor progression and metastasis; indeed, being the tumor vasculature leaky, platelets may come in contact with the tumor and deposit in situ several of these angiogenic factors, which in turn can promote tumor vascularization.
In light of this evidence, and considering that BALB/NeuT tumors are virtually lacking infiltrating CD4+ T cells, especially at early phases of transformation (21 and unpublished data), we have focused our attention on platelets as the likely source of CD40L. Once confirmed that CD40L is expressed on activated mouse platelets, and that platelets are present in the vessels of BALB/NeuT tumors, we functionally tested their involvement in the tumorigenic process. The ADP receptor antagonist clopidogrel (22) is an anti-platelet drug that, among its actions on platelet activation, has been found to specifically inhibit ADP-induced CD40L expression on the platelet surface (23). We used clopidogrel to test whether platelets, and likely CD40L expressed by them, may have any role in tumor development in our model by treating young BALB/NeuT mice chronically. We hypothesized that clopidogrel, by inhibiting CD40L expression on the platelet surface, could delay/reduce tumor outgrowth in BALB/NeuT mice to the rate shown by CD40-KO/NeuT animals.
Because the clopidogrel effect on platelets is wider than inhibiting CD40L expression, we also treated CD40-KO/NeuT mice to distinguish the effect due to CD40 engagement from all other CD40-independent effects. CD40-KO/NeuT mice treated or not with clopidogrel did not show any statistically significant difference, whereas a highly significant difference was observed between treated and untreated BALB/NeuT mice. These results indicate that the major effect of clopidogrel is on the contribution of CD40L, whereas other effects on platelets are less involved in our system, especially if affecting other players that are redundant in the angiogenic process. For example, VEGF, one of the key molecules in the angiogenic process, is already released by the mammary tumors (44).
The BM origin of CD40L donors was confirmed with BMT experiments using CD40L-KO (C57BL/6 background) donors to transplant lethally irradiated transgenic (C57BL/6×BALB/NeuT)F1 mice. In the CD40LKO > NeuT chimeras, tumor development is delayed compared with C57BL/6 > NeuT control chimeras (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20060844/DC1).
In conclusion, our results sustain the hypothesis of using anticoagulants in cancer therapy to prevent platelet interaction with tumor vasculature (43), and highlights CD40/CD40L interaction as an additional mechanism that platelets use to promote neo-angiogenesis. At the same time, our findings, revealing an underestimated role of CD40 in fostering tumor neo-angiogenesis, raise a concern with using Abs to CD40 to enhance antitumor immune responses. Stimulation of CD40 may be a double-edged sword, and therefore a careful evaluation of the pros (antitumor effect by means of APC activation) and cons (angiogenesis promotion through EC activation) of CD40 triggering is needed in the design of an immunotherapeutic approach if prophylactic (45) or directed to incipient tumors.
MATERIALS AND METHODS
Animal models.
BALB/cAnNCrl mice were purchased from Charles River Laboratories. CD40-KO mice (46) were provided on a BALB/c background by L. Adorini (Bioxell, Milan, Italy). Congenic, female BALB/NeuT mice have been described elsewhere (47, 48). The CD40-KO/NeuT strain has been obtained by introducing the HER2/neu transgene into CD40-KO mice. CD40 deficiency has been tested by FACS analysis on PBMCs using an anti-CD40 FITC-conjugated mAb (clone 3/23; BD Biosciences). The presence of the HER2/neu transgene has been checked by PCR on tail DNA as described previously (48). Mice were bred and maintained at the Istituto Nazionale Tumori and treated according to the European Union guidelines. Animal studies were approved by the Animal Ethical Committee appointed by the Istituto Nazionale Tumori.
Mammary gland histology.
Whole mount preparations were performed as described at http://tgmouse.compmed.ucdavis.edu/HistoLab/wholmt1.htm. Digital photos were acquired with a Nikon Coolpix 995 (Nital SpA) mounted on a stereoscopic microscope (MZ6; Leica).
BMT.
5–6-wk-old mice were lethally γ-irradiated with 900 cGy, and BMT was performed as described previously (49).
To verify engraftment, 8 wk after BMT, PBMCs were stained with FITC-conjugated anti-CD40 mAb and PE-conjugated B220 mAb, as well as isotype control FITC/PE-conjugated rat IgG2a (all from BD Biosciences), and analyzed by flow cytometry.
FACS analysis.
The analysis of T reg cell numbers was performed by cytofluorimetry with anti–CD4-FITC and anti–CD25-PE Ab (both from BD Biosciences). Percentage of T reg cells was calculated on the total number of CD4+ cells.
For analysis of activated platelets, platelet-rich plasma (PRP) was obtained from PBMCs, collected with acid-citrate-dextrose, and centrifuged twice at 1,800 rpm to eliminate erythrocytes and other cells. PRP was activated with 0.2 U/ml human thrombin (Sigma-Aldrich) for 1 h at 37°C. Resting and activated platelets were stained for CD62P and CD40L (both from BD Biosciences).
For FACS analysis of CD40 expression on 1G11 ECs (provided by A. Vecchi, Mario Negri Institute, Milan, Italy), anti–CD40-FITC Abs and clones 3/23 and HM-40-3 (both from BD Biosciences) were used.
Dual radiolabeled mAb technique.
The mAbs used for in vivo assessment of CD40 and PECAM-1 expression were 3/23 for mouse CD40, MEC 13.3 against mouse PECAM-1 (both from BD Biosciences), and P-23, a nonspecific, nonbinding murine IgG1 directed against human P-selectin (provided by D.C. Anderson, Pharmacia-Upjohn, Kalamazoo, MI). The specific binding (3/23 and MEC 13.3) and nonbinding (P23) mAbs were labeled with 125I and 131I, respectively (Du Pont-New England Nuclear). Mice were anesthetized intramuscularly with 150 mg/kg ketamine and 7.5 mg/kg xylazine. The right jugular vein and right carotid artery were cannulated with polyethylene tubing (PE-10). To measure CD40 or PECAM-1 expression, a mixture of 125I-labeled binding mAb (either 20 μg anti-CD40 or 10 μg anti–PECAM-1) and 0.5–5 μg of nonbinding 131I mAb (adjusted to ensure a total 131I injected activity of 500,000 ± 100,000 cpm), was injected through the jugular vein catheter (total volume 200 μl). Blood samples (200 μl) were obtained from the carotid artery catheter 5 min after injection of the mAb mixture for measurement of plasma 125I and 131I activity. Thereafter, an isovolemic blood exchange was rapidly performed by perfusion with 6 ml of bicarbonate-buffered saline through the jugular vein catheter with simultaneous blood withdrawal through the carotid artery catheter. This was followed by perfusion of 15 ml of bicarbonate-buffered saline through the carotid artery catheter after severing the inferior vena cava at the thoracic level. Organs were harvested and weighed before radioactivity measurements. The method for calculating CD40 and PECAM-1 expression has been described previously (17).
In vivo Matrigel plug assay.
Matrigel plug assay has been performed in BALB/c and CD40-KO mice by s.c. injection of growth factor–reduced Matrigel (BD Biosciences) containing 25 ng/ml bFGF (R&D Systems), 40 μg/ml anti-CD40 mAb (clone FGK; Qbiogene), isotype-matched control mAb (rat IgG2a; BD Biosciences), 500 ng/ml of msCD40L plus enhancer (CD40L soluble Set; Qbiogene) or without any additive. 6–10 d later, animals were killed and Matrigel plugs were recovered in OCT for immunostaining with rat anti–mouse mAb CD31 (see below), or fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin. The percentage of CD31+ cells is calculated over the total number of cells recruited in the external area of the plugs (very few cells are present in the most inner part of the plug), and the mean of 10 fields per each sample is calculated.
Immunohistochemistry.
Tumor samples were embedded in OCT compound, snap frozen, and stored at −80°C. Immunohistochemical analysis on 5-μm cryostat sections was performed as described previously (49). The following Abs have been used: rat anti–mouse mAb CD31, rabbit polyclonal Ab anti–mouse collagen type IV (Chemicon), rat anti–mouse mAb Laminin (Chemicon), rat anti–mouse CD41 (Integrin IIb chain; BD Biosciences), and biotinylated goat anti–rat or anti–rabbit IgG as secondary Ab. Avidin–peroxidase complex (Sigma-Aldrich) was used and antigens were revealed with 3,3′-diaminobenzidine (Sigma-Aldrich) according to the manufacturer's instructions.
Images were digitally captured on a microscope (Eclipse E1000; Nikon) equipped with a digital camera (DXM1200; Nikon) and analyzed using ACT1 software. The percentage of the area occupied by blood vessels in tumors is calculated as CD31 positive pixel number/tumoral area pixel number × 100 in 200× fields. Mean ± SD is performed on nine samples per each group.
In vivo clopidogrel treatment.
BALB/NeuT and CD40-KO/NeuT mice have been treated chronically, from 4 wk of age, with clopidogrel (Plavix) given continuously via the drinking water at a concentration of 0.25 mg/ml (equivalent to an oral dose of ∼30 mg/kg/day). The inhibitory effect of clopidogrel treatment on platelet activation was assessed by FACS analysis with anti-CD62P Ab on PRP obtained from treated and untreated mice, restimulated in vitro with thrombin (Fig. S4).
Statistical analysis.
Data were expressed as the mean plus SD or SE. Differences between groups were analyzed for statistical significance by means of an unpaired t test, with P < 0.05 as significant cutoff.
Online supplemental material.
Fig. S1 illustrates comparative whole mount analysis of mammary glands from BALB/c and CD40-KO mice at different time points. Fig. S2 shows X-gal staining of tumor vessels in double transgenic (FVBTieβ-galxBALB/NeuT)F1 mice in comparison with BALB/NeuT mice transplanted with (FVBTieβ-galxBALB/c)F1 BM cells (FVBTieβ-galxB/c > BALB/NeuT). Fig. S3 shows functional CD40 expression on mouse 1G11 ECs. Fig. S4 shows the efficacy of clopidogrel treatment in inhibiting platelet activation. Figs. S1–S4 are available at http://www.jem.org/cgi/content/full/jem.20060844/DC1.
Supplemental Material
Acknowledgments
We thank M. Parenza, I. Arioli, and J. Russell for their expert technical assistance.
This work was supported by Associazione Italiana Ricerca sul Cancro, Italian Ministry of Health, Italian Ministry for University and Scientific and Technological Research, Fondo per gli Investimenti di Base (contract RBNE017B4C, FIRB), and the European Union (contract 005033-EICOSANOX). S. Sangaletti and I. Alessandrini are recipients of FIRC fellowships.
The authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; ADP, adenosine diphosphate; BMT, BM transplantation; EC, endothelial cell; PECAM, platelet EC adhesion molecule; PRP, platelet-rich plasma; T reg, T regulatory; VEGF, vascular endothelial growth factor.
References
- 1.Coussens, L.M., and Z. Werb. 2002. Inflammation and cancer. Nature. 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill, F., and L.M. Coussens. 2004. Cancer: an inflammatory link. Nature. 431:405–406. [DOI] [PubMed] [Google Scholar]
- 3.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2–17. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan, B., and R. Thomas. 2003. CD40 and dendritic cell function. Crit. Rev. Immunol. 23:83–107. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, G.A., and B.S. Hostager. 2003. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 14:297–309. [DOI] [PubMed] [Google Scholar]
- 6.Grewal, I.S., and R.A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135. [DOI] [PubMed] [Google Scholar]
- 7.Melter, M., M.E. Reinders, M. Sho, S. Pal, C. Geehan, M.D. Denton, D. Mukhopadhyay, and D.M. Briscoe. 2000. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 96:3801–3808. [PubMed] [Google Scholar]
- 8.Young, L.S., A.G. Eliopoulos, N.J. Gallagher, and C.W. Dawson. 1998. CD40 and epithelial cells: across the great divide. Immunol. Today. 19:502–506. [DOI] [PubMed] [Google Scholar]
- 9.de Visser, K.E., L.V. Korets, and L.M. Coussens. 2005. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 7:411–423. [DOI] [PubMed] [Google Scholar]
- 10.Di Carlo, E., S. Rovero, K. Boggio, E. Quaglino, A. Amici, A. Smorlesi, G. Forni, and P. Musiani. 2001. Inhibition of mammary carcinogenesis by systemic interleukin 12 or p185neu DNA vaccination in Her-2/neu transgenic BALB/c mice. Clin. Cancer Res. 7:830s–837s. [PubMed] [Google Scholar]
- 11.Rovero, S., A. Amici, E.D. Carlo, R. Bei, P. Nanni, E. Quaglino, P. Porcedda, K. Boggio, A. Smorlesi, P.L. Lollini, et al. 2000. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 165:5133–5142. [DOI] [PubMed] [Google Scholar]
- 12.Kumanogoh, A., X. Wang, I. Lee, C. Watanabe, M. Kamanaka, W. Shi, K. Yoshida, T. Sato, S. Habu, M. Itoh, et al. 2001. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J. Immunol. 166:353–360. [DOI] [PubMed] [Google Scholar]
- 13.Gothert, J.R., S.E. Gustin, J.A. van Eekelen, U. Schmidt, M.A. Hall, S.M. Jane, A.R. Green, B. Gottgens, D.J. Izon, and C.G. Begley. 2004. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 104:1769–1777. [DOI] [PubMed] [Google Scholar]
- 14.Schlaeger, T.M., S. Bartunkova, J.A. Lawitts, G. Teichmann, W. Risau, U. Deutsch, and T.N. Sato. 1997. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. USA. 94:3058–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong, Q.G., S. Bernasconi, S. Lostaglio, R.W. De Calmanovici, I. Martin-Padura, F. Breviario, C. Garlanda, S. Ramponi, A. Mantovani, and A. Vecchi. 1997. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler. Thromb. Vasc. Biol. 17:1599–1604. [DOI] [PubMed] [Google Scholar]
- 16.Panes, J., M.A. Perry, D.C. Anderson, A. Manning, B. Leone, G. Cepinskas, C.L. Rosenbloom, M. Miyasaka, P.R. Kvietys, and D.N. Granger. 1995. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am. J. Physiol. 269:H1955–H1964. [DOI] [PubMed] [Google Scholar]
- 17.Vowinkel, T., K.C. Wood, K.Y. Stokes, J. Russell, C.F. Krieglstein, and D.N. Granger. 2005. Differential expression and regulation of murine CD40 in regional vascular beds. Am. J. Physiol. Heart Circ. Physiol. 290:H631–H639. [DOI] [PubMed] [Google Scholar]
- 18.Grewal, I.S., and R.A. Flavell. 1996. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol. Today. 17:410–414. [DOI] [PubMed] [Google Scholar]
- 19.Nannizzi-Alaimo, L., V.L. Alves, and D.R. Phillips. 2003. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 107:1123–1128. [DOI] [PubMed] [Google Scholar]
- 20.Akbiyik, F., D.M. Ray, K.F. Gettings, N. Blumberg, C.W. Francis, and R.P. Phipps. 2004. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 104:1361–1368. [DOI] [PubMed] [Google Scholar]
- 21.Nanni, P., G. Nicoletti, C. De Giovanni, L. Landuzzi, E. Di Carlo, F. Cavallo, S.M. Pupa, I. Rossi, M.P. Colombo, C. Ricci, et al. 2001. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J. Exp. Med. 194:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savi, P., P. Nurden, A.T. Nurden, S. Levy-Toledano, and J.M. Herbert. 1998. Clopidogrel: a review of its mechanism of action. Platelets. 9:251–255. [DOI] [PubMed] [Google Scholar]
- 23.Hermann, A., B.H. Rauch, M. Braun, K. Schror, and A.A. Weber. 2001. Platelet CD40 ligand (CD40L)–subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 12:74–82. [DOI] [PubMed] [Google Scholar]
- 24.Quinn, M.J., D.L. Bhatt, F. Zidar, D. Vivekananthan, D.P. Chew, S.G. Ellis, E. Plow, and E.J. Topol. 2004. Effect of clopidogrel pretreatment on inflammatory marker expression in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 93:679–684. [DOI] [PubMed] [Google Scholar]
- 25.Lanzavecchia, A. 1998. Immunology. Licence to kill. Nature. 393:413–414. [DOI] [PubMed] [Google Scholar]
- 26.Guiducci, C., B. Valzasina, H. Dislich, and M.P. Colombo. 2005. CD40/CD40L interaction regulates CD4+CD25+ Treg homeostasis through dendritic cell-produced IL-2. Eur. J. Immunol. 35:557–567. [DOI] [PubMed] [Google Scholar]
- 27.Reinders, M.E., M. Sho, S.W. Robertson, C.S. Geehan, and D.M. Briscoe. 2003. Proangiogenic function of CD40 ligand-CD40 interactions. J. Immunol. 171:1534–1541. [DOI] [PubMed] [Google Scholar]
- 28.Mach, F., U. Schonbeck, G.K. Sukhova, T. Bourcier, J.Y. Bonnefoy, J.S. Pober, and P. Libby. 1997. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA. 94:1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karmann, K., C.C. Hughes, J. Schechner, W.C. Fanslow, and J.S. Pober. 1995. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc. Natl. Acad. Sci. USA. 92:4342–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pammer, J., A. Plettenberg, W. Weninger, B. Diller, M. Mildner, A. Uthman, W. Issing, M. Sturzl, and E. Tschachler. 1996. CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi's sarcoma. Am. J. Pathol. 148:1387–1396. [PMC free article] [PubMed] [Google Scholar]
- 31.Biancone, L., V. Cantaluppi, M. Boccellino, L. Del Sorbo, S. Russo, A. Albini, I. Stamenkovic, and G. Camussi. 1999. Activation of CD40 favors the growth and vascularization of Kaposi's sarcoma. J. Immunol. 163:6201–6208. [PubMed] [Google Scholar]
- 32.Russo, S., B. Bussolati, I. Deambrosis, F. Mariano, and G. Camussi. 2003. Platelet-activating factor mediates CD40-dependent angiogenesis and endothelial-smooth muscle cell interaction. J. Immunol. 171:5489–5497. [DOI] [PubMed] [Google Scholar]
- 33.Henn, V., J.R. Slupsky, M. Grafe, I. Anagnostopoulos, R. Forster, G. Muller-Berghaus, and R.A. Kroczek. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 391:591–594. [DOI] [PubMed] [Google Scholar]
- 34.Slupsky, J.R., M. Kalbas, A. Willuweit, V. Henn, R.A. Kroczek, and G. Muller-Berghaus. 1998. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemost. 80:1008–1014. [PubMed] [Google Scholar]
- 35.Roselli, M., T.C. Mineo, S. Basili, F. Martini, S. Mariotti, S. Aloe, G. Del Monte, V. Ambrogi, A. Spila, R. Palmirotta, et al. 2004. Soluble CD40 ligand plasma levels in lung cancer. Clin. Cancer Res. 10:610–614. [DOI] [PubMed] [Google Scholar]
- 36.Holzer, G., T. Pfandlsteiner, H. Blahovec, K. Trieb, and R. Kotz. 2003. Serum concentrations of sCD30 and sCD40L in patients with malignant bone tumours. Wien. Med. Wochenschr. 153:40–42. [DOI] [PubMed] [Google Scholar]
- 37.Nierodzik, M.L., A. Klepfish, and S. Karpatkin. 1995. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb. Haemost. 74:282–290. [PubMed] [Google Scholar]
- 38.Hejna, M., M. Raderer, and C.C. Zielinski. 1999. Inhibition of metastases by anticoagulants. J. Natl. Cancer Inst. 91:22–36. [DOI] [PubMed] [Google Scholar]
- 39.Nieswandt, B., M. Hafner, B. Echtenacher, and D.N. Mannel. 1999. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 59:1295–1300. [PubMed] [Google Scholar]
- 40.Borsig, L., R. Wong, J. Feramisco, D.R. Nadeau, N.M. Varki, and A. Varki. 2001. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA. 98:3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verheul, H.M., A.S. Jorna, K. Hoekman, H.J. Broxterman, M.F. Gebbink, and H.M. Pinedo. 2000. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood. 96:4216–4221. [PubMed] [Google Scholar]
- 42.Mohle, R., D. Green, M.A. Moore, R.L. Nachman, and S. Rafii. 1997. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA. 94:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinedo, H.M., H.M. Verheul, R.J. D'Amato, and J. Folkman. 1998. Involvement of platelets in tumour angiogenesis? Lancet. 352:1775–1777. [DOI] [PubMed] [Google Scholar]
- 44.Melani, C., C. Chiodoni, G. Forni, and M.P. Colombo. 2003. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 102:2138–2145. [DOI] [PubMed] [Google Scholar]
- 45.Colombo, M.P., and G. Forni. 1994. Cytokine gene transfer in tumor inhibition and tumor therapy: where are we now? Immunol. Today. 15:48–51. [DOI] [PubMed] [Google Scholar]
- 46.Castigli, E., F.W. Alt, L. Davidson, A. Bottaro, E. Mizoguchi, A.K. Bhan, and R.S. Geha. 1994. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc. Natl. Acad. Sci. USA. 91:12135–12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller, W.J., E. Sinn, P.K. Pattengale, R. Wallace, and P. Leder. 1988. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 54:105–115. [DOI] [PubMed] [Google Scholar]
- 48.Boggio, K., G. Nicoletti, E. Di Carlo, F. Cavallo, L. Landuzzi, C. Melani, M. Giovarelli, I. Rossi, P. Nanni, C. De Giovanni, et al. 1998. Interleukin 12–mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 188:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sangaletti, S., A. Stoppacciaro, C. Guiducci, M.R. Torrisi, and M.P. Colombo. 2003. Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J. Exp. Med. 198:1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.