Abstract
Pathogen persistence after clinical cure is a hallmark of many chronic infections. Previously, we showed that naturally occurring CD4+CD25+ regulatory T (nTreg) cells rapidly accumulate within chronic dermal sites of Leishmania major infection where they suppress anti-pathogen CD4+ T cell responses, favor parasite persistence and dermal pathology, and consequently control concomitant immunity. Here, we postulated that chemokines might direct nTreg cell homing in sites of infection and show that CD4+CD25+ nTreg cells, compared with normal CD4+ T cells, preferentially express the CCR5 chemokine receptor, which enables them to migrate in response to CCR5 ligands in vitro. We show that in contrast to their wild-type (WT) counterparts, CCR5−/− CD4+CD25+ nTreg cells resulted in an increased magnitude of parasite-specific, interferon γ–producing CD4+ T cells within infection sites, dramatically reduced parasite numbers, and potent resistance to infection, a finding consistent with the clinical outcome of infected CCR5−/− mice. Interestingly, this resistance was related to an inefficient migration of CCR5−/− nTreg cells to infected dermal sites compared with WT nTreg cells. Thus, this study shows that CCR5 directs the homing of CD4+CD25+ nTreg cells to L. major–infected dermal sites where they promote the establishment of infection and long-term survival of the parasite in the immune host.
Naturally occurring CD4+ regulatory T (nTreg) cells that represent 5–10% of peripheral CD4+ T cells constitutively express the IL-2R α chain (CD25) and the Foxp3 transcription factor, and play a central role in maintaining self-tolerance in several models of autoimmunity (1–3). These nTreg cells acquire their immunosuppressive properties during normal thymopoesis, and abrogation of their development or function correlates with increased immunity to tumors, allergens, grafts, and pathogens and provokes the induction of multiorgan autoimmunity. Thus, CD4+ nTreg cells survive in the periphery poised to prevent potential autoimmune responses and immunopathology (4–8).
Persistent infections may represent a compromise between pathogen and host. While the host attempts to maintain strong effector immunity against reinfection and limit pathology due to an uncontrolled inflammatory response (9), pathogens strive to subvert host immunity to actively promote immunosuppression and pathogen persistence (10). Many studies show that persistent pathogens establish chronic infections by engaging nTreg cells, which function to suppress host immunity and control excessive effector immune responses (11–15). We have previously shown that nTreg cells are essential for the development and maintenance of chronic cutaneous infection in Leishmania major–resistant C57BL/6 mice (7, 12). In this model, disease is initiated by an acute phase hallmarked by pathogen replication and followed by the establishment of a chronic phase characterized by the stable maintenance of a low number of parasites at the site of primary challenge, the absence of overt pathology, and resistance to reinfection. Regulatory T cells rapidly accumulate at sites of infection, where they suppress anti-pathogen CD4+ T cell responses, favor the persistence of a small number of parasites within cutaneous lesions, and consequently control concomitant immunity, as mice lacking nTreg cells achieve sterile cure and lose immunity to a secondary infection. In this model, regulation was antigen specific and partially dependent on nTreg cell–derived IL-10 production (16, 17). However, the molecular basis dictating the homing of nTreg cells to infected sites is not fully understood.
The differential ability of CD4+CD25− and CD4+CD25+ T cell subsets to resolve disease may in fact reflect differences in their homing to lymphoid organs and sites of inflammation. Lymphocyte homing and trafficking in inflamed lymphoid and nonlymphoid tissues may be facilitated by the expression of distinct sets of chemokine receptors, which provide directional cues for the migration and recruitment of T cells into sites of inflammation (18). Currently, the chemokine basis of nTreg cell trafficking to sites of inflammation is not clearly understood (19). In vitro studies have shown that a large fraction of CD4+CD25+ T cells from human peripheral blood selectively express CCR4 and CCR8 and show a strong chemotactic response to CCR4 ligands(20), consistent with a CCR4-dependent recruitment of regulatory T cells in human ovarian carcinoma (21). Another study showed that CD4+CD25+CD62L+ T cells in prediabetic nonobese diabetic mice express high levels of CCR7, thus potentially migrating toward the lymphoid chemokines CCL19 and CCL21 (22). Recently, Kleinewietfeld et al. (23) have shown that CCR6 is expressed on a distinct subset of mouse and human nTreg cells with an effector–memory phenotype and function that is enriched in peripheral blood and rapidly accumulates in the central nervous system after the induction of experimental allergic encephalomyelitis. Bystry et al. (24) has shown that murine CD4+CD25+ T cells express CCR5 and display in vitro responsiveness to CCR5 chemokines, whereas another study has indicated that CCR5 may be modulating nTreg cell activity in graft-versus-host disease (25, 26). Thus, chemokine receptors may endow nTreg cells with a competitive advantage over effector T cells to migrate more efficiently to inflammatory sites where they prevent immune responses.
In this study, we sought to determine whether CCR5 is selectively expressed on nTreg cells and further characterize its functional significance in the context of an L. major infection in vivo. First, we show that CD4+CD25+ nTreg cells, in contrast to conventional CD4+ T cells, preferentially express the CCR5 chemokine receptor, which enables them to migrate in response to the CCR5 ligands in vitro. We also demonstrate that CCR5 deficiency is associated with an increase in anti–L. major effector T cell expansion and IFN-γ production within infection sites. Furthermore, we show that CCR5−/− CD4+CD25+ nTreg cells migrated less efficiently to infected dermal sites compared with WT nTreg cells, thus allowing the development of a high number of parasite-specific, CD4+ effector T cells and potent resistance to infection. Collectively, this study shows that CCR5 directs the homing of CD4+CD25+ nTreg cells to L. major–infected dermal sites where they promote the establishment of infection and ensure the long-term survival of the parasite in the immune host.
RESULTS
CD4+CD25+ nTreg cells express CCR5
Previously, we have shown that a rapid influx of CD4+CD25+ nTreg cells occurs in infected dermal sites during the silent phase of disease, which is characterized by peak parasite numbers in the site of L. major infection before the onset of adaptive immunity (7). The early and preferential recruitment of nTreg cells to infected sites might be critical to the ultimate establishment of a state of immunosuppression that dampens anti-parasite immunity and favors the parasite persistence (7, 12). As CCR5 has been proposed to influence nTreg activity in graft-versus-host disease and tumor immunity, we hypothesized that CCR5 may drive the recruitment of nTreg cells to infectious sites.
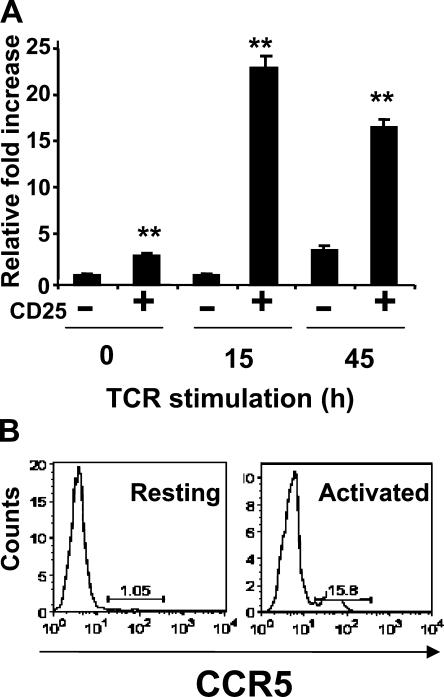
To validate the CCR5 expression on nTreg cells, we performed quantitative real-time PCR analysis on total RNA isolated from resting or TCR-stimulated CD4+CD25− or CD4+CD25+ T cells (Fig. 1 A). In freshly isolated, unstimulated CD4+CD25+ T cells, CCR5 mRNA levels were approximately fivefold greater than their CD4+CD25− T cell counterparts. By 12 h after TCR stimulation, CCR5 mRNA levels in CD4+CD25+ nTreg cells were ∼20-fold greater than their CD4+CD25− T cell counterparts. After 48 h of TCR engagement, CCR5 expression levels on CD4+CD25+ T cells, albeit fivefold lower than the 12-h time point, were nonetheless significantly greater (fourfold increase) than their CD4+CD25− counterparts. This preferential CCR5 gene expression on nTreg cells is also confirmed by our finding that 1% of resting and 15% of activated CD4+CD25+ nTreg cells preferentially express cell surface CCR5 (Fig. 1 B). Thus, although the expression of CCR5 on nTreg cells is detectable in the absence of activation, its expression is significantly increased upon antigen stimulation, consistent with a recent study from Bystry et al. (24).
Figure 1.
CD4+CD25+ regulatory T cells preferentially express CCR5. (A) CD4+CD25− (−) or CD4+CD25+ (+) T cells were isolated from the LNs of C57BL/6 mice by FACS and stimulated with 10 μg/ml of plate-bound anti-CD3 and 5 ng/ml IL-2. At various time points after stimulation, total RNA was isolated and real-time PCR analysis of CCR5 expression relative to 18S rRNA expression was performed. Similar results were obtained in three additional experiments performed. Results represent the mean ± SD. **, P < 0.001, difference from WT mice. (B) CCR5 expression on resting (left) and anti-CD3–activated (right) CD4+Foxp3+ T cells. Cells were stained for intranuclear Foxp3 and surface CD4 and CCR5, and analyzed by FACS. Similar results were obtained in three separate experiments performed.
CCR5 is required for CD4+CD25+ nTreg cell chemoattraction, but not suppression, in vitro
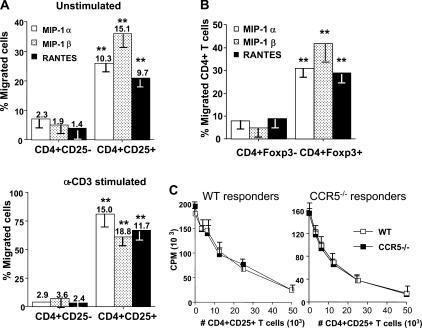
To directly determine whether the CCR5 mRNA is translated into the functional protein in nTreg cells, we explored the chemotactic response profile of highly purified CD4+CD25− and CD4+CD25+ T cells to CCR5 ligands MIP-1α, MIP-1β, or RANTES (100 ng/ml) in transwell chambers (Fig. 2). CD4+CD25+ T cells exhibited almost a 2.5-fold increased migratory capacity in the absence of chemokine stimulation compared with CD4+CD25− T cells (not depicted). Our results show that both resting and activated CD4+CD25− T cells migrated very poorly to every chemokine (Fig. 2 A). In stark contrast, 26, 36, and 21% of resting, unstimulated CD4+CD25+ T cells migrated in response to MIP-1α, MIP-1β, or RANTES, respectively (P < 0.003). Similarly, 81, 61, and 67% of anti-CD3–activated CD4+CD25+ T cells showed preferential chemotactic activity to MIP-1α, MIP-1β, or RANTES, respectively (P < 0.007). The addition of neutralizing antibodies to these chemokines completely abrogated the migration of nTreg cells in this assay (not depicted). Using total CD4+ T cells, we observed that MIP-1α, MIP-1β, or RANTES strongly induced the migration of a substantial population of Foxp3+CD4+ T cells (Fig. 2 B). This confirms that nTreg cells, in contrast to CD4+CD25− T cells, preferentially express CCR5 and respond to its ligands.
Figure 2.
CCR5 is required for CD4+CD25+ regulatory T cell chemoattraction, but not suppressive activity, in vitro. (A) CD4+CD25− or CD4+CD25+ T cells were isolated as described in Materials and methods. Resting or anti-CD3 activated (10 μg/ml in the presence of irradiated, T cell–depleted spleen cells for 72 h) T cells were subjected to transwell migration assays in the presence or absence of 100 ng/ml MIP-1α, MIP-1β, or RANTES. Histograms show percentage of CD4+CD25− or CD4+CD25+ T cells that migrated toward CCR5 chemokines without (top) and with (bottom) anti-CD3 stimulation. Values above each histogram indicate chemotactic index for each chemokine. Results represent the mean ± SD. **, P < 0.001, difference from WT mice. (B) Chemotaxis assays were performed as in A, and migrated T cells were stained intranuclearly for Foxp3. Histograms show percentage of CD4+ T cells that are Foxp3+ or Foxp3− that migrated toward CCR5 ligands. Results represent the mean ± SD. **, P < 0.001, difference from WT mice. (C) CCR5 is not required for suppressive activity of CD4+CD25+ T regulatory cells. 5 × 104 WT (left) or CCR5−/− (right) CD4+ responder T cells were stimulated with 0.5 μg/ml anti-CD3 and 2 × 105 irradiated, T cell–depleted spleen cells in the presence of titrated numbers of WT (□) or CCR5−/− (▪) CD4+CD25+ nTreg cells. Similar results were obtained in three additional experiments performed.
As nTreg cell suppression occurs via a contact-dependent and cytokine-independent manner in vitro, we postulated that these cells could use CCR5 to localize in physical proximity to target cells and engage in suppression. To this end, we isolated nTreg cells from WT or CCR5−/− mice and analyzed their ability to suppress proliferation of WT (Fig. 2 C, left) and CCR5−/− (Fig. 2 C, right) responder T cells. In all cases, CD4+CD25+ nTreg cells were equally capable of suppressing anti-CD3–induced proliferation regardless of nTreg cell/responder ratios. In vitro neutralization of each chemokine failed to abrogate the suppressive activity of CD4+CD25+ T cells (not depicted). Collectively, these results show that CCR5 is not required for nTreg cell suppressive function in vitro but does promote nTreg cell chemoattraction to CCR5 ligands.
CCR5−/− mice are resistant to L. major infection
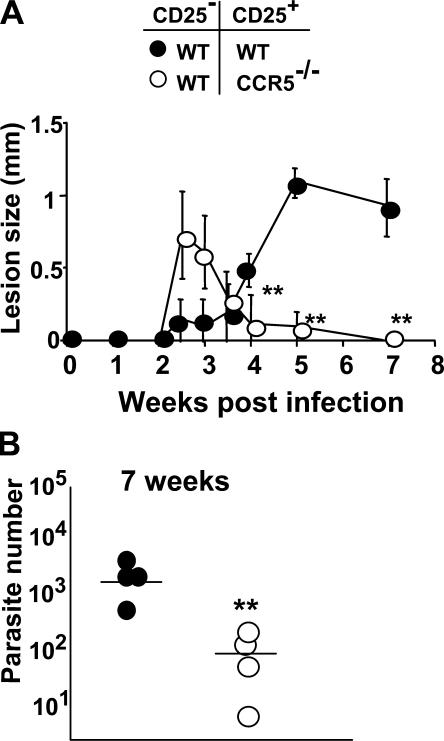
We next sought to examine the relative contribution of CCR5 expression on CD4+CD25+ nTreg cells in a low-dose intradermal (i.d.) model of L. major infection in C57BL/6 mice. WT and CCR5−/− C57BL/6 mice were infected with 103 L. major metacyclics in one ear, and the parasite burden and disease severity were monitored 6 wk after infection. In WT mice, infection resulted in a transient pathology that coincided with an increase in the parasite burden (Fig. 3 A, left and right). Interestingly, CCR5−/− mice were readily able to control L. major infection since the parasite burden in infectious sites was significantly reduced compared with WT (P < 0.009) and correlated with a reduced pathology throughout the course of infection (Fig. 3 A, left and right). These findings show that CCR5 is essential for parasite persistence and the establishment of L. major infection in genetically resistant mice, consistent with the observation made by Sato et al. (27, 28).
Figure 3.
CCR5−/− mice are resistant to L. major infection. (A) Parasite load and lesion sizes in L. major–infected WT or CCR5−/− mice. Mice were infected i.d. in each ear with 103 L. major promastigotes, and at various time points after infection parasite load (left) and lesion size (right) were determined. For each genotype, four to five mice were analyzed at every time point. Results represent the mean ± SD. **, P < 0.001, difference from WT mice. (B) Accumulation of dermal CD4+CD3+ (•) and TCRβ+ IFN-γ–producing cells (⋄) at 3 and 12 wk after infection in WT and CCR5−/− mice. Purified dermal cells were incubated overnight with L. major–infected BMDCs before labeling for CD4, CD3, or TCRβ surface markers and intracytoplasmic staining for IFN-γ. Numbers represent the absolute number of CD4+CD3+or TCRβ+IFNγ+ cells per ear. Dermal cells from six or eight ears were pooled. This experiment is representative of two distinct experiments. Results represent the mean ± SD. *, P < 0.01 and **, P < 0.01, difference from WT mice at 3 and 12 wk. (C) Accumulation of IL-10–producing dermal CD3+ cells 12 wk after infection in WT and CCR5−/− mice. Purified dermal cells were incubated overnight with L. major–infected BMDCs before labeling for CD3 surface marker and intracytoplasmic staining for IL-10. Numbers represent the percentage of CD3+ IL-10+ cells per ear. Results represent the mean ± SD. **, P < 0.001, difference from WT mice. Similar results were obtained in three separate experiments performed.
In our low-dose murine model of L. major infection, the long-term persistence of parasites in infected sites is exquisitely determined by an equilibrium that is established between IFN-γ–producing CD4+ effector T cells and IL-10–producing CD4+CD25+ nTreg cells (7). In this model, parasite growth or elimination is rapidly triggered if the activities of IFN-γ or IL-10 are, respectively, impaired. To determine if the increased resistance in CCR5−/− mice occurred as a result of a functional imbalance in the IFN-γ/IL-10 regulatory axis, WT and CCR5−/− mice were infected i.d. with L. major, and the degree of anti-pathogen Th1 immunity was determined at 3 and 12 wk after infection (Fig. 3 B). At 3 wk after infection, CCR5−/− mice displayed a 2.7-fold increase in i.d. CD4+ T cells compared with infected WT mice (6.3 × 106 vs. 2.3 × 106 CD4+CD3+). Surprisingly, this increased accumulation of T cells in CCR5−/− mice also correlated with a 3.6-fold increased number of IFN-γ–producing T cells in infected dermal sites compared with WT mice (79 × 103 vs. 22 × 103 TCRβ+ cells) at 3 wk after infection. CCR5−/− mice were highly resistant to infection as they had a significant reduction in the number of parasites in dermal sites and surprisingly developed little or no overt pathology in contrast to WT mice (Fig. 3 A). This increased ability to control the parasite load in infected CCR5−/− mice compared with WT mice at 12 wk was associated with a twofold increased accumulation of T cells in i.d. sites, which paralleled the kinetics of accumulation of IFN-γ–producing T cells in the site (48 × 103 vs. 23 × 103 TCRβ+ cells) before parasite killing (Fig. 3 B). Interestingly, CCR5−/− mice also had a 6.2-fold decrease in the frequency of IL-10–producing T cells in dermal sites compared with WT mice (8.7 vs. 1.4% of CD3+ T cells), consistent with the decreased susceptibility to L. major typically seen in WT mice devoid of nTreg cells (Fig. 3 C). Thus, CCR5 deficiency perturbs the equilibrium between effector and nTreg cells within sites of infection, amplifies local immune responses in favor of anti-pathogen immunity, and promotes parasite clearance.
CCR5−/− CD4+CD25+ regulatory T cells fail to promote parasite persistence
To directly assess the relative contribution of CCR5 expressed by nTreg cells in host immunity and parasite persistence, freshly isolated WT or CCR5−/− CD4+CD25− effector T cells were transferred into RAG−/− recipients either alone or in combination with CD4+CD25+ T cells from naive WT or CCR5−/− C57BL/6 mice, and the severity of disease was monitored at various time points after infection (Fig. 4). At 3.5 wk after infection, the approximate time of peak parasite load establishment in C57BL/6 mice, recipients of either WT or CCR5−/− CD4+CD25− T cells alone harbored very few parasites in the site and developed minimal or no dermal pathology (not depicted). At the same time point, RAG−/− recipient mice reconstituted with either CD4+CD25−/CCR5−/− CD4+CD25+ or CD4+CD25−/WT CD4+CD25+ cell populations displayed a similar ability to control infection and developed a transient dermal pathology, which in each case was greater than in recipients of either WT or CCR5−/− CD4+CD25− alone (Fig. 4 A and not depicted). At 7 wk after infection, recipients reconstituted with CD4+CD25−/WT CD4+CD25+ T cells developed a lesion similar to that seen in chronically infected WT mice with >1,000 parasites persisting in the site (Fig. 4 B). In contrast, in the presence of CCR5−/− CD4+CD25+ T cells, RAG−/− recipient mice healed faster and completely cleared the parasites from the site of infection to an extent similar to WT mice devoid of nTreg cells (Fig. 4). Thus, CCR5 expression on nTreg cells contributes directly to controlling anti-parasite immunity within infected sites.
Figure 4.
CCR5−/− CD4+CD25+ regulatory T cells fail to promote parasite persistence. RAG2−/− mice were infected in one ear with 103 L. major promastigotes. At the time of infection, WT CD4+CD25− T cells (3 × 105/mouse) were adoptively transferred together with CD4+CD25+ T cells (3 × 104/mouse) obtained from either WT (•) or CCR5−/− mice (○). (A) Lesion sizes were measured at various time points after infection. Results are shown as the mean ± SD of the induration (four to six mice per time point). (B) Parasite numbers were assessed at 7 wk after infection. Numbers represent the mean parasite number per ear (four mice per group). Results represent the mean ± SD. **, P < 0.01, difference from WT mice. Similar results were obtained in three separate experiments performed.
CCR5-dependent homing of CD4+CD25+ regulatory T cells in infected dermal sites
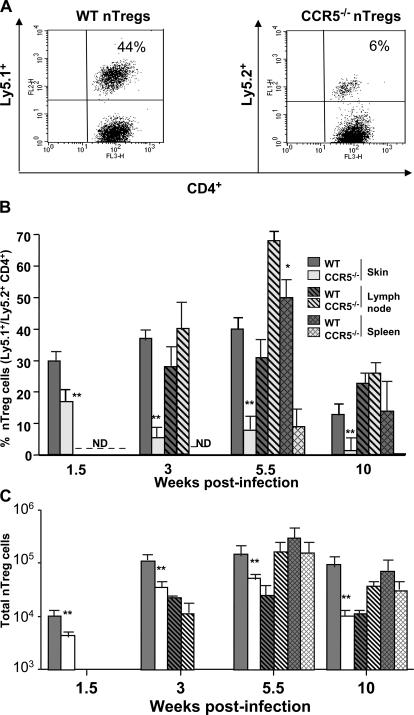
Our studies clearly show that CCR5 deficiency alters the ability of nTreg cells to control L. major immunity. To directly address whether CCR5 dictates their migration from peripheral lymphoid compartments to sites of persistent infection, we used a Ly5 congenic transfer system to independently monitor nTreg and effector T cells from WT and CCR5−/− mice at various time points after L. major infection. We cotransferred at a 1:10 ratio CD4+CD25+ nTreg cells from naive Ly5.1+ WT or Ly5.2+ CCR5−/− mice and CD4+CD25− T cells from naive Ly5.1+ or Ly5.2+ WT mice into RAG−/− recipients, and the numbers of each subset were analyzed during the accumulation of peak parasite numbers in infected dermal sites. By 3 wk after infection, the frequency of WT Ly5.1+CD4+CD25+ nTreg cells represented 44% of total CD4+ T cells in the infected dermis (Fig. 5 A). In contrast, the frequency of CCR5−/− Ly5.2+CD4+CD25+ T cells decreased to 6%, representing a 7.3-fold decrease compared with the WT condition (Fig. 5 A), suggesting that CCR5 may be directing the homing of nTreg cells in L. major–infected sites during the silent phase of disease.
Figure 5.
CCR5-dependent homing of CD4+CD25+ regulatory T cells to infected dermal sites. Naive CD4+CD25+ T cells from Ly5.1 WT or Ly5.2 CCR5−/− C57BL/6 mice and CD4+CD25− T cells from Ly5.1 WT or Ly5.2 WT C57BL/6 mice were purified and coinjected i.v. at a ratio of 1 CD4+CD25+ for 10 CD4+CD25− (3 × 104/3 × 105) into RAG2−/− mice 1 d before i.d. infection with L. major. (A) Percentage of WT (Ly5.1+) or CCR5−/− (Ly5.2+) CD4+ nTreg cells in the infected ear 3 wk after infection. Values represent the mean of four to six ears. The numbers represent the percentage of positive events in the quadrant. (B) Percentage of WT or CCR5−/− nTreg cells in the skin, LN, and spleen during the course of the infection. Single cell suspensions from each tissue of individual mice were extracted and stained with anti–TCRβ chain, anti-CD4, and anti-Ly5.2/Ly5.1 antibodies. Percentage of Ly5.2+/Ly5.1+ cells (nTreg) was calculated on TCRβ+CD4+ gated events. (C) Absolute numbers of WT or CCR5−/− nTreg cells in the skin, LN, and spleen during the course of the infection. Cells were analyzed as in B. Results represent the mean ± SD. *, P < 0.001 and **, P < 0.001, difference from WT mice. Similar results were obtained in three experiments performed.
To examine the evolution of nTreg cell recruitment into inflamed sites, we monitored the number of nTreg cells at different time points in the spleen, draining LN, and infected dermal sites (Fig. 5 B). At 1.5 wk after infection, during the silent phase of disease characterized by parasite growth, WT nTreg cells (Ly5.1+) represented 30% of CD4+ T cells in dermal sites, although they were undetectable in draining LN or spleen. The frequency of WT nTreg cells steadily increased to ∼30–40% of CD4+ T cells in the dermis and draining LN at 3 wk and remained at these levels at 5.5 wk after infection, during the stage of lesion formation and immune clearance of parasites, with the exception of the spleen where the percentage of WT nTreg cells represented 50% of T cells. At 10 wk, around the onset of the chronic phase, the Ly5.1+ cells (WT nTreg) represented ∼15% of the CD4+TCRβ+ T cells in the dermis and ∼25% in the LN. In addition, WT nTreg cells continued to accumulate efficiently in the spleen during acute and chronic phases of disease (50 vs. 15% at 10 wk). These quantitative changes were greatly due to the expansion or recruitment of Ly5.2+ CD4+ effector T cells in the site and not to a decrease in the absolute number of Ly5.1+ CD4+ nTreg cells (not depicted). In stark contrast, CCR5−/− nTreg cells (Ly5.2+), while being undetectable in the LN and spleen, represented 15–20% of CD4+TCRβ+ T cells in dermal sites at 1.5 wk, with the frequency drastically declining to ∼5–7% of total CD4+ T cells at 3 and 5.5 wk after infection. During the chronic phase (10 wk), CCR5−/− nTreg cells (Ly5.2+) represented <5% of the CD4+TCRβ+ T cells in the site of infection. Surprisingly, CCR5−/− nTreg cells accumulated more efficiently than WT cells in the draining LN of infected mice at 3 wk (40 vs. 30%, respectively) and 5.5 wk (70 vs. 30%, respectively) after infection. At 10 wk after infection, CCR5−/− nTreg cells migrated less efficiently than their WT counterparts in the spleen during acute and chronic phases of disease (10 vs. 50% at 5.5 wk, and 1 vs. 15% at 10 wk, respectively). These frequency changes correlated with a decrease in the absolute number of Ly5.2+ T cells in these sites, particularly during the chronic phase of infection (10 wk; Fig. 5 C). Thus, WT nTreg cells home initially to the infected site where they maintain themselves until the chronic phase of the infection, whereas CCR5−/− nTreg cells have a reduced capacity to home to similar environments and appear to accumulate in the draining LN. In summary, these studies show that CCR5 is directly responsible for the recruitment of nTreg cells to L. major–infected dermal sites where they promote infection and parasite persistence.
L. major infection induces CCR5 chemokines
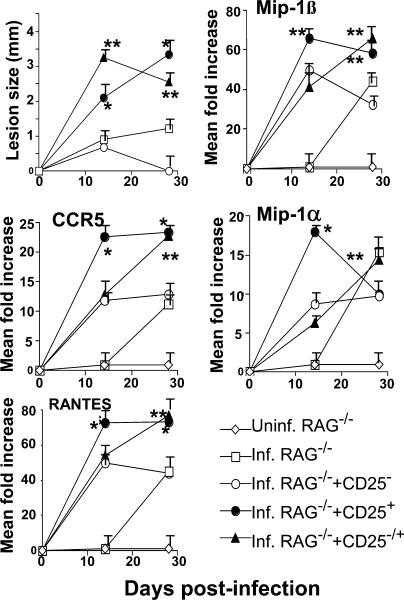
Our results show that CCR5 expression on CD4+CD25+ nTreg cells is essential for their trafficking from peripheral lymphoid tissues into infected dermal sites. We next wished to demonstrate that CCR5 ligands, such as MIP-1α, MIP-1β, or RANTES, are produced within the dermis after L. major infection and are responsible for the chemoattraction of CD4+CD25+ T cells to these sites. To this end, we transferred WT CD4+CD25− T cells from naive mice to infected RAG−/− recipients either alone or in the presence of WT CD4+CD25+ nTreg cells at a 1:10 ratio. To assess chemokine production within infected sites, we isolated the infected dermis at 14 and 28 d after infection and performed RT-PCR analysis for CCR5, MIP-1α, MIP-1β, or RANTES gene expression (Fig. 6). In contrast to uninfected RAG−/− recipients, which never expressed any chemokines, L. major infection of similar hosts resulted in a significant induction of MIP-1α, MIP-1β, or RANTES with gene expression levels being, respectively, 15-, 40-, and 45-fold higher than uninfected controls and peaking at 28 d after infection. Transfer of CD4+CD25− T cells resulted in a more rapid induction of MIP-1α, MIP-1β, or RANTES with gene expression levels being, respectively, 7-, 50-, and 45-fold higher than uninfected controls and peaking at 14 d after infection. Interestingly, cotransfer of CD4+CD25−/CD4+CD25+ T cells resulted in a significantly greater induction of MIP-1α, MIP-1β, or RANTES gene expression levels (respectively, 17-, 70-, and 75-fold higher than uninfected controls), peaking at 14 d after infection and correlating temporally with the increase in parasite burden and loss of disease control. Strikingly, RAG−/− recipient mice transferred with CD4+CD25+ nTreg cells alone also resulted in significant expression of each chemokine at every time point and paralleled the expression of CCR5 (Fig. 6) and Foxp3 (not depicted) in these sites, suggesting that nTreg cells themselves may paradoxically be responsible for the induction of CCR5 chemokines. Overall, these studies demonstrate that expression of the CCR5 ligands are actively induced after L. major infection and are temporally correlated with the recruitment of nTreg cells in infected skin where they promote the establishment of infection.
Figure 6.
L. major infection induces CCR5 chemokines. Infected RAG2−/− mice (n = 5 per group) received either no cells (□), WT CD4+CD25− T cells (3 × 105/mouse) alone (○), or CD4+CD25+ T cells (3 × 104/mouse) alone (•), or with a combination of CD4+CD25−/CD4+CD25+ T cells (▴; 3 × 105/3 × 104/mouse). Uninfected RAG2−/− mice served as a negative control (⋄). Lesion sizes were monitored weekly. RT-PCR analysis of CCR5, RANTES, MIP-1α, and MIP-1β gene expression relative to G3PDH expression was performed on total RNA isolated from the ears of various experimental groups obtained 14 or 27 d after infection. Results represent the mean ± SD. *, P < 0.01 and **, P < 0.01, difference from recipient mice with no nTreg cells. Similar results were obtained in two separate experiments performed.
DISCUSSION
CD4+ nTreg cells represent a critical peripheral switch in the control of immune responses to self-antigens as well as to a variety of pathogenic microorganisms (6, 29–37). Previously, we showed that the establishment of pathogen persistence within L. major–infected sites is dependent on a tight equilibrium between effector and nTreg cells (7). The abrogation of nTreg cell function or IL-10 production promotes clearance of the parasite, whereas depletion of effector cells or inflammatory cytokines like IFN-γ promotes disease reactivation. Interestingly, nTreg cells preferentially accumulate in sites of L. major infection during phases of parasite expansion and establishment of chronicity, in contrast to effector T cells, which increase during the acute phase of disease characterized by increased anti-pathogen immunity. Thus, differential chemokine receptor expression profiles between different T cell subsets could explain potential trafficking differences in vivo, as shown in other models (20, 23, 26, 38, 39).
In this study, we establish a novel relationship between CCR5-dependent chemotaxis and the development of immunity in L. major infection, and show that this receptor regulates critical aspects of the disease. First, we show that CD4+CD25+ nTreg cells preferentially express CCR5 and respond very efficiently in vitro to its ligands MIP-1α, MIP-1β, or RANTES. Consistent with the enhanced control of systemic Leishmania donovani infection and dermal L. major infection, we then show that CCR5−/− mice are resistant to L. major infection in contrast to their WT counterparts (27, 28). We also show that this increased resistance correlates with a significant reduction in the production of IL-10 by dermal CD4+ T cells and a predominant Th1 response in CCR5−/− mice. More importantly, CCR5−/− nTreg cells, in contrast to WT nTreg cells, migrated less efficiently to infected dermal sites and failed to suppress parasite-specific, IFN-γ–producing CD4+ T cells, thus resulting in potent resistance to infection. Collectively, this study shows that CCR5 directs the recruitment of nTreg cells to L. major–infected dermal sites where they promote the establishment of chronic infection.
We establish CCR5 as a critical checkpoint influencing the balance between regulatory and effector T cells in infectious sites. The rapid control of parasite load in CCR5−/− mice was associated with a significantly increased infiltration of CD4+ effector T cells in infected dermal sites as well as an enhanced magnitude of IFN-γ–producing CD4+ T cells in response to L. major in CCR5−/− mice compared with control mice. IFN-γ could contribute indirectly to the resolution of L. major infection in CCR5−/− mice by sustaining the inflammatory response in these mice. Although CCR5 may contribute to the pathogenesis of infectious diseases by promoting IFN-γ–producing Th1 responses, CCR5 deficiency actually augmented IFN-γ production by dermal CD4+ T cells, further supporting the notion that disease resistance is likely caused by a lack of nTreg cell–mediated regulation in infected sites. These results are consistent with the predominant Th1 response seen in a high-dose model reported by Sato et al. (28), but are contrasted by the apparent reduction of early antigen-specific IFN-γ responses in the spleen of outbred CCR5−/− mice infected i.v. with a high dose of L. donovani. Our results show no impairment in the accumulation of nTreg cells in the LN; however, we did see a reduction of parasite numbers in this site, without any apparent alterations in nTreg cell numbers in the LN (not depicted). This may suggest that the primary site is necessary for parasite maintenance in the LN, possibly by the creation of specific priming conditions or favorable cytokine environment. The ability of CCR5−/− nTreg cells to migrate efficiently to the LN but not to the infected dermal site suggests that CCR5 ligands produced in infected sites are needed to fully engage nTreg cells in these sites. Overall, CCR5 signals during primary challenge dampen anti-pathogen effector mechanisms and control the intensity of the inflammatory response within L. major–infected sites.
Our data indicates that CCR5 deficiency results in a drastic reduction in the production of IL-10 by dermal CD4+ T cells, an observation consistent with a reduction of nTreg cell function in infectious sites. A function for IL-10 in promoting parasite persistence and susceptibility to L. major infection in both susceptible and resistant strains is well documented (40–42). We have previously shown that IL-10, which is produced by nTreg cells, contributes directly to parasite persistence and concomitant immunity (7). The mechanism by which IL-10 allows parasite growth and survival is not fully defined, but it is likely due to its potent deactivating role of infected APCs that would become unresponsive to activation by IFN-γ. These results support the notion that during the chronic phase of disease, IL-10–producing nTreg cells dominate over effector mechanisms producing IFN-γ. This immunosuppressive role for IL-10 is also applicable to other animal models of infection, in which the levels of IL-10 are highly predictive of the outcome of the clinical course infection (13). The development of nTreg cells in CCR5−/− mice is similar to WT mice, thus excluding the possibility that inherent differences in nTreg cell development or maturation in WT and CCR5−/− mice underlie our findings. Our results do not exclude the possibility that CCR5 may have a direct impact on the expansion, survival, or function of nTreg cells in inflammatory settings. Although CCR5 deficiency decreases the cellular frequency of nTreg cells and consequential IL-10 production in dermal sites, CCR5 signals may be required for the terminal differentiation of IL-10–producing nTreg cells within infected sites, as recently suggested for conventional CD4+ T cell effector functions (43).
We also show that the CCR5 chemokines MIP-1α, MIP-1β, or RANTES are actively induced after a low-dose L. major infection, consistent with those made in other studies using high-dose footpad infection models of other Leishmania strains (44–47). We also make the novel observation that this expression is temporally correlated with the recruitment of nTreg cells in infected dermal sites. The cellular sources of these chemokines are unknown and may be produced by a variety of cell types throughout the course of an immune response, including activated effector T cells, infected macrophages, and dendritic cells (44). It is plausible that the initial inflammatory events after pathogen infection result in chemokine production by these cell types, which subsequently attracts nTreg cells to dampen local immune responses. Strikingly, the presence of nTreg cells alone or in the presence of effector T cells appeared to significantly increase the expression of each chemokine in infected sites, suggesting that nTreg cells themselves may paradoxically produce or induce the synthesis of the chemokines. Thus, CD4+ nTreg cells may represent a functionally diversified population consisting of different subsets, such that some are responsible for the initial chemokine release whereas others are endowed with chemokine responsiveness.
Persistent infections such as AIDS, tuberculosis, and leishmaniasis may represent a compromise between pathogen and host, such that the former subverts host immune responses to promote pathogen replication, whereas the latter maintains strong effector immunity against reinfection while limiting potential immunopathology (10). The cellular and molecular mechanisms underlying the establishment, maintenance, and disruption of the homeostatic balance between host and pathogen remain poorly understood. Many studies indicate that pathogens may establish chronic infections in immunocompetent hosts by engaging various regulatory T cells to promote immunosuppression and pathogen persistence. Our findings show that CCR5 expression on nTreg cells is a critical requirement for the establishment of chronicity in an immune host and shed light into the overall dynamics of T cell responses in infected sites. On the basis of these results, it can be envisaged that transient CCR5 blockade may lead to the development of novel immunotherapeutic strategies for various infectious diseases.
MATERIALS AND METHODS
Mice.
WT, CCR5−/−, Ly5.1 congenic C57BL/6, and C57BL/10 RAG2−/− mice were obtained from the National Cancer Institute and The Jackson Laboratory. All mice were bred and maintained in a specific pathogen-free animal facility. All mice used were generally 6–8 wk of age. All procedures conformed to the norms of the McGill University Animal Care Committee.
L. major infections and parasite quantitation.
Infective, metacyclic L. major promastigotes (clone V1 MHOM/IL/80/Friedlin) were grown and isolated from 4–5-d-old stationary cultures by negative selection of infective forms using peanut agglutinin (Vector Laboratories) as described previously (48). Mice were infected in the ear dermis with 103 L. major metacyclic promastigotes in a volume of 10 μl, and parasitic loads in the ears were determined as described previously (12, 16). In brief, the ventral and dorsal sheets of the infected ears were separated and deposited dermal side down in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml of liberase CI enzyme blend (Boehringer). Ears were incubated for 2 h at 37°C. The sheets were cut into small pieces using a Medimachine (Becton Dickinson). The tissue homogenates were filtered using a 70-μm cell strainer (Falcon Products Inc.) and serially diluted (increment 2) in a 96-well flat-bottom microtiter plate containing biphasic medium prepared using 50 μl NNN medium containing 20% of defibrinated rabbit blood overlaid with 100 μl M199/S. The number of viable parasites in each ear was determined from the highest dilution at which promastigotes could be grown out after 7 d of incubation at 26°C. The number of parasites was also determined in the local draining LNs (retromaxilar). The LNs were mechanically dissociated and the parasite load in LN cells was determined by limiting dilution as described above.
Flow cytometry of dermal T cells.
For the analysis of surface markers and intracytoplasmic staining for IFN-γ or IL-10, dermal single cell suspensions were stimulated with L. major–infected or soluble leishmania antigen–stimulated bone marrow–derived dendritic cells (BMDCs) for 12–18 h as described previously (16). During the last 4–6 h, cells were cultured with 10 μg/ml brefeldin A, fixed in 4% paraformaldehyde, and incubated with an anti–Fcγ III/II receptor in staining buffer as described previously (21). Cells were permeabilized and stained with a variety of antibodies: CD3 (145-2C11, FITC), CD4 (RM4-5, PE), IFN-γ (XMG1.2, PE), IL-10 (JES5-16E3, PE), TCRβ (H57, APC), or rat IgG2b (A95-1) and rat IgG2a (R35-95; all from BD Biosciences) isotype controls. Staining for the nuclear marker Foxp3 was performed as per the manufacturer's protocol (eBioscience). Cell acquisition was performed using a FACSCalibur flow cytometer (Becton Dickinson) and CELLQuest software.
Purification of CD4+ T cell subsets.
CD4+CD25+ or CD4+CD25− T cells from appropriate mice were purified from a pool of LN cells on a FACSVantage cell sorter to a final purity of >98% as described previously (12). Irradiated (3,000 R), T-depleted spleen cells were used as APCs and were prepared by negative selection of Thy1.2+ on the AutoMACS magnetic separation system (Miltenyi Biotec). Responder T cells were purified by negative depletion of CD8+, DX5+, B220+, and I-Ab+ cells from LNs by AutoMACS.
In vitro proliferation assays.
Proliferation assays were performed by culturing 5 × 104 CD4+ T cells in 96-well flat-bottom microtiter plates in complete RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated FCS, with 1–2 × 105 irradiated T-depleted spleen cells and 0.5 μg/ml of soluble anti-CD3 for 72 h at 37°C in 7% CO2. Cell cultures were pulsed with 1 μCi 3H-TdR for the last 6–12 h. All data represent the average counts per minute of triplicate determinations. All proliferation experiments were repeated at least three times.
Adoptive cell transfers.
CD4+CD25+ or CD4+CD25− T cells (2–3 × 105/mouse) were transferred i.v. to RAG−/− mice at various time points after i.d. L. major infection as described previously (7).
RT-PCR.
Evaluation of CCR5 gene expression was performed as follows. Total RNA was prepared from ear tissues or T cell cultures with the Trizol RNA extraction reagent, and cDNA was made using Superscript II with random hexamer primers (Invitrogen). Real-time PCR was performed using primers and a 6-carboxyfluorescein (FAM)-labeled probe for CCR5: forward primer, 5′-TGACGTCACTGGAGTTGTACGG-3′; reverse primer, 5′-GGTTCATGTCATGGATGGTGC-3′; probe, 5′-FAM-TTCAGCGCTCACTGCTCTTGTGACAG-TAMRA-3′ (Applied Biosystems). All PCR reactions were performed in triplicate using a TaqMan universal PCR master mix amplified with an ABI Prism 7700 Sequence Detection System for 40 cycles and quantified using standard curves for CCR5 and TaqMan Ribosomal RNA Control Reagents (Applied Biosystems). RT-PCR primers for MIP-1α, MIP-1β, RANTES, CCR5, Foxp3, and G3PDH were obtained from BD Biosciences. PCR products were electrophoresed, and semiquantitative analysis of gene expression was achieved by normalizing the test amplicon densitometric value with the intensity of the G3PDH amplicon for each sample by the Quantity One 4,4,1 software (Bio-Rad Laboratories) as described previously (29).
Migration assays.
Migration assays were done in 6.5-mm diameter, 5.0-μm pore size polycarbonate membrane filter transwell plates (Costar Corning). The lower well chamber contained 0.6 ml RPMI medium with respective chemokines (100 ng/ml). All chemokines were purchased from PeproTech. T cell suspensions (0.1 ml at 107 cells/ml) were added to the upper chamber. After incubation for 3 h at 37°C and 10% CO2, the cells migrated into the lower chamber, were stained with anti-CD4 (L3T4, FITC) and anti-CD25 (PC61, PE) or anti-Foxp3 (PE), and analyzed by FACS.
Statistical analysis.
Results are expressed as mean ± SD. To determine whether differences were statistically significant, the Student's t test was performed using a two-tailed distribution with unpaired samples. In some instances, group comparisons were made by ANOVA or Kruskal-Wallis, followed by Dunnet's test to determine differences between genotypes.
Acknowledgments
We are grateful for the McGill Flow Cytometry Facilities for their effort in cell sorting. We would also like to thank the excellent technical assistance provided by the animal care staff at the McGill Animal Center.
We acknowledge the financial support of the Canadian Institutes for Health Research (CIHR MOP 67211), Canadian Diabetes Association (CDA), and Foundation for Innovation (CFI). E. Yurchenko is a recipient of fellowships from the CIHR Training Grant in Neuroinflammation and the Strategic Training Centre in Infectious Diseases and Autoimmunity (McGill University). C.A. Piccirillo is the recipient of the Canada Research Chair in Regulatory Lymphocytes of the Immune System.
The authors have no conflicting financial interests.
Abbreviations used: BMDC, bone marrow–derived dendritic cell; i.d., intradermal; nTreg, naturally occurring CD4+ regulatory T.
References
- 1.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 2.Shevach, E.M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 4.Shevach, E.M. 2001. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 193:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein, L., K. Khazaie, and H. von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccirillo, C.A., and E.M. Shevach. 2004. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16:81–88. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 8.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 16:115–123. [DOI] [PubMed] [Google Scholar]
- 9.O'Garra, A., P.L. Vieira, P. Vieira, and A.E. Goldfeld. 2004. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J. Clin. Invest. 114:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay, B.B., and G. McFadden. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 124:767–782. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid, Y., and B.T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360. [DOI] [PubMed] [Google Scholar]
- 12.Mendez, S., S.K. Reckling, C.A. Piccirillo, D. Sacks, and Y. Belkaid. 2004. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J. Exp. Med. 200:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse, M., C.A. Piccirillo, Y. Belkaid, J. Prufer, M. Mentink-Kane, M. Leusink, A.W. Cheever, E.M. Shevach, and T.A. Wynn. 2004. The pathogenesis of Schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172:3157–3166. [DOI] [PubMed] [Google Scholar]
- 14.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat. Med. 10:29–30. [DOI] [PubMed] [Google Scholar]
- 15.Long, T.T.A., S. Nakazawa, S. Onizuka, M.C. Huaman, and H. Kanbara. 2003. Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. Int. J. Parasitol. 33:175–183. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid, Y., E. Von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M.C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992–4000. [DOI] [PubMed] [Google Scholar]
- 17.Suffia, I.J., S.K. Reckling, C.A. Piccirillo, R.S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charo, I.F., and R.M. Ransohoff. 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354:610–621. [DOI] [PubMed] [Google Scholar]
- 19.Zou, W. 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6:295–307. [DOI] [PubMed] [Google Scholar]
- 20.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curiel, T.J., G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J.R. Conejo-Garcia, L. Zhang, M. Burow, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949. [DOI] [PubMed] [Google Scholar]
- 22.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 23.Kleinewietfeld, M., F. Puentes, G. Borsellino, L. Battistini, O. Rotzschke, and K. Falk. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T-cell subset. Blood. 105:2877–2886. [DOI] [PubMed] [Google Scholar]
- 24.Bystry, R.S., V. Aluvihare, K.A. Welch, M. Kallikourdis, and A.G. Betz. 2001. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2:1126–1132. [DOI] [PubMed] [Google Scholar]
- 25.Luster, A.D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129–135. [DOI] [PubMed] [Google Scholar]
- 26.Wysocki, C.A., Q. Jiang, A. Panoskaltsis-Mortari, P.A. Taylor, K.P. McKinnon, L. Su, B.R. Blazar, and J.S. Serody. 2005. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 106:3300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato, N., S.K. Ahuja, M. Quinones, V. Kostecki, R.L. Reddick, P.C. Melby, W.A. Kuziel, and S.S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major–resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato, N., W.A. Kuziel, P.C. Melby, R.L. Reddick, V. Kostecki, W. Zhao, N. Maeda, S.K. Ahuja, and S.S. Ahuja. 1999. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR)5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J. Immunol. 163:5519–5525. [PubMed] [Google Scholar]
- 29.Kullberg, M.C., V. Hay, A.W. Cheever, M. Mamura, A. Sher, J.J. Letterio, E.M. Shevach, and C.A. Piccirillo. 2005. TGF-β1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur. J. Immunol. 35:2886–2895. [DOI] [PubMed] [Google Scholar]
- 30.Piccirillo, C.A., and A.M. Thornton. 2004. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 25:374–380. [DOI] [PubMed] [Google Scholar]
- 31.Herman, A.E., G.J. Freeman, D. Mathis, and C. Benoist. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mottet, C., H.H. Uhlig, and F. Powrie. 2003. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170:3939–3943. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone, J.A., and Q. Tang. 2005. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 17:638–642. [DOI] [PubMed] [Google Scholar]
- 34.Belkaid, Y. 2003. The role of CD4+CD25+ regulatory T cells in Leishmania infection. Expert Opin. Biol. Ther. 3:875–885. [DOI] [PubMed] [Google Scholar]
- 35.Manigold, T., E.-C. Shin, E. Mizukoshi, K. Mihalik, K.K. Murthy, C.M. Rice, C.A. Piccirillo, and B. Rehermann. 2006. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees recovered from Hepatitis C. Blood. 107:4424–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahlenkamp, T.W., M.B. Tompkins, and W.A.F. Tompkins. 2005. The role of CD4+CD25+ regulatory T cells in viral infections. Vet. Immunol. Immunopathol. 108:219–225. [DOI] [PubMed] [Google Scholar]
- 37.Walther, M., J.E. Tongren, L. Andrews, D. Korbel, E. King, H. Fletcher, R.F. Andersen, P. Bejon, F. Thompson, and S.J. Dunachie. 2005. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 23:287–296. [DOI] [PubMed] [Google Scholar]
- 38.D'Ambrosio, D., F. Sinigaglia, and L. Adorini. 2003. Special attractions for suppressor T cells. Trends Immunol. 24:122–126. [DOI] [PubMed] [Google Scholar]
- 39.Putheti, P., M. Morris, L. Stawiarz, N. Teleshova, P. Kivisakk, M. Pashenkov, M. Kouwenhoven, M.K. Wiberg, L. Bronge, Y.M. Huang, et al. 2003. Multiple sclerosis: a study of chemokine receptors and regulatory T cells in relation to MRI variables. Eur. J. Neurol. 10:529–535. [DOI] [PubMed] [Google Scholar]
- 40.Belkaid, Y., K.F. Hoffmann, S. Mendez, S. Kamhawi, M.C. Udey, T.A. Wynn, and D.L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti–IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemos de Souza, V., J. Ascencao Souza, T.M. Correia Silva, P. Sampaio Tavares Veras, and L.A. Rodrigues de-Freitas. 2000. Different Leishmania species determine distinct profiles of immune and histopathological responses in CBA mice. Microbes Infect. 2:1807–1815. [DOI] [PubMed] [Google Scholar]
- 42.Kane, M.M., and D.M. Mosser. 2001. The role of IL-10 in promoting disease progression in Leishmaniasis. J. Immunol. 166:1141–1147. [DOI] [PubMed] [Google Scholar]
- 43.Molon, B., G. Gri, M. Bettella, C. Gomez-Mouton, A. Lanzavecchia, C. Martinez-A, S. Manes, and A. Viola. 2005. T cell costimulation by chemokine receptors. Nat. Immunol. 6:465–471. [DOI] [PubMed] [Google Scholar]
- 44.Luther, S.A., and J.G. Cyster. 2001. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2:102–107. [DOI] [PubMed] [Google Scholar]
- 45.Teixeira, M.J., J.D. Fernandes, C.R. Teixeira, B.B. Andrade, M.L. Pompeu, J. Santana da Silva, C.I. Brodskyn, M. Barral-Netto, and A. Barral. 2005. Distinct Leishmania braziliensis isolates induce different paces of chemokine expression patterns. Infect. Immun. 73:1191–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antoniazi, S., H.P. Price, P. Kropf, M.A. Freudenberg, C. Galanos, D.F. Smith, and I. Muller. 2004. Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infect. Immun. 72:5168–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977. [DOI] [PubMed] [Google Scholar]