Abstract
Peritoneal B1 cells are known to generate large amounts of antibodies outside their residential site. These antibodies play an important role in the early defense against bacteria and viruses, before the establishment of adaptive immune responses. Although many stimuli, including antigen, lipopolysaccharide, or cytokines, have been shown to activate B1 cells and induce their differentiation into plasma cells, the molecular signals required for their egress from the peritoneal cavity are not understood. We demonstrate here that direct signals through Toll-like receptors (TLRs) induce specific, rapid, and transient down-regulation of integrins and CD9 on B1 cells, which is required for detachment from local matrix and a high velocity movement of cells in response to chemokines. Thus, we revealed an unexpected role for TLRs in governing the interplay between integrins, tetraspanins, and chemokine receptors required for B1 cell egress and, as such, in facilitating appropriate transition from innate to adaptive immune responses.
Toll-like receptors (TLRs), a family of pattern-recognition receptors that detect conserved molecular products of microorganisms, have been shown to play an essential role in the induction of immune responses (1). Recognition of microbial products by TLR expressed on “classic” innate cells, such as dendritic cells, triggers their maturation leading to initiation of antigen-specific adaptive immune responses through T cell activation. Furthermore, direct signals through TLRs expressed on B cells play an important role in the activation and optimal antibody production to T-dependent antigens (2).
However, adaptive immune responses take days to weeks to fully develop, which is too much of delay to combat quickly replicating microorganisms. To facilitate prompt antibody responses, a special subset of B cells, marginal zone (MZ) B and B1 cells, appears to be evolutionarily selected and maintained (3). These cells, named innate-like B cells (4, 5), bridge the innate and adaptive immunity and make an optimal transition between the two immune responses by producing the first wave of antibodies required for antigenic clearance. Indeed, B1 cells are known to participate in a very early T-independent phase of immune responses against bacteria, viruses, and certain parasites (6–12). This characteristic is partly explained by their lower threshold than conventional B2 cells for activation, proliferation, and differentiation into plasma cells. Besides functional characteristics, B1 cells are distinguished from conventional B (B2) cells by their anatomical location, self-renewing capacity, and surface phenotype (13–15). B1 cells are located mainly in the peritoneal and pleural cavities and express high levels of surface IgM and low levels of IgD, CD23, and B220. In addition to Mac-1, a significant fraction of peritoneal B1 cells, known as B1a cells, expresses CD5, whereas the remaining fraction constitutes B1b cells.
Multiple studies on B1 cells have been focusing on developmental origins, repertoire selection, and activation requirements of this subset of cells compared with conventional B2 cells. However, despite the importance of B1 cells in protection from infections, surprisingly little is known about how these cells are retained in the body cavities and even less is understood about the molecular signals required for their recruitment out of their compartment for antigenic clearance.
Molecules that are universally involved in cell adhesion are integrins. These are heterodimeric (αβ) transmembrane glycoproteins essential for many fundamental processes, like self-renewal and differentiation of hematopoietic cells, cell migration, and tissue retention (16–18). They bind cellular receptors, such as vascular cell adhesion molecule 1 or intercellular adhesion molecule 1, and extracellular matrix components, such as laminin or fibronectin. Some integrins physically associate with small proteins called tetraspanins (19) that were also implicated in a broad range of biological activities, including cell fusion, motility, metastasis, proliferation, and differentiation (20, 21). For example, CD9 plays a critical role in sperm–egg fusion (22) and a reverse correlation of CD9 expression and cancer metastasis is well documented (21, 23, 24). Interestingly, expression of CD9 on B cells is restricted to innate-like cells such as B1 and MZ B cells as well as plasma cells (25).
Besides integrins and their associating partners, chemokines and chemokine receptors are known to play important roles for cell migration and localization (26). They are not only participating in the recruitment of cells into the lymphoid tissues, but also, as revealed in recent studies, in the egress of cells from these lymphoid tissues (27).
Here we show that B1 cells express extremely high levels of integrins and that direct signals through TLRs induce a massive egress of B1 cells from the peritoneal cavity that is associated with coordinated down-regulation of integrins and CD9. We have also demonstrated that modulation of adhesion/motility-related proteins on B1 cells allows them to migrate with enhanced motility in response to chemokines. Our studies have thus identified key requirements for mobilization of nonrecirculating cells and established a role for TLRs in control of B1 cell egress.
RESULTS
Bacteria and bacterial components induce B1 cell egress
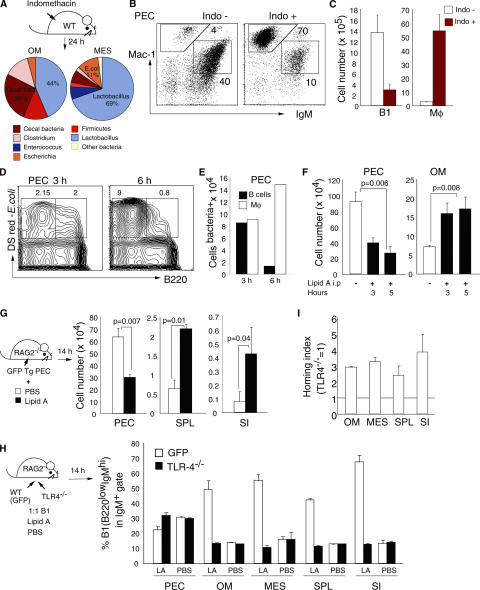
One function of peritoneal B1 cells is to survey the abdominal cavity and to join forces with innate cells, such as macrophages (MΦ), for a rapid and efficient bacterial clearance. We mimicked an acute infection from the gut by administration of high doses of indomethacin, an antiinflammatory drug that disrupts the gut epithelial barrier (28). Translocation of bacteria, predominantly from the small intestine (as indicated by 16SrRNA sequences, with Lactobacillus being the predominant bacteria in the small intestine in our mouse colony) into the peritoneum-associated tissues (omentum and mesenterium; Fig. 1 A) induced dramatic changes in the cellular composition of the peritoneal cavity. These changes consisted of a depletion of resident B1 cells and a massive influx of monocytes/MΦ or granulocytes from the blood (Fig. 1, B and C). To monitor the kinetics of B1 cell egress from the peritoneal cavity, we injected fluorescence-labeled live bacteria i.p. We found that 3 h after injection, similar percentages and numbers of B cells (B220+IgM+) and MΦ (B220−IgM−) were loaded with bacteria (Fig. 1 D). However, 6 h after peritonitis, the number of bacteria+ B cells as well as the total number of B1 cells (B220lowIgMhi Mac-1+) present in the peritoneal cavity drastically decreased, whereas the number of B220−Mac-1+ cells containing bacteria increased (Fig. 1 E).
Figure 1.
Bacteria and bacterial components induce peritoneal B1 cell egress. (A) Bacteria identified by sequence analyses of the 16S rRNA genes in omentum (OM) and mesenterium (MES) after s.c. injection of indomethacin. Each color represents a specific sequence, and the numbers indicate percentages in sequenced clones. (B) Representative flow cytometric profiles of peritoneal cavity cells in control (Indo−) or mice treated 24 h previously with indomethacin (Indo+). Numbers indicate the percentages of B1 cells (IgMhiMac-1+) and monocytes-MΦ (IgM−Mac-1hi) in total cells. (C) Absolute numbers of B1 cells and monocytes-MΦ in the peritoneal cavity before and 24 h after indomethacin treatment. (D) Representative flow cytometric profiles and (E) total numbers of B220+DsRed+(B cells) and B220−DsRed+(MΦ) of cells from the peritoneal cavity 3 and 6 h after i.p. injection of 107 E. coli (DH5α) expressing Ds-Red. (F) Total numbers of B220lowIgMhi Mac-1+ B1 cells from the peritoneal cavity and omentum at the indicated time points after i.p. injection of 10 μg lipid A. Mean ± standard error, n = 4, Student's t test. (G) Total numbers of B1 cells in the peritoneal cavity (PEC), spleen (SPL), and small intestine (SI) 14 h after i.p. injection of 107 peritoneal cells from GFP Tg mice without (white bars) or with 10 μg lipid A (black bars). Mean ± standard error, n = 5, Mann-Whitney test. Competitive homing experiments between peritoneal B1 cells from GFP Tg mice (WT) and TLR-4−/− mice. (H) Percentages of B220lowGFP+ (white bars) and B220lowGFP− (black bars) B1 cells in the IgM+ gate, and (I) the homing index calculated by total B1 numbers in the indicated tissues 14 h after i.p. injection of peritoneal cells (1:1 ratio) into RAG2−/− mice together with 10 μg lipid A. Error bars represent 95% confidence intervals. Data are representative of two independent experiments.
A similar egress of B1 cells from the peritoneal cavity was not only associated with bacteria phagocytosis, but also with cell stimulation by pure bacterial components. As shown in Fig. 1 F, i.p. injection of lipid A induced a significant depletion of B1 cells from the peritoneal cavity. We suspected that the omentum, an entry site for B1 cells (29), might also represent an exit route. In agreement with this, we found that at 3 and 5 h after lipid A stimulation the numbers of B1 cells in the omentum increased, which were probably in transit out from the peritoneal cavity (Fig. 1 F). Target organs for B1 cell migration outside the peritoneum-associated tissues were evaluated 14 h after i.p. injection of GFP+ peritoneal cells into RAG2−/− mice. Stimulation with lipid A or peptidoglycan by i.p injection induced a significant increase in the numbers of B1 cells that migrated to the spleen or small intestine lamina propria (Fig. 1 G and not depicted). Thus, the presence of bacteria or bacterial components in the abdominal cavity drives a large fraction of B1 cells out of the peritoneal cavity and thereby facilitates their migration to effector sites.
Impaired egress of B1 cells from TLR4−/− mice
We next asked if the egress of peritoneal B1 cells induced by lipid A or peptidoglycan required direct signals through TLRs. The migration properties of peritoneal B1 cells isolated from TLR4−/− mice were compared with those from normal mice in competitive transfer experiments after stimulation with lipid A. Although equal numbers of B1 cells from GFP transgenic (Tg) and TLR4−/− mice were injected into the peritoneal cavity of RAG2−/− mice, much less TLR4−/−-derived B1 cells could be detected outside the peritoneal cavity 14 h after transfer (Fig. 1 H). The majority of B1 cells that migrated to the omentum, mesenterium, spleen, or small intestine were derived from GFP Tg mice, and their homing index was three to four times higher as compared with that of B1 derived from TLR4−/− mice (Fig. 1 I). To ensure that TLR4−/− B1 cells were not innately deficient in their ability to home to peripheral tissues, we performed competitive transfer experiments in the absence of stimulation. As shown in Fig. 1 H, similar percentages of B1 cells from GFP Tg and TLR4−/− mice could be detected outside the peritoneal cavity, clearly indicating that the impaired egress of TLR4-deficient B1 cells after acute stimulation was due to their unresponsiveness to TLR ligands.
The results demonstrate that signals through TLRs on B1 cells are responsible for a rapid and efficient mobilization of B1 cells from the peritoneal cavity to other lymphoid organs.
TLR stimulation induces integrin down-regulation on B1 cells
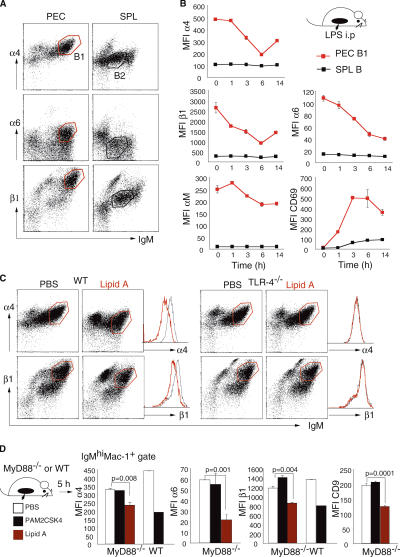
We next wanted to elucidate the mechanisms involved in TLR-induced B1 cell egress. We found that peritoneal B1 cells express much higher levels of α4, α6, and β1 integrins as compared with freely recirculating spleen B2 cells (Fig. 2 A). This observation raised the possibility that a high expression level of integrins is responsible for retention of B1 cells in the peritoneal cavity and would make pertinent the prediction that TLR-induced egress involves integrin down-regulation on B1 cells. Indeed, analyses of integrin levels after LPS stimulation revealed a striking and selective down-regulation of surface integrins on B1 cells (Fig. 2 B). Stimulation with LPS i.p. reduced the mean fluorescence intensity level of α4, α6, and β1 integrins on B1 cells to <50% by 6 h after injection, in parallel with cell activation, as assessed by CD69 expression (Fig. 2 B). In contrast, it did not affect the expression level of Mac-1 (αM) on B1 cells nor of any integrins expressed on spleen B2 cells (Fig. 2 B). i.v. injection of LPS, although strongly activated spleen B2 cells, as assessed by CD69 up-regulation, failed to induce changes of integrin levels, whereas B1 cells responded by a similar down-regulation as seen after i.p. challenge (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20061041/DC1). It is therefore unlikely that the nonresponsiveness of spleen B2 cells is attributed to a lack of sufficient activation by LPS injection i.p.
Figure 2.
TLR stimulation induces integrin down-regulation on B1 cells. (A) Representative flow cytometric profiles of cells isolated from the peritoneal cavity (PEC) and spleen (SPL) stained for IgM and the indicated integrins. Gates were set for PEC B1 (red) and SPL B2 (black). (B) Kinetics of surface integrin modulation on peritoneal B220lowIgMhi Mac-1+ B1 cells (red squares) and spleen (SPL) B220+IgM+ cells (black squares) after i.p. stimulation with 20 μg LPS. Mean ± standard error, n = 3 mice. (C) Representative flow cytometric profiles of peritoneal cavity cells stained for IgM and α4 and β1 integrins isolated from WT and TLR-4−/− mice 5 h after i.p injection of PBS or 10 μg lipid A. Histograms show mean fluorescence intensities of surface integrins on IgMhi Mac-1+ B1 cells (red gate) without (black line) and with (red line) stimulation. (D) Mean fluorescence intensities of α4, α6, and β1 integrins and CD9 of peritoneal B1 cells from MyD88−/− mice and WT mice 5 h after i.p. injection of PBS (white bars), 1 μg PAM2CSK4 (black bars), or 10 μg lipid A (red bars). Mean ± standard error, n = 3 mice, Student's t test.
Integrin modulation on B1 cells was the consequence of direct signaling through TLRs and not of engagement of B cell receptors because injection of lipid A had no effect on peritoneal cells from TLR4−/− mice, whereas it induced a clear integrin down-regulation in WT mice (Fig. 2 C). We further investigated whether the adaptor molecule myeloid differentiation primary response gene 88 (MyD88) was critical for TLR-dependent down-modulation of surface integrins on B1 cells. Consistent with complete MyD88 dependency for TLR2 but not TLR4 signaling described for other aspects of innate and adaptive immune responses (30–34), we found that MyD88−/− mice did not respond to synthetic TLR2 ligand (Pam2CSK4) but clearly down-regulated integrins on peritoneal B1 cells after TLR4 ligand (lipid A) stimulation (Fig. 2 D). We further confirmed that integrin down-modulation on peritoneal B1 cells was a direct effect of TLRs on B cells (rather than an indirect one through cytokines secreted by activated peritoneal MΦ) because purified B cells stimulated in vitro had an identical response with those from nonsorted cultures (Fig. S1 B).
Coordinated regulation of integrin and CD9 through TLRs
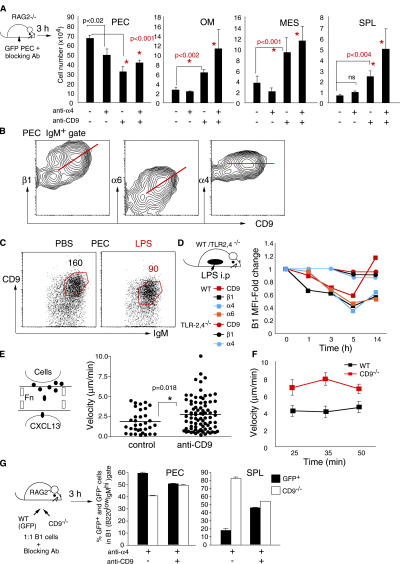
To further prove the involvement of integrins in B1 cell migration, we examined the effect of blocking integrin function with antibodies. Thus, we performed short-term transfer experiments with peritoneal cells preincubated in vitro with integrin-blocking antibodies in the absence of any stimulation. Anti-α4 antibody treatment resulted in a modest decrease in B1 cell number in the peritoneal cavity and a slight increase in B1 cells that migrated to the spleen (Fig. 3 A). Similar results were obtained when peritoneal cells were preincubated with a combination of anti-α4, α6, and β1 antibodies (not depicted). Thus, down-modulation of integrins alone cannot explain the efficient egress of peritoneal B1 cells seen after TLR stimulation.
Figure 3.
Egress of B1 cells requires coordinated down-regulation of integrins and CD9. (A) Total number of B220lowIgMhi B1 cells recovered from the peritoneal cavity (PEC), omentum (OM), mesenterium (MES), and spleen (SPL) 3 h after i.p. injection (without any TLR stimulation) into RAG-2−/− mice of peritoneal cells preincubated for 20 min with the indicated blocking antibodies. Mean ± standard error, n = 4–7, Student's t test. (B) CD9 and integrin α4, α6, and β1 flow cytometric profiles of IgM+ gated peritoneal cavity cells. (C) Representative IgM and CD9 profiles of B220+ gated peritoneal cavity cells 5 h after i.p. injection of PBS or 20 μg LPS. Numbers indicate the mean fluorescence intensity of CD9 in gated cells. (D) Kinetics of surface expression of CD9 (red line), integrin α4 (blue line), α6 (orange line), and β1 (black line) in WT mice (squares) and TLR-2,4−/− mice (circles) after i.p. injection of 20 μg LPS. (E) Frequency distribution of peritoneal cell velocities toward CXCL13 after incubation with isotype control or 200 μg/ml anti-CD9 antibodies at the 15-min time point. p-value was measured by Student's t test. (F) Velocity of peritoneal cells from WT mice (black) or CD9−/− mice (red) determined by measuring cell displacements with successive video frames (1 min) during 50-min chemotaxis. Data represent the mean ± standard error of a minimum of 40 cells tracked for each time point and cell phenotype. (G) Competitive migration between peritoneal B1 cells from GFP Tg mice (WT) and CD9−/− mice. B1 cells (1:1 ratio) were preincubated for 30 min with anti-α4 or anti-α4 and 200 μg/ml anti-CD9 antibodies and injected i.p. into RAG2−/− mice. Percentages of GFP+ (black bars) and GFP− (white bars) cells in the B220lowIgMhi gate from the indicated tissues 3 h after injection. Error bars represent 95% confidence intervals, n = 2 mice/group.
CD9 is known to be selectively expressed by B1 cells (25) and to regulate cell motility (20). We found that CD9 expression on the surface of peritoneal B1 cells is correlative with α6 and β1 although not with α4 integrins (Fig. 3 B). These observations led us to examine if TLR stimulation induces modifications in CD9 expression that can be related with B1 cell migration out of the peritoneal cavity or peritoneum-associated tissues. As shown in Fig. 3 (C and D), i.p. stimulation with LPS induced a considerable down-regulation of CD9 expression on B1 cells 5 h after activation in WT mice but not in TLR2,4−/− mice. LPS-induced CD9 changes on B1 cells paralleled modulation of α4, α6, and β1 integrin expression, including a coordinated up-regulation 14 h after stimulation (Fig. 3 D). Furthermore, similar to α4, α6, and β1 integrins, CD9 down-regulation after TLR4 stimulation with lipid A was MyD88 independent, whereas that induced through TLR2 after PAM2CSK4 stimulation was dependent on MyD88 (Fig. 2 D). Strikingly, in vitro preincubation of peritoneal cells with both anti-α4 and anti-CD9 antibodies induced a significant depletion of B1 cells from the peritoneal cavity and a parallel increase in the number of B1 cells that migrated to the omentum, mesenterium, or spleen 3 h after transfer (Fig. 3 A). Anti-CD9 antibody treatment alone exerted a strong effect on peritoneal cell migration, with increased numbers of B1 cells being either in transit or already homed to the spleen.
The enhanced egress of B1 cells observed after CD9 blocking was not due to the down-regulation of surface integrins (not depicted) but most likely to increased cell motility. This is based on the observations that both anti-CD9 antibody–treated cells or peritoneal cells from CD9−/− mice migrated much faster than control or WT cells on fibronectin-coated microchannels in response to CXCL13 (Fig. 3, E and F, and Videos S1 and S2, which are available at http://www.jem.org/cgi/content/full/jem.20061041/DC1). To further confirm a direct involvement of CD9 in B1 cell migration, we performed in vivo competitive transfer experiments between normal and CD9−/− B1 cells. Cells (1:1 ratio of CD9−/−/GFP Tg B1) were preincubated in vitro with anti-α4 alone or anti-α4 and anti-CD9 antibodies, and B1 cell migration was evaluated 3 h after injection into the peritoneal cavity of RAG2−/− mice. As shown in Fig. 3 G, the majority of B1 cells that migrated to the spleen after integrin α4 blocking were derived from CD9−/− mice. In contrast, similar percentages of CD9−/− and GFP B1 cells were detected in the spleen after preincubation with both anti-α4 and anti-CD9 antibodies. This result clearly indicates that anti-CD9 antibodies enhanced the migration of B1 cells from GFP Tg mice by blocking the CD9 function, and that modulation of integrins and CD9 together is required to induce B1 cell mobilization. Thus, coordinated integrin-CD9 down-regulations induced by TLR stimulation cause efficient emigration of B1 cells from the peritoneal cavity.
G protein–coupled receptors are required for B1 cell egress
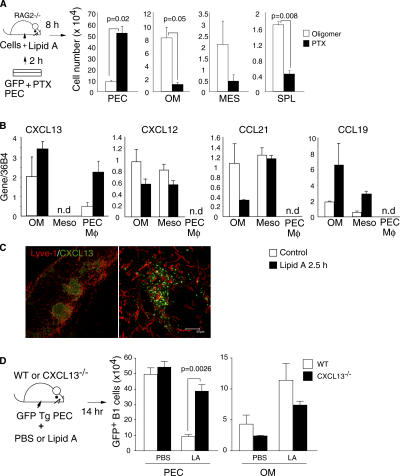
Because the B1 cell migration under steady-state conditions was shown to require intact signaling through chemokine receptors (35), we next inquired if chemokines and their receptors are also involved in B1 cell egress induced by TLR stimulation. Therefore, we tested the effect of pertussis toxin (PTX) treatment on the TLR-induced egress of B1 cells. Peritoneal cavity cells were incubated in vitro for 2 h with PTX, an enzyme that ADP ribosylates and inactivates Gαi proteins, or, as a control, with the non-ADP–ribosylating oligomer B subunit of PTX, and then transferred into the peritoneal cavity of RAG2−/− mice with lipid A. As shown in Fig. 4 A, PTX treatment caused a substantial (∼85–90%) inhibition of B1 cell migration to the omentum, mesenterium, and spleen, with their accumulation in the peritoneal cavity 8 h after cell transfer.
Figure 4.
Involvement of chemokine and chemokine receptors for B1 cell egress. (A) Total number of IgMhiMac1+B1 cells recovered from the peritoneal cavity (PEC), omentum (OM), mesenterium (MES), and spleen (SPL) of RAG2−/− mice 8 h after i.p. injection (with 10 μg lipid A) of peritoneal cells treated for 2 h with 100 ng/ml oligomer B (white bars) or PTX (black bars). Mean ± standard error, n = 2. (B) Quantitative PCR analyses of the indicated chemokines in omentum (OM), mesothelial cells (Meso), and peritoneal MΦ (PEC MΦ) expressed as relative amounts of mRNA normalized to 36B4. Bars represent the mean ± standard error of three experiments. (C) Whole mount pictures of omentum stained for CXCL13 and lymphatic endothelium marker (Lyve-1). Bar, 50 μm. (D) Total numbers of B1 cells in the peritoneal cavity (PEC) and omentum (OM) 14 h after i.p. injection of 107 peritoneal cells from GFP Tg mice without or with 10 μg lipid A into WT mice (white bars) or CXCL13−/− mice (black bars). Mean ± standard error, n = 3, Student's t test.
Several lymphoid chemokines were constitutively produced by omentum and mesothelial cells of the peritoneal wall (Fig. 4 B). Among these, the most abundantly expressed were CXCL13 and CCL19, and these were also the only chemokines of which we detected up-regulation at mRNA levels after stimulation (Fig. 4 B). Furthermore, CXCL13-producing cells were mainly located in the lymphoid aggregates of omentum (Fig. 4 C), and B1 cells showed robust chemotactic response to CXCL13, both in vivo and in vitro (29, 35–37). Consistent with published results (29), except for the CXCL13, the mRNA levels for CXCL12, CCL19, and CCL21 were very low to undetectable in peritoneal MΦ. To determine the contribution of omentum-derived CXCL13 for emigration of peritoneal B1 cells, an equal number of GFP+ peritoneal cells were injected without or with lipid A into the peritoneal cavity of WT and CXCL13−/− mice. The efficiency of GFP+ B1 cell egress was evaluated 14 h after the transfer. As shown in Fig. 4 D, fewer B1 cells migrated after lipid A stimulation to the omentum in CXCL13−/− mice than in WT mice, and the number of GFP+ B1 cells recovered from the peritoneal cavity of CXCL13−/− mice was significantly higher than the number of B1 cells recovered from WT mice. In contrast, similar numbers of B1 cells were recovered from the peritoneal cavity of both CXCL13−/− and WT mice in the absence of stimulation (Fig. 4 D). CCR7-CCL19 receptor–ligand pair might also be involved in peritoneal B1 cell egress because CCR7−/− B1 cells tend to accumulate more than WT B1 cells in the peritoneal cavity after transfer into RAG2−/− mice (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20061041/DC1) and CCR7−/− mice accumulate much more peritoneal cells as compared with WT mice (38 and unpublished data). Collectively, these results clearly indicate that G protein–coupled receptors are required for TLR-induced peritoneal B1 cell egress and that CXCL13 is an important chemokine involved in this emigration process.
DISCUSSION
We demonstrate here that independent from antigenic recognition through specific B cell receptors, direct signals through TLRs induce B1 cell egress from the peritoneal cavity. The sensing of bacteria or bacterial components triggers a series of coordinated events on B1 cells, consisting of rapid, transient, and parallel down-regulation of integrins and CD9. These changes are required for B1 cell egress because the lack of integrin-CD9 down-modulation is associated with a significant impairment in B1 cell egress in TLR4−/− mice after lipid A stimulation (Fig. 1 H). Involvement of integrins and CD9 on regulation of retention versus migration of peritoneal B1 cells was confirmed by antibody treatment as well as in competitive migration studies with WT and CD9-deficient B1 cells (Fig. 3, A and G). Furthermore, peritoneal B1 cells appear to use similar molecular machinery with classic innate cells to transduce signals from TLRs. Thus, the TLR4 signaling cascade involves both MyD88-dependent and -independent pathways, whereas signals through TLR2 are strictly dependent on MyD88 function (Fig. 2, C and D).
Besides changes in integrin-CD9 expression, the exit of B1 cells from their compartment requires chemokine receptors. This is clearly demonstrated by the fact that treatment with PTX almost completely inhibits migration out of peritoneal B1 cells, even under activation conditions. CXCR5-CXCL13 appears to be the receptor–ligand pair used by B1 cells not only for homing into the peritoneum, but also for their egress out of the peritoneal cavity. This conclusion is supported by the observation that a much larger fraction of B1 cells (injected directly into the peritoneal cavity, thus circumventing the involvement of CXCL13 for homing) remained in the peritoneal cavity of CXCL13−/− mice than in WT mice, even after stimulation through TLR (Fig. 4 D).
The responsiveness to CXCL13, abundantly produced in the omentum, appears to be influenced by modulation of CD9 because CD9-blocked peritoneal cells as well as those from CD9−/− mice migrated both faster and more efficiently to CXCL13 (Videos S1 and S2, and Fig. 3, E and F).
Sphingosine-1-phosphate receptor 1 (S1P1) was shown to be involved in lymphocyte egress from the lymphoid organs (39). Peritoneal B1 cells express S1P1 and show a moderate chemotactic response to S1P1 ligand (S1P) under nonactivated conditions (Fig. S2, B and C). However, after TLR stimulation, both S1P1 expression and the chemotactic response of B1 cells to S1P decreased (Fig. S2, B and C), making it unlikely that S1P1-S1P plays a significant role for peritoneal B1 cell egress.
TLR signals may control B1 cell recruitment and participation not only in acute responses, but also in steady-state conditions for the maintenance of immune system homeostasis. Indeed, we found that germ-free mice accumulate significantly more numbers of B1 cells in the peritoneal cavity or peritoneum-associated tissues compared with mice kept under specific pathogen-free conditions harboring a diverse gut microbiota (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20061041/DC1). We speculate that a significant fraction of B1 cells that survey the abdominal cavity are sensing the gut microbiota and are constantly induced to migrate out of the peritoneal cavity. They are likely to participate in maintenance of homeostasis of the gut barrier by producing secretory IgAs against commensal bacteria. The B1-derived IgAs are generated through a primitive T-independent and follicular-independent pathway and play an important role in the regulation of bacterial communities in the intestine (40–43). Our findings fully support the conclusion that B1 cells are innate-like B cells with specialized functions that are poised to react rapidly to gut-associated antigens and pathogens (3).
Thus, the egress of B1 cells from the peritoneal cavity is a complex, multistep process governed by interplay between integrins, tetraspanins, and chemokines that would allow these innate-like B cells to coordinate efficient immune responses against infections.
The results presented here reveal an unexpected requirement for TLR signaling in the down-modulation of integrin and tetraspanin expression on innate-like B1 cells, which is critical for their rapid mobilization and participation in immune responses. TLRs are thus involved in the control of antibody responses in multiple steps: activation and recruitment of B1 cells to effector sites of immune responses; relocalization of splenic MZ B cells from the MZ to white pulp cords (6, 44, 45); and proliferation and differentiation of B1 MZ B cells into antibody-secreting cells (6). These sequential TLR-orchestrated events lead to efficient removal of pathogens soon after infection and facilitate optimal transition from innate to adaptive immune responses.
MATERIALS AND METHODS
Mice and adoptive cell transfers.
Mice, all on a C57BL/6 background, were bred and maintained in specific pathogen-free conditions at the animal facility of the Research Center for Allergy and Immunology and used at 8–16 wk of age. Germ-free mice were obtained from Yakult. For cell transfer, WT, RAG2−/−, and CXCL13−/− mice were injected i.p. with 107 peritoneal cells obtained from age- and sex-matched GFP Tg mice. For competitive transfer experiments, peritoneal cavity cells obtained from GFP Tg mice and TLR4−/− or CD9−/− mice were counted, stained for surface markers, mixed to a 1:1 ratio of B1 cells (∼106 B220lowIgMhi cells), and injected into RAG2−/− mice. All animal experiments were performed in accordance with approved protocols from the Institutional Animal Care at RIKEN.
Stimulation with TLR ligands.
Mice were stimulated with lipid A and endotoxin-free PAM2CSK4, both purchased from InvivoGen, and LPS (Escherichia coli O26:B6) from Sigma-Aldrich. For bacteria-induced peritonitis, the E. coli DH5α strain was transformed with pGex-4T-1 (GE Healthcare) vector expressing DsRed2 (original vector pDsRed2 was from CLONTECH Laboratories, Inc.). After 10–12 h of aerobic growth at 37°C, the cultures were maintained overnight under micro-aerophilic conditions at room temperature. After this period, 70–80% of the bacteria expressed detectable levels of DsRed2. After two washes with PBS, DsRed2+ bacteria were quantified using a fluorescence microscope (Axioplan2; Carl Zeiss MicroImaging, Inc.), and 1–10 × 107 fluorescent units were injected into mice i.p. For disruption of the gut barrier, 10-wk-old BALB/c mice (Clea) were subcutaneously injected twice at 12-h intervals with 25 mg/kg indomethacin (Sigma-Aldrich). Mice were killed and analyzed after 1 d. DNA was extracted from cell suspensions obtained after the digestion of omentum and mesenterium, and the 16S rRNA was amplified by universal primers described previously (43).
Cell preparation and antibodies.
Peritoneal cells, spleen, and mesenteric lymph node cell suspensions were prepared as described previously (35). Cells from parietal peritoneum, omentum, mesenterium, and small intestine lamina propria were isolated after stirring for 45 min with 1.5 mg/ml collagenase (Wako) and 0.5 U/ml dispase at 37°C. Cells were washed twice in RPMI medium with 2% FCS and stained for flow cytometry. Allophycocyanin (APC) anti-B220 (RA3-6B2), PE anti-CD49d (integrin α4, R1-2), PE anti-CD49f (integrin α6, GoR3), biotin anti-CD29 (integrin β1, Ha2-5), biotin anti-CD9 (KMC8), and FITC anti-CD21 were from BD Biosciences. APC anti-CD11b (Mac-1) and PE anti-CD69 (H1.2F3) were from eBioscience, and FITC or PE anti-IgM was from SouthernBiotech.
Cell sorting and in vitro cultures.
Peritoneal B1 cells (B220lowIgMhiMac-1+) and spleen B cells (B220hiIgM+) were sorted, (FACSAria; Becton Dickinson) or run through the nozzle without sorting, after staining with APC anti-B220, FITC anti-IgM, and PE anti–Mac-1. Cells were incubated with RPMI medium supplemented with 10% FCS and 20 μM 2-ME without or with 20 μg/ml LPS. After 12 h, cells were recovered, washed, and stained for surface integrins.
Blocking experiments.
For integrin or/and CD9 blocking, cells were treated before i.p. injection for 20–30 min with 100 μg/ml of purified anti-integrin α4 antibody (clone R1-2) and 200 μg/ml anti-CD9 antibody (clone KMC8). All antibodies were obtained from BD Biosciences. For G protein inactivation, peritoneal cells were treated with 100 ng/ml oligomer B or PTX (Sigma-Aldrich) for 2 h in RPMI medium supplemented with 10% FCS and 20 μM 2-ME, washed, and injected i.p. into RAG-2−/− mice together with 10 μg lipid A.
Whole mount microscopy.
Omenta were dissected from animals, placed in 24-well plates, fixed with Cytofix/Cytoperm solution (BD Biosciences) for 30 min at 4°C, and incubated for 60 min with the following antibodies: goat IgG anti–mouse CXCL13 (R&D Systems), rabbit IgG anti–Lyve-1 (Abcam), FITC-conjugated donkey anti–goat IgG (H+L), and Cy3-conjugated donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories). Stained tissues were placed on slides, mounted (Vectashield; Vector Laboratories), and analyzed with a confocal laser-scanning microscope (model DMRXA2; Leica).
Chemotaxis assays and locomotion analysis.
The KK chamber (Effector Cell Institute) was used to detect real-time chemotaxis of peritoneal lymphocytes (46). Peritoneal cells were incubated in the RPMI media with 10% FCS for 1.5 h at 37°C to remove the MΦ, harvested, and incubated for an additional 20 min with 200 μg/ml anti-CD9 (KMC8) or isotype control antibodies. Cells were washed in warm RPMI 1640, 2% FCS, and 10 mM Hepes, and then loaded (1 μl of 106 cells/ml) into the top wells. CXCL13 (PeproTech) was loaded into the bottom well (10 μg/ml). The micro cover glass was coated with 40 μg/ml fibronectin (Biogenesis). A charge-coupled device camera was used to record the migration of peritoneal B cells for 1.5 h at 37°C. The migration velocity of peritoneal lymphocytes on the KK chamber was analyzed using Volocity software (Improvision Inc.). Frames of video images were captured and digitized by the computer. To measure the cell displacement, the screen x and y coordinates of individual cells were tracked over the time, followed by the calculation of cell velocity. For each sample, ∼40 cells were tracked over a 30-min period. The frame-by-frame velocity data were used to calculate the mean velocity and the variance of velocity. Chemotactic response to S1P (Sigma-Aldrich) was performed using Transwell membranes as described previously (35).
RT-qPCR.
Total RNA was isolated from cells using the RNeasy mini-kit (QIAGEN). After the spectrometric analysis, equal amounts of RNA were used for cDNA synthesis. After DNase treatment, oligo dT primers were used for first-strand cDNA synthesis (RT). All procedures were performed according to the manufacturer's instructions (Invitrogen). Quantitative PCR was performed on an iCycler thermal cycler using SYBR Green Supermix according to instructions and analyzed by software (all Bio-Rad Laboratories). All primers were determined by BEACON DESIGNER (v2.1; Premier Biosoft International). Sequences were as follows: 36B4 forward: 5′-CACTGGTCTAGGACCCGAGAAG, reverse: 5′-GGTGCCTCTGGAGATTTTCG; CXCL13 forward: 5′-TGAAGTTGTGATCTGGACCAAGA, reverse: 5′-ACAGACTTTTGCTTTGGACATGTC; CXCL12 forward: 5′-AAGGTCGTCGCCGTGCTG, reverse: 5′-GATGCTTGACGTTGGCTCTGG; CCL21 forward: 5′-GGCTATAGGAAGCAAGAACCAAGT, reverse: 5′-TCCTCAGGGTTTGCACATAGCT; CCL19 forward: 5′-CCTGGGAACATCGTGAAAGC, reverse: 5′-GCACAGAGCTGATAGCCCCTTA; and S1P1 forward: 5′-TTCCGCAAGAACATCTCCAAGG, reverse: 5′-CAGCCCACATCTAACAGTAGTAGG.
Starting quantity of cDNA was 800 ng RNA for omentum and mesothelial cell samples. Optimized final primer concentrations ranged from 150 to 300 nM, and reactions were run for 3 min at 95°C, followed by 40 cycles of 15 s at 95°C, 20 s at 60°C, 20 s at 72°C, and one cycle of 1 min at 95°C. A serially diluted positive control (bulk cDNA) and RT minus controls of each tested sample were included in each PCR reaction. Specificity of PCR products was initially confirmed by sequence analysis and subsequently by melt-curve analysis. All qPCR reactions were repeated three times with different preparations of cDNA.
Online supplemental material.
Fig. S1 shows the down-regulation of integrin α4 and β1 on peritoneal B1 cells after in vivo (i.v.) and in vitro LPS stimulation. Fig. S2 shows possible involvement of CCR7 but not S1P1 for B1 cell egress from the peritoneal cavity. Fig. S3 presents the total B1 cell numbers in the peritoneal cavity and omentum in germ-free and specific pathogen-free mice. Videos S1 and S2 show real-time chemotaxis to CXCL13 of peritoneal B cells from WT and CD9−/− mice, respectively. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061041/DC1.
Supplemental Material
Acknowledgments
We wish to thank O. Kanagawa, M. Tomura, and the common facilities staff at RCAI for advice and constant technical assistance. We also thank the Yakult Central Institute for Microbiological Research and Dr. E. Mekada for providing germ-free mice and CD9−/− mice, respectively. We are especially grateful to T. Honjo, T. Kinashi, P. Kincade, and R. Triendl for suggestions and critical reading of the manuscript.
This work was funded by RIKEN, RCAI.
The authors have no conflicting financial interests.
Abbreviations used: MΦ, macrophages; MyD88, myeloid differentiation primary response gene 88; MZ, marginal zone; PTX, pertussis toxin; S1P1, sphingosine-1-phosphate receptor 1; Tg, transgenic; TLR, Toll-like receptor.
References
- 1.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 2.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature. 438:364–368. [DOI] [PubMed] [Google Scholar]
- 3.Martin, F., and J.F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195–201. [DOI] [PubMed] [Google Scholar]
- 4.Kearney, J.F. 2005. Innate-like B cells. Springer Semin. Immunopathol. 26:377–383. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac, A., M. Bonneville, and J.F. Kearney. 2001. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 1:177–186. [DOI] [PubMed] [Google Scholar]
- 6.Martin, F., A.M. Oliver, and J.F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629. [DOI] [PubMed] [Google Scholar]
- 7.Benedict, C.L., and J.F. Kearney. 1999. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 10:607–617. [DOI] [PubMed] [Google Scholar]
- 8.Boes, M., A.P. Prodeus, T. Schmidt, M.C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas, K.M., J.C. Poe, D.A. Steeber, and T.F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 23:7–18. [DOI] [PubMed] [Google Scholar]
- 10.Baumgarth, N., O.C. Herman, G.C. Jager, L.E. Brown, L.A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell–derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochsenbein, A.F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R.M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159. [DOI] [PubMed] [Google Scholar]
- 12.Alugupalli, K.R., J.M. Leong, R.T. Woodland, M. Muramatsu, T. Honjo, and R.M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 21:379–390. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, R.R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621. [DOI] [PubMed] [Google Scholar]
- 14.Kantor, A.B., and L.A. Herzenberg. 1993. Origin of murine B cell lineages. Annu. Rev. Immunol. 11:501–538. [DOI] [PubMed] [Google Scholar]
- 15.Herzenberg, L.A., and J.W. Tung. 2006. B cell lineages: documented at last! Nat. Immunol. 7:225–226. [DOI] [PubMed] [Google Scholar]
- 16.Potocnik, A.J., C. Brakebusch, and R. Fassler. 2000. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 12:653–663. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo, A.G., J.T. Yang, H. Rayburn, and R.O. Hynes. 1996. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 85:997–1008. [DOI] [PubMed] [Google Scholar]
- 18.Arroyo, A.G., D. Taverna, C.A. Whittaker, U.G. Strauch, B.L. Bader, H. Rayburn, D. Crowley, C.M. Parker, and R.O. Hynes. 2000. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J. Immunol. 165:4667–4675. [DOI] [PubMed] [Google Scholar]
- 19.Berditchevski, F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 114:4143–4151. [DOI] [PubMed] [Google Scholar]
- 20.Hemler, M.E. 2005. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6:801–811. [DOI] [PubMed] [Google Scholar]
- 21.Wright, M.D., G.W. Moseley, and A.B. van Spriel. 2004. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 64:533–542. [DOI] [PubMed] [Google Scholar]
- 22.Miyado, K., G. Yamada, S. Yamada, H. Hasuwa, Y. Nakamura, F. Ryu, K. Suzuki, K. Kosai, K. Inoue, A. Ogura, et al. 2000. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 287:321–324. [DOI] [PubMed] [Google Scholar]
- 23.Miyake, M., H. Inufusa, M. Adachi, H. Ishida, H. Hashida, T. Tokuhara, and Y. Kakehi. 2000. Suppression of pulmonary metastasis using adenovirally motility related protein-1 (MRP-1/CD9) gene delivery. Oncogene. 19:5221–5226. [DOI] [PubMed] [Google Scholar]
- 24.Miyake, M., K. Nakano, Y. Ieki, M. Adachi, C.L. Huang, S. Itoi, T. Koh, and T. Taki. 1995. Motility related protein 1 (MRP-1/CD9) expression: inverse correlation with metastases in breast cancer. Cancer Res. 55:4127–4131. [PubMed] [Google Scholar]
- 25.Won, W.J., and J.F. Kearney. 2002. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J. Immunol. 168:5605–5611. [DOI] [PubMed] [Google Scholar]
- 26.Springer, T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- 27.Cyster, J.G. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23:127–159. [DOI] [PubMed] [Google Scholar]
- 28.Yamada, T., E. Deitch, R.D. Specian, M.A. Perry, R.B. Sartor, and M.B. Grisham. 1993. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 17:641–662. [DOI] [PubMed] [Google Scholar]
- 29.Ansel, K.M., R.B. Harris, and J.G. Cyster. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 16:67–76. [DOI] [PubMed] [Google Scholar]
- 30.Kaisho, T., K. Hoshino, T. Iwabe, O. Takeuchi, T. Yasui, and S. Akira. 2002. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int. Immunol. 14:695–700. [DOI] [PubMed] [Google Scholar]
- 31.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688–5694. [DOI] [PubMed] [Google Scholar]
- 32.Schnare, M., G.M. Barton, A.C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947–950. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 34.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. [DOI] [PubMed] [Google Scholar]
- 35.Fagarasan, S., R. Shinkura, T. Kamata, F. Nogaki, K. Ikuta, K. Tashiro, and T. Honjo. 2000. Alymphoplasia (aly)-type nuclear factor κB–inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J. Exp. Med. 191:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowman, E.P., J.J. Campbell, D. Soler, Z. Dong, N. Manlongat, D. Picarella, R.R. Hardy, and E.C. Butcher. 2000. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 191:1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen, L., S.A. Shinton, R.R. Hardy, and K. Hayakawa. 2005. Association of B-1 B cells with follicular dendritic cells in spleen. J. Immunol. 174:6918–6926. [DOI] [PubMed] [Google Scholar]
- 38.Hopken, U.E., A.H. Achtman, K. Kruger, and M. Lipp. 2004. Distinct and overlapping roles of CXCR5 and CCR7 in B-1 cell homing and early immunity against bacterial pathogens. J. Leukoc. Biol. 76:709–718. [DOI] [PubMed] [Google Scholar]
- 39.Matloubian, M., C.G. Lo, G. Cinamon, M.J. Lesneski, Y. Xu, V. Brinkmann, M.L. Allende, R.L. Proia, and J.G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360. [DOI] [PubMed] [Google Scholar]
- 40.Macpherson, A.J., D. Gatto, E. Sainsbury, G.R. Harriman, H. Hengartner, and R.M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 288:2222–2226. [DOI] [PubMed] [Google Scholar]
- 41.Fagarasan, S., K. Kinoshita, M. Muramatsu, K. Ikuta, and T. Honjo. 2001. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 413:639–643. [DOI] [PubMed] [Google Scholar]
- 42.Fagarasan, S., M. Muramatsu, K. Suzuki, H. Nagaoka, H. Hiai, and T. Honjo. 2002. Critical roles of activation-induced cytidine deaminase (AID) in the homeostasis of gut flora. Science. 298:1424–1427. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, K., B. Meek, Y. Doi, M. Muramatsu, T. Chiba, T. Honjo, and S. Fagarasan. 2004. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA. 101:1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsson, M.C., R. Guinamard, S. Bolland, M. Sankala, R.M. Steinman, and J.V. Ravetch. 2003. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 198:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu, T.T., and J.G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 297:409–412. [DOI] [PubMed] [Google Scholar]
- 46.Kanegasaki, S., Y. Nomura, N. Nitta, S. Akiyama, T. Tamatani, Y. Goshoh, T. Yoshida, T. Sato, and Y. Kikuchi. 2003. A novel optical assay system for the quantitative measurement of chemotaxis. J. Immunol. Methods. 282:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.